ABSTRACT
Interleukin (IL)-25, a member of the IL-17 cytokine superfamily, is produced by immune and non-immune cells and exerts type 2 pro-inflammatory effects in vitro and in vivo. The IL-25 receptor(R) is composed of the IL-17RA/IL-17RB subunits. Previous work showed that germinal centre (GC)-derived B-cell non Hodgkin lymphomas (B-NHL) expressed IL-17AR, formed by IL-17RA and IL-17RC subunits, and IL-17A/IL-17AR axis promoted B-NHL growth by stimulating neoangiogenesis. Here, we have investigated expression and function of IL-25/IL-25R axis in lymph nodes from human GC-derived B-NHL, i.e. Follicular Lymphoma (FL,10 cases), Diffuse Large B Cell Lymphoma (6 cases) and Burkitt Lymphoma (3 cases). Tumor cells expressed IL-25R and IL-25 that was detected also in non-malignant cells by flow cytometry. Immunohistochemical studies confirmed expression of IL-25R and IL-25 in FL cells, and highlighted IL-25 expression in bystander elements of the FL microenvironment. IL-25 i) up-regulated phosphorylation of NFkBp65, STAT-1 and JNK in B-NHL cells; ii) inhibited in vitro proliferation of the latter cells; iii) exerted anti-tumor activity in two in vivo B-NHL models by dampening expression of pro-angiogenic molecules as VEGF-C, CXCL6 and ANGPT3.
In conclusion, IL-25, that is intrinsically pro-angiogenic, inhibits B-NHL growth by reprogramming the angiogenic phenotype of B-NHL cells.
Introduction
The lymphoma microenvironment plays a critical role in tumor initiation, progression, immune escape, and chemoresistance.Citation1–4 In this vein, our group has recently demonstrated a role of IL-17A, the most renowned member of the IL-17 family, in B cell non-Hodgkin lymphoma (B-NHL) progression.Citation5
IL-17A is produced by T helper-17 CD4+ T cells and plays pivotal roles in protection from bacterial and fungal infections and development of autoimmune diseases.Citation6,7 IL-25, also known as IL-17E, diverges from the other members of the IL-17 cytokine family since it promotes Th2 immune responses.Citation8–12 Thus, overexpression of IL-25 resulted in increased production of IL-4, IL-5, IL-9 and IL-13,Citation13 increased antibody class switching to IgG1 and IgE, and recruitment of eosinophils, basophil, mast cells, along with CD4+Th2 cells.Citation13,14 IL-25 is produced by immune cells, such as CD4+, CD8+ T cells, macrophages, dendritic cells, mast cells, eosinophils, and non-immune cells, such as epithelial and endothelial cells.Citation15 In murine models, IL-25 deficiency results in defective Th2 response and higher susceptibility to infections.Citation10,16,17
IL-25 binds the heterodimeric receptor composed of IL-17RA and IL-17RB subunits.Citation15 IL-17RA is expressed ubiquitously, with high levels in the hematopoietic cell compartment.Citation7,18 IL-17RB is highly expressed in kidney, liver and brain.Citation19 Both IL-17RA and IL-17RB are involved in IL-25 driven signal transduction.Citation20
IL-25 is highly expressed in lungs of patients with idiopathic pulmonary fibrosis and induces collagen production by human fibroblasts.Citation21 In a mouse model of allergen-induced airway response, blockade of IL-25 abolished airway remodelling.Citation22 Intranasal challenge with IL-25 increased peribronchial endothelial vessels in the airways of mice with chronic asthma and strongly upregulated expression of various pro-angiogenic molecules.Citation23 Endothelial cells express IL-25R,Citation24 and IL-25 induced angiogenesis in vitro by increasing basic Fibroblast Growth Factor (bFGF) mRNA and protein expression in human vascular endothelial cells through IL-17RB signalling.Citation25 IL-25 produced by synoviocytes antagonized the pro-inflammatory effects of IL-17A by acting as receptor antagonist.Citation26
