ABSTRACT
Introduction: Some studies have suggested that baseline tumor-infiltrating-lymphocytes (TILs), such as CD8+ and FoxP3+ T-cells, may be associated with a better prognosis in colorectal cancer. We sought to investigate modulation of the immune response by preoperative radiotherapy (preopRT) and its impact on survival in locally advanced rectal cancer (LARC).
Materials & Methods: We analyzed data for 237 patients with LARC who received RT. Density of TILS (CD8+ and FoxP3+) in intraepithelial (iTILs) and stromal compartments (sTILs) were evaluated from surgery pathological specimens and biopsies performed at baseline. The primary endpoint was to assess the impact of infiltration of the tumor or tumor site after preopRT on progression-free survival (PFS) and overall survival (OS). Secondary endpoints were the impact of dose fractionation scheme on TILs.
Results: In univariate analysis, several factors significantly correlated (p<0.05) with PFS and/or OS (T-stage, M-stage, the delay between RT and surgery). A high level of post-treatment FoxP3+ TIL density correlated significantly with a better PFS (p = 0.007). In multivariate analysis, a decrease in the CD8+/FoxP3+ iTILs ratio after preopRT correlated with better PFS and OS (p = 0.049 and p = 0.024, respectively). More particularly, patients with a delta CD8+/FoxP3+ <−3.8 had better PFS and OS. Interestingly, the dose fractionation scheme significantly influenced the CD8+/FoxP3+ ratio after treatment (p = 0.027) with a lower ratio with hypofractionated RT (≥2 Gy).
Conclusion: Patients with LARC who had a significant decrease in the CD8+/FoxP3+ ratio after preopRT were more likely to live longer. This ratio needs to be validated prospectively to guide physicians in adjuvant treatment decision-making.
Introduction
Short-course preoperative radiotherapy (sc-preopRT) or long-course preoperative radiotherapy (lc-preopRT) with or without chemotherapy followed by total mesorectal excision (TME) is the backbone in the management of locally advanced rectal adenocarcinoma (LARC).Citation1,2
Baseline tumor-infiltrating lymphocytes (TIL) in colorectal tumors were described as prognostic factors associated with better survival for CD8+ TILs and surprisingly also for Treg TILs expressing Fork-head box P3 (FoxP3+).Citation3–6
Treg cells, a subgroup of CD4+ T helper cells expressing the FoxP3 transcription factor, are able to suppress T-cell immunity in both physiological and pathological environments. On one hand, in most varieties of human cancers, a high density of FoxP3+ TILs is associated with an unfavorable prognosis.Citation7 On the other hand, a recent meta-analysis highlighted that FoxP3+ TILs were associated with an improvement in overall survival (OS) in colorectal cancer (CRC).Citation8 This opposite effect can be explained by FoxP3+ TILs in CRC may preferentially control the T-Cell immune response driven by microbial rather than by tumor antigens.Citation5
We aimed to investigate the impact of preoperative sc-preopRT or lc-preopRT on the immune response, expressed by CD8+ and FoxP3+TILs, and the impact of this response on progression-free survival (PFS) and OS in rectal cancer managed with TME.
Materials and methods
Study patients
We retrospectively studied cancer-tissue specimens from 237 patients who underwent preoperative RT or concomitant preoperative chemoradiotherapy (preopCRT) in LARC in 3 French centers (Georges François Leclerc Cancer Center, Dijon, University Hospital of Besançon and Institut de Cancérologie de Lorraine), Nancy from 1995 to 2007. Patients received lc-preopRT (≤ 2 Gy/fraction) or sc-preopRT (> 2 Gy / fraction). Patients with cancer-tissue specimens fixed using a buffer other than formaldehyde were excluded from this study.
This study was approved by the IRB and the French CCTIRS committee (Comité consultatif sur le traitement de l'information en matière de recherche et de santé) and CNIL (Commission nationale de l'informatique et des libertés).
TIL assessment
TILs (CD8+ and FoxP3+) were retrospectively analyzed in collected formalin-fixed paraffin of surgical samples for all patients and in rectal biopsy samples at diagnosis for 135 patients. Immunohistochemistry used monoclonal antibodies against T-cell marker CD8 (dilution 1/200, clone C8/144B, Dako, France) and the Treg cell marker FoxP3 (dilution 1/100, clone 236 A/E7, Abcam, France,) using the same portal as previously described.Citation9 Every slide was digitized using the NDP Nanozoomer scanner (Hamamatsu Photonics, Japan).
CD8+ and Foxp3+ cells were quantified using 20X magnification on at least three distinct and representative fields of 0.46 mm2 at the level of tumor, tumor regression or tumor site (in cases with a complete response) for surgical samples.
Histopathologic evaluation of TILs was performed by a pathologist and a biologist who were blinded to clinical information. Using the generalized kappa test, the biologist evaluation was validated by a substantial agreement (data not shown).
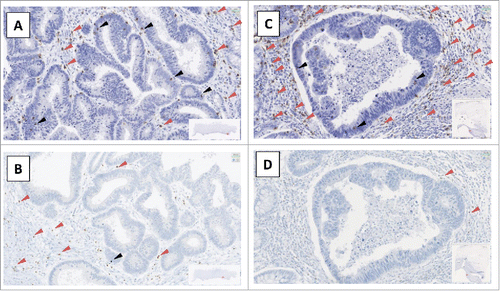
Mean values of these three fields were analyzed. Using criteria described by Denkert et al.,Citation10 intraepithelial TILs (iTILs) were defined as the number of lymphocytes per field in direct contact with tumor cells, whereas stromal TILs (sTILs) were defined as the number of lymphocytes, per field, in the tumor stroma (, Supplementary Fig. 1).
Figure 1. Example of slides of FoxP3 and CD8 labeling A and C: CD8+ labeling, B and D: FoxP3+ labeling, A and B patient N°1; C and D: patient N°2, red arrow: sTILs (stromal Tumor-Infiltrating Lymphocytes), black arrow: iTILS (intraepithelial Tumor-Infiltrating Lymphocytes).
The effect of neoadjuvant treatment on TILs was evaluated using delta parameters which were the subtraction of TIL values after treatment minus TIL values at baseline.
Statistical analyses
All analyses were performed using Stata V13 software (StataCorp LP, College Station, TX). Quantitative variables were described using mean with SD (standard deviation) or median with range. Qualitative variables were described using percentages. Cox multivariate models, adjusted for chemotherapy, T, N, M and the delay between surgery and RT, were performed to evaluate the impact of each TILs on PFS and OS.
A Kruskall-Wallis test was used to study the effect of RT fractionation on tumor lymphocyte infiltration. Effect of neoadjuvant treatments on TILs quantity was determined using paired Wilcoxon test. P < 0.05 was considered statistically significant. All confidence intervals (CIs) were stated at the 95% confidence level.
Results
Characteristics of patients and treatments
The baseline clinical characteristics of patients and treatment details are listed in . There were 159 men and 78 women with a median age of 66.9 years [28.7–85.5]. Regarding the radiotherapy, 174 patients received lc-preopRT and 60 patients received sc-preopRT. Nearly half of the patients received concomitant chemotherapy (CT) (46.9%). The most frequent concomitant CT regimens were Capecitabine (36.9%), Folic Acid+5FU (28.8%) and Oxaliplatin+Capecitabine (22.5%). Seventy-three patients (30.8%) received adjuvant chemotherapy.
Table 1. Characteristics of patients included in LYMPHOREC study.
Description of TILs at baseline and after preopRT
At baseline, CD8+ and FoxP3+ TILs in biopsy samples were observed in the same proportion (mean 54.0±44.5 and 50.9±57.4 positive cells per field, respectively ()). Both CD8+ and FoxP3+ TILs infiltration were higher in stromal (median 35.7 [0–203.5] and 32 [0–245.7], respectively) compared with intra-epithelial areas (median 4.05 [0–87.2] and 0 [0–20.7], respectively).
Table 2. Effect of neo-adjuvant treatments on TILs quantity.
After preopRT, we found no variation in CD8+ TILs (p = 0.496) whereas a significant decrease in FoxP3+ TILs was observed (p<0.001) for both FoxP3+ iTILs and FoxP3+ sTILs (p<0.001) but with a lower infiltration intensity concerning FoxP3+ iTILs (≤6.6 cells per field) ().
The CD8+/FoxP3+ TILs ratio was significantly increased by preopRT (p = 0.008). This increase concerned CD8+/FoxP3+ sTILs (p = 0.012) but not CD8+/FoxP3+ iTILs (p = 0.753) ().
Effect of short-course versus long-course preopRT on TILs
Quantities of CD8+ TILS and FoxP3+ TILs were comparable for patients treated with either sc-preopRT or lc-preopRT ().
Table 3. Effect of radiotherapy scheme on TILs.
Conversely, the radiotherapy scheme significantly modulated the CD8+/FoxP3+ TILs ratio (p = 0.027), and more particularly at the level of stroma (p = 0.019).