Conflicting results have been published on the role played by IL-25 in tumor growth. Thus, in breast cancer, IL-25 produced by normal epithelial cells was shown to bind IL-25R expressed on adjacent neoplastic cells and to induce selective apoptosis of the latter cells.Citation27 Moreover, IL-25 treatment reduced tumor growth in xenograft models of melanoma, breast, lung, colon and pancreatic cancers.Citation28 In contrast, IL-25 had a pro-oncogenic role in breast cancer and hepatocarcinoma, stimulating proliferation and survival of malignant cells, promoting metastasis, and contributing to drug resistance.Citation29–31
In this study, we have investigated expression and function of IL-25 in the tumor microenvironment of different GC-derived B-NHL, i.e. Follicular Lymphoma (FL), Diffuse Large B-cell Lymphoma (DLBCL) and Burkitt Lymphoma (BL). FL presents a follicular growth pattern that is partially retained for a long time together with most of the microarchitectural characteristics of normal GCs.Citation32,33 In contrast, BL and DLBCL are high-grade malignancies showing diffuse effacement of the lymphoid tissue architecture and displaying aggressive behavior and unfavorable outcome.Citation33,34 Both DLBCL and FL occur commonly in adults and rarely in children or adolescents.Citation34 DLBCL is the most frequent B-NHL subtype, with approximately one third of cases originating from the transformation of FL.Citation33 BL affects predominantly children or young adults, with frequent intra-abdominal or extranodal involvement.Citation34
Here, we show that B-NHL cells expressed IL-25R and IL-25, and that the latter was expressed also in the non-malignant components of the tumor microenvironment. Moreover, IL-25 was found to exert anti-tumor activity in two different in vivo models of B-NHL lymphomas of GC origin, mainly through potent inhibition of neo-angiogenesis and consequent induction of ischemic necrosis.
Results
Expression of IL-25R/IL-25 in GC-derived B-NHL cells
We investigated by flow cytometry the expression of the heterodimeric IL-25R (composed of IL-17RA and IL-17RB chains) on tumor cells from lymph node biopsies of patients with FL (n = 10), DLBCL (n = 6) and BL (n = 3). Malignant cells were detected and gated according to the expression of monoclonal Ig κ or λ light chains. We showed IL-17RB expression and confirmed IL-17RA expressionCitation5 in B-NHL cells () (Mean of Mean Relative Fluorescence Intensity (MRFI) ± SD for IL-17RA: FL = 3.1 ± 1.4; DLBCL = 2.4 ± 1.2 and BL = 2.4 ± 0.8; MRFI ± SD for IL-17RB: FL = 3.4 ± 1.5; DLBCL = 2.9 ± 0.9 and BL = 1.8 ± 0.4). Accordingly, immunohistochemical analysis of five FL lymph nodes documented the expression of IL-17RB in neoplastic cells ().
Figure 1. Expression of IL-25R and IL-25 in primary tumor cells from patients with FL, DLBCL or BL. (A) MNCs from B-NHL lymph nodes were double stained with anti-Ig κ or anti λ mAbs in combination with anti IL-17RA or anti IL-17RB mAbs, and analyzed by flow cytometry gating on the cell fraction expressing monoclonal κ or λ chains. Results for 10 FL, 6 DLCBL and 3 BL cases are shown in histograms, as mean % positive cells +SD. (B) Immunohistochemical analysis of IL-17RB in representative lymph nodes from patients with FL (X200 left panel and X400 right panel). IL-17RB is expressed in the neoplastic GC of FL. (C) Immunohistochemical analysis of IL-25 in representative lymphnodes from patients with FL. IL-25 expression is diffusely distributed among FL cells and associated microenvironment components (X200 left panel and X400 right panel). (D) Cell suspensions from B-NHL lymph nodes were surface stained with anti-Ig κ and anti λ mAbs, permeabilized, stained intracellularly with anti-IL-25 mAb and analyzed by flow cytometry gating on the cell fraction expressing monoclonal κ or λ chains. Results are means % positive cells from 5 FL, 3 DLCBL and 2 BL cases pooled together +SD.