Indeed, the CD8+/FoxP3+ TILs ratio and more particularly sTILs were significantly lower after sc-preopRT (mean 4.3 ± 5.9 and 3.9 ± 5.8, respectively) than after lc-preopRT (mean 8.2 ± 15.0 and 7.4 ± 13.2, respectively) with p = 0.027 and p = 0.017 for TILs and sTILs, respectively ().
Impact of TILs parameters on survival
The median follow-up was 8.1 years (95% CI [7.4–8.8]). The median PFS was 6.3 years (95% CI [4.3–8.3]) and median OS was 7.9 years (95% CI [6.4–10.9]).
Concerning TILs at baseline, CD8+ TILs were associated with neither PFS (p = 0.432) nor OS (p = 0.119). In contrast, patients who had FoxP3+ TILs and more particularly FoxP3+ sTILs at baseline had a better OS than patients with no FoxP3+ TILs in univariate (p = 0.016 and p = 0.027, respectively) and multivariate analysis (p = 0.040 and p = 0.035 respectively) ( and ).
Table 4. Relation between TILs at the baseline and after preopRT and progression free survival.
Table 5. Relation between TILs at the baseline and after preopRT and overall survival.
After preoperative RT, CD8+ TILs and FoxP3+ TILs were associated with better PFS (p = 0.011 and p = 0.003). After preopRT, a high FoxP3+ infiltration (≥36.5) was related to a better PFS and a low FoxP3+ infiltration (<6.5) was related to a much worse PFS. In a similar fashion, a high CD8+ infiltration (≥83.0) was related to a better PFS and a low CD8+ infiltration (<24.6) was related to a much worse PFS. A similar trend was found with multivariate analysis on PFS and OS (p = 0.081 and p = 0.059, respectively).
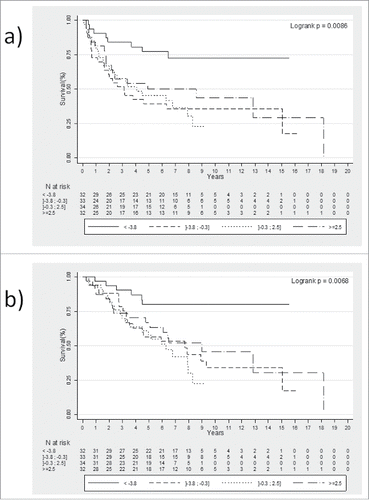
The delta of CD8+ TILs, delta of FoxP3+ TILs or delta of the CD8+/FoxP3+ TILs ratio did not correlate with PFS or OS, whereas delta of the CD8+/Foxp3+ iTILs ratio between baseline and after preoperative RT was significantly associated with better PFS and OS in univariate (p = 0.015 and p = 0.014, respectively) and multivariate analysis (p = 0.049 and p = 0.024, respectively) ( and ).
Thus, using quartile value analysis, we found that patients with a delta CD8+/FoxP3+ iTILS <−3.8 had better PFS (, Logrank p = 0.0086) and OS (, Logrank p = 0.0068).
Discussion
Immune cell infiltration, particularly T lymphocyte infiltration, has been investigated in various tumor types and displays a strong correlation with improved outcomes in colorectal, and other types of cancer.Citation9,11–13 While clinical outcomes are largely dependent on stage at diagnosis and treatment, mounting evidence suggests that host immune infiltration at baseline may also be a highly informative prognostic indicator.Citation14,15 in colorectal cancers. It has been understood for years that individuals with colorectal cancers containing many TILs have a survival advantage over those that do not.Citation11,16–18
In colorectal cancers, Galon et al. has developed “Immunoscores” based on CD3+ and CD8+ TILs density. These scores completed the validated AJCC/UICC TNM classification to improve prognostic values.Citation19 In a study based on 426 patients with colorectal cancer, Ling et al. showed the impact of a disparity in immune infiltrates on survival according to intratumoral subsite localization.Citation3 The authors found that intraepithelial infiltrations of CD8+ and FoxP3+ T lymphocytes at the tumor front and center were major prognostic factors in cancer-specific survival but they suggested that FoxP3+ TILs had a stronger correlation with survival than did CD8+ TILs. Moreover, these authors and others found a positive impact on survival of a high expression of CD8+ iTILs whatever the expression of FoxP3 whereas patients with low CD8+ iTILs expression who had a high infiltration of FoxP3+ at the tumor invasive front had a better prognosis.Citation20
While colon cancers and rectal cancers are often mixed and regarded as harboring the same pathology, the management of each differs. Colon cancers are recognized as non-radiosensitive cancers and are managed with surgery only, with or without adjuvant chemotherapy depending on stage whereas rectal cancers require radiotherapy before surgery to avoid local and regional failure while the impact of neoadjuvant or adjuvant chemotherapy on survival is still debated. It therefore seems important to study the impact of TILs variation on survival in patients with the same pathology.