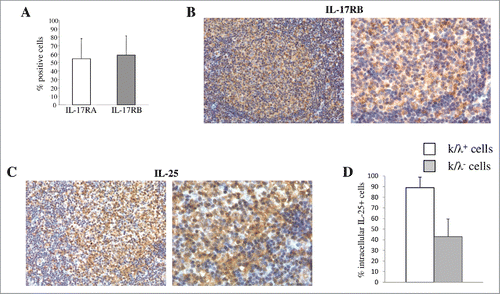
Next, we asked whether IL-25 was expressed in the B-NHL microenvironment. Cell suspensions from 5 FL, 3 DLCBL and 2 BL lymph node biopsies were stained with anti-Ig κ and anti λ mAbs on the cell surface and anti-IL-25 mAb in the cytoplasm, and analyzed by flow cytometry gating on cells expressing monoclonal κ or λ chains. shows the results of all B-NHL cases pooled together. B-NHL cells (clonal Ig light chain positive) were the major source of IL-25 that was also detected in non-malignant cells (clonal Ig light chain negative) (). Immunohistochemical analysis of five FL lymph nodes revealed diffuse expression of IL-25 both in neoplastic cells and bystander elements of the neoplastic GC microenvironment ().
Functional Activity of IL-25 on B-NHL cells
IL-25 signalling involves the canonical NF-kB pathway and the MAPK or JNK pathways.Citation35 Signaling initiated by binding of hrIL-25 to IL-25R was investigated by flow cytometry in primary FL and DLBCL (assessing 3 cases each). Isolated tumor cells incubated from 0 to 60 minutes with 50ng/ml hrIL-25 up-regulated significantly phosphorylated (p)NF-kBp65 (with a peak at 5 minute) (, left panel), but not unphosphorylated NF-kBp65 (similar levels of 91 ± 5% expression in treated and untreated cells). Furthermore, IL-25 enhanced pSTAT1 and pJNK, with peaks at 30 and 5 minutes, respectively (, middle and right panels, respectively), while leaving unphosphorylated STAT-1 and JNK unaltered (similar levels of 94 ± 5% and 96 ± 2% expression, respectively, in treated and untreated cells).
Figure 2. IL-25 signaling and function in B-NHL cells. (A) Flow cytometric analysis of pNFkBp65, pSTAT1 and pJNK in purified lymphoma cells (3 FL and3 DLBCL) treated for 0, 5 or 30 min with hrIL-25 (50 ng/ml). Results are shown as median MRFI, maximum, minimum and first and third quartile. (p = 0.0089 for pNF-kBp65, p = 0.0071 for pSTAT-1 at five minutes of treatment and p = 0.0024 for pJNK at 30 minutes of treatment). (B-C) Primary neoplastic cells from 5 FL and 3 DLBCL patients were cultured without (Med) or with rhIL-25 or hrCD40L and tested for proliferation by 3H-TdR incorporation (B) or by intracellular staining for PCNA (C). Results are expressed in box plot, as median cpm (B) or % positive cells (C), maximum, minimum, first and third quartile. (D) CAM assay. Gelatin sponges were adsorbed with: vehicle alone-PBS (left panel), hrIL-25 (middle panel) or VEGF-A (right panel). Original magnification: x 50.
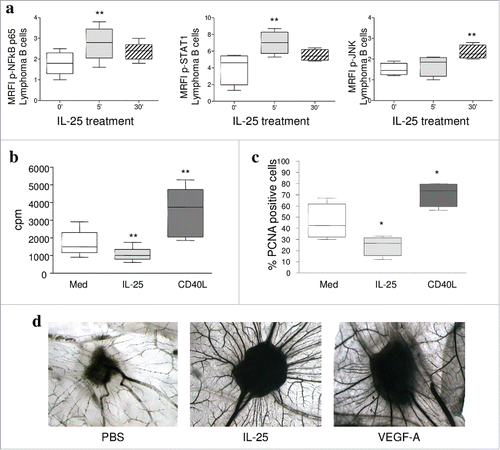
Next, tumor cells purified from 5 FL and 3 DLBCL lymph nodes were incubated with 50 ng/ml hrIL-25 and tested for proliferation. IL-25 significantly dampened 3H-thymidine incorporation in tumor cells, whose proliferation was increased by hrCD40L tested as control ().Citation36 These results were confirmed by intracellular flow cytometric analysis of PCNA ().The proportions of apoptotic cells, as assessed by AnnexinV/PI staining, were similar in IL-25 treated and untreated lymphoma cells (Supplemental Fig. 1). Finally, IL-25 did not attract lymphoma cells (Supplemental Fig. 1) nor enhanced their migration to CXCL12 or CXCL13 (data not shown), as shown for normal GC B cells.Citation37
We next performed CAM experiments to test the direct angiogenic activity of hrIL-25. At day 12, hrIL-25 induced a significant angiogenic response in the form of numerous allantoic neovessels developing radially towards the implant in a ‘spoked-wheel’ pattern (mean number of vessels = 24+/−4 for hrIL-25) (, middle panel). The pro-angiogenic activity of hrIL-25 was comparable to that of Vascular Endothelial Growth Factor (VEGF)-A tested as positive control (mean number of vessels = 24+/−3) (, right panel). PBS, used as negative control, did not induce any angiogenic response (mean number of vessels = 7+/−1) (, left panel).
Expression and function of IL-25 in GC derived B-NHL cell lines
The expression and function of the heterodimeric IL-25R was next investigated in five different malignant cell lines derived from GC B-NHL, i.e. SU-DHL-4, DoHH2, OCI-Ly8, Raji and Ramos. We have recently demonstrated that these cell lines expressed IL-17RA.Citation5 In the present study we found that all the cell lines investigated were positive for IL-17RB, as demonstrated by flow cytometry (MRFI IL-17RB: SU-DHL-4 = 2.6 ± 0.2, DoHH2 = 2.5 ± 0.4, OCI-Ly8 = 3.4 ± 0.5, Raji = 2.1 ± 0.1 and Ramos = 2.1 ± 0.2). GC B-NHL lymphoma cell lines cultured for different time with 50 ng/ml hrIL-25 significantly increased pNF-kBp65 (p = 0.0013 at 1 minute and p = 0.0067 for 30 minute), pJNK and pSTAT1 (p = 0.02 for both at 1 and 30 minute), as compared to cells incubated with medium alone (data not shown). The signaling activity of IL-25 on GC B-NHL cell lines supported in vivo experiments aimed at investigating the role of IL-25 in the control of tumor growth.
IL-25 inhibits growth of human B-NHL cells in mouse xenografts
Subcutaneous (s.c) injection of SUDHL-4 and OCI-Ly8 cell lines in SCID/NOD mice is a suitable model to investigate the effects of cytokines on B-NHL growth.Citation5 We inoculated 5 × 106 tumor cells from SUDHL-4 or Oci-Ly8 in the flank of two groups of 20 animals each (for both cell lines), and treated mice with 3 weekly doses s.c. of rhIL-25 (1µg/doses) or PBS (control). All animals developed tumors, in the absence of any evidence for increased size of internal organs (spleen, liver, lymph nodes, lung, kidney, brain). Tumors formed by either lymphoma cell line in mice treated with hrIL-25 were significantly smaller than tumors grown in PBS treated animals (p = 0.0295 for SUDHL-4 and p = 0.0008 for OCI-Ly8) ( and Supplemental Fig. 2). Histological examination of tumors formed by SU-DHL-4 in hrIL-25 treated mice revealed: i) areas of ischemic necrosis (, panel e); ii) defective vascularization (, panel f), as assessed by CD31 staining, and iii) reduced proliferative activity (, panel g), as assessed by Ki-67 staining, compared with tumors from PBS treated mice (, panel a, b and c and ). Apoptotic events, as assessed by TUNEL staining, were comparable in hrIL-25 treated and PBS treated mice (, and ). Likewise, a scanty reactive cell infiltrate, mainly composed of monocytes/macrophages, was detected in tumors from both cohorts of mice (not shown). Decreased tumor cell proliferation in mice treated with IL-25 was confirmed flow cytometric analysis of Ki67 expression ex-vivo ().