Conversely, the effect of radiotherapy on TILs deserves to be explored in greater depth in particular for rectal cancer, contrary to the effect of chemotherapy on TILs in breast cancer.Citation9,21 Recent retrospective studies performed in small cohorts of patients with rectal cancer have highlighted an increase in CD8+ TILs after preoperative radiotherapy, which was associated with survival.Citation22–24 To the best of our knowledge, our study is the largest to date about LARC alone to demonstrate the advantage of preoperative RT on survival with respect to baseline TILs but also the response of immune infiltrates to RT (i.e. an increase in CD8+/FoxP3+ ratio).
A meta-analysis performed from 30 studies, concluded that a high tumor inflammatory infiltrate could be a good prognostic marker for colorectal cancer.Citation15 In the tumor stroma, high CD8+ TILs were associated with increased OS.
Thus, to take into account these previous studies, we chose to evaluate the number of each TIL and their stromal or intraepithelial localization.
A large proportion of studies evaluated the impact of TILs on tumor regression (for example.Citation24,25) Using the validated TRG scale, we found no correlation between TILs before preopRT (data not shown) or after preopRT (supplementary ) and complete or not tumor regression.
In our present study, we found no correlation between PFS or OS and CD8+ TILs at baseline. These results are in agreement with a recent study conducted in 557 patients with colorectal cancer. These authors analyzed the impact of TILs at baseline on survival according to the initial tumor location. They highlighted that a high level of TILs CD8+ at baseline was related to better OS only when the tumor was located in the right colon (p<0.01) and not when tumors were located in the left colon or in the rectum (p = 0.087 and p = 0.656, respectively).Citation26
However, in multivariate analysis, we demonstrated that FoxP3+ TILs at baseline, and more particularly FoxP3 sTILS, correlated with OS. Nonetheless, among the four quartiles analyzed we did not find a cut-off which could better guide neoadjuvant and adjuvant strategies.
Very few studies have attempted to establish the role of preoperative RT in local anti-tumor immunity in rectal cancer.
We found that FoxP3+ TILs were significantly decreased by RT. The effect of radiotherapy on FoxP3+ TILs has been little studied. Recently, McCoy et al. studied levels of FoxP3+TILs before and after CRT but their analysis used two TIL intensity levels (high vs. low) and it was not a quantitative analysis as in the present study. As a result, they were not able to highlight a TILs modification with high precision.Citation27 In contrast to previous studies, which analyzed smaller numbers of patients, we did not find a significant increase in CD8+ TILs after RT.Citation23,24 Surprisingly, a significant decrease in the CD8+/FoxP3+ ratio was highlighted after preoperative RT suggesting that an immunological balance between CD8+ TILs and FoxP3+ TILs occurs after exposure to ionizing radiation. This decrease expressed using the delta CD8+/FoxP3+ iTILs parameter correlated significantly with OS and PFS.
In the present study, the modulation of local immunity observed after preoperative treatment was independent to chemotherapy as no difference in CD8+ and Foxp3+ TILs was observed between patients treated with preoperative RT or preoperative RT/CT (with respectively p = 0.124 and p = 0.694, data not shown). Among the concomitant chemotherapy drugs administered in 111 patients, some are able to trigger immunogenic cell death, as is the case for Oxaliplatin.Citation28 or to decrease MDSC as is the case for 5FU.Citation29 Thus, these CT may have induced such effects, but the biological variability between the patients and the small number of patients treated with these drugs prevented us from conducting a subgroup analysis.
Interestingly, we highlighted a significant difference in the effect of RT on TILs according to the RT administration schedule. Indeed, the CD8+/FoxP3+ TILs ratio, and more particularly sTILs, were significantly higher after lc-preopRT than after sc-preopRT. These results are in keeping with the results reviewed by Hellevik et al.,Citation30 who reported that the effect of RT on the microenvironment, and more particularly on tumor immunity, was RT schedule dependent according to dose and fractionation. We observed a significant difference for the delay between RT and surgery according to whether patient had lc-preopRT or sc-preopRT (means (SD) were respectively 44.5 (29.5) days and 35.1 (25.1) days, data not shown). However, the delay had no significant impact on TILs variations after preopRT (data not shown).