Figure 3. Role of IL-25 on in vivo B-NHL growth. (A) Volume of tumors grown s.c. in mice treated with PBS or IL-25 (1µg) 20 days after SU-DHL-4 cell injection. Results are expressed in box plot, as median tumor volume mm3, maximum, minimum and first and third quartile (**p = 0.002). (B) Tumors developed after s.c. injection of SU-DHL-4 cells in PBS treated SCID/NOD mice consisted of a mixture of small and large lymphoid cells with centrocyte and centroblast morphology (a). These tumors displayed a distinct vascularization (b), a strong proliferative activity (c) and some apoptotic events (d). The histologic features of SU-DHL-4 tumors appeared heavily compromised by rhIL-25 treatment, since these tumors were characterized by wide areas of ischemic necrosis (N) (e), defective vascularization (f), and decreased proliferation (g), whereas apoptotic events (h) were comparable to those observed in control tumors (d) (Magnification: X400). (C) Flow cytometric analysis of cell proliferation in cell suspensions from explanted SU-DHL-4 cell tumors, as assessed by Ki67 staining. Results for 6 PBS and 6 IL-25-treated mice are expressed as median % positive cells, maximum, minimum and first and third quartile (**p = 0.0049).
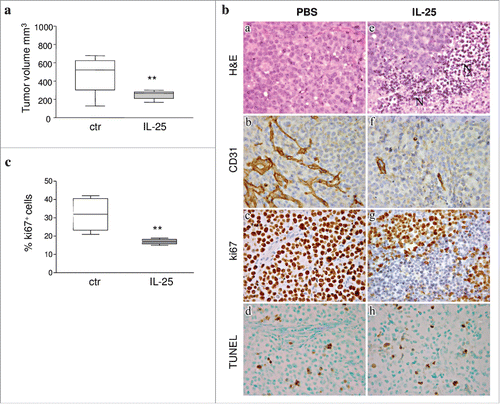
Table 1. Immunohistochemical assessment of microvessels, apoptotic cells and pro-angiogenic molecules in tumors developed after SU-DHL-4 cell injection in PBS or hrIL-25 treated mice.
The in vivo anti-angiogenic activity of IL-25, that has a physiological pro-angiogenic effect,Citation23 prompted further experiments. Small fragments of tumor masses explanted from hrIL-25 or PBS treated mice were tested in the CAM assay. CAMs treated with tumor xenografts from the latter group of mice showed numerous allantoic vessels (mean number of vessels = 25+/−7) radiating in a “spoked wheel” pattern toward the sponges, whereas CAMs treated with tumor xenografts from IL-25-treated mice formed a significantly lower number of vessels (mean number of vessels = 17+/−4, p<0.001 vs tumor xenograft alone) ().
Figure 4. Role of IL-25 on in vivo tumor angiogenesis. (A) CAM assay performed ex vivo with tumor masses explanted from PBS (left panel) or hrIL-25 (right panel) treated mice. (B) Gene expression profiling of human angiogenesis related genes in SU-DHL-4 tumors explanted from SCID/NOD mice as assessed by PCR Array. Results represent fold differences in individual mRNA expression between PBS or hrIL-25 treated mice. Pooled results from 4 different experiments are shown. (C) Immunohistochemical analysis of the expression of selected pro-angiogenic molecules in SU-DHL-4 tumors from PBS or hrIL-25 treated mice. In comparison with tumors developed in PBS treated mice (a-c), the small tumor masses from hrIL-25 treated mice showed very low expression of VEGF-C (d), CXCL6 (e) and ANGPT3 (f).
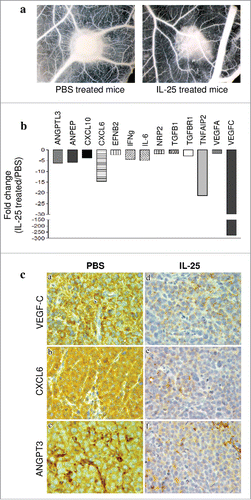
We next investigated by PCR array the expression of a panel of 84 genes involved in angiogenesis in four tumors from hrIL-25 treated mice and 4 tumors from control mice. Treatment with IL-25 downregulated the expression of different angiogenesis-related genes, i.e. VEGF-C, VEGF-A, angiopoietin like (ANGPTL)-3, alanylaminopeptidase (ANPEP), chemokine C-X-C motif ligand (CXCL