Unlike the majority of others studies, we did not choose to perform a semi-quantitative evaluation of TILs. We were thus able to determine a cutoff for delta CD8+/FoxP3+, which could be validated in a future prospective study.
If these results were confirmed by a prospective study, a routine assessment and quantification of TILs before and after treatment for rectal cancer patients could provide clinically meaningful prognostic information and may give an early indication of treatment efficacy. Thus from these results, clinicians could determine whether patients would benefit from additional CT.
Disclosures of interest
The authors report no conflict of interest.
supp_data.zip
Download Zip (2.9 MB)Acknowledgments
This work was supported by the “Cancéropôle Grand Est”, and the “Conseils Régionaux de Bourgogne, de Franche Comté et de Lorraine”. The authors thank Laura Guyard for tissue sample organizations; Zélie Barthod for the clinical data collection, Cécile Dalban for inter-observer correlation analysis and Mélanie Gauthier and Julie Blanc for their significant help to statistical analysis. We thank Dr L. Arnould and his team for their kind help in the IHC experiment. They also thank Philip Bastable for his review and corrections of the wording in this manuscript.
References
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23. doi:10.1056/NEJMoa060829. PMID:16971718.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi:10.1056/NEJMoa010580. PMID:11547717.
- Ling A, Edin S, Wikberg ML, Oberg A, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110:2551–9. doi:10.1038/bjc.2014.161. PMID:24675384.
- Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, Sinicrope FA. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One. 2012;7:e42274. doi:10.1371/journal.pone.0042274. PMID:22879926.
- Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–18. doi:10.1007/s00262-011-1046-y. PMID:21644034.
- Salama P, Stewart C, Forrest C, Platell C, Iacopetta B. FOXP3+ cell density in lymphoid follicles from histologically normal mucosa is a strong prognostic factor in early stage colon cancer. Cancer Immunol Immunother. 2012;61:1183–90. doi:10.1007/s00262-011-1191-3. PMID:22210551.
- Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F. Human FOXP3 and cancer. Oncogene. 2010;29:4121–9. doi:10.1038/onc.2010.174. PMID:20498631.
- Huang Y, Liao H, Zhang Y, Yuan R, Wang F, Gao Y, Wang P, Du Z. Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: A meta analysis. PLoS One. 2014;9:e94376. doi:10.1371/journal.pone.0094376. PMID:24827118.
- Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi:10.1002/path.2866. PMID:21437909.
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi:10.1200/JCO.2009.23.7370. PMID:19917869.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi:10.1126/science.1129139. PMID:17008531.
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi:10.1056/NEJMoa051424. PMID:16371631.
- Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi:10.1038/onc.2009.416. PMID:19946335.
- Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi:10.1186/1479-5876-10-1. PMID:22214470.
- Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–605. doi:10.1038/bjc.2014.46. PMID:24504370.
- Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J Pathol. 2010;222:350–66. doi:10.1002/path.2774. PMID:20927778.
- Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi:10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. PMID:9349235.
- Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585–9. doi:10.1136/jcp.39.6.585. PMID:3722412.
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi:10.1002/path.4287. PMID:24122236.
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi:10.1200/JCO.2008.18.7229. PMID:19064967.
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi:10.1200/JCO.2013.55.0491. PMID:25071121.
- Lim SH, Chua W, Cheng C, Descallar J, Ng W, Solomon M, Bokey L, Wong K, Lee MT, de Souza P, et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34:6505–13. PMID:25368252.
- Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S414–21. doi:10.1245/s10434-014-3584-y.
- Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, Zhang J, Yu J. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5:2064–74. PMID:26269765.
- Vacchelli E, Semeraro M, Enot DP, Chaba K, Poirier Colame V, Dartigues P, Perier A, Villa I, Rusakiewicz S, Gronnier C, et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget. 2015;6:20840–50. doi:10.18632/oncotarget.4428. PMID:26369701.
- Berntsson J, Svensson MC, Leandersson K, Nodin B, Micke P, Larsson AH, Eberhard J, Jirström K. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A cohort study. Int J Cancer 2017;141:1654–66. doi:10.1002/ijc.30869. PMID:28677162.
- McCoy MJ, Hemmings C, Anyaegbu CC, Austin SJ, Lee-Pullen TF, Miller TJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, et al. Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8:19803–13. PMID:28177891.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–91. doi:10.1038/onc.2009.356. PMID:19881547.
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi:10.1158/0008-5472.CAN-09-3690. PMID:20388795.
- Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front Oncol. 2014;4:1. doi:10.3389/fonc.2014.00001. PMID:24478982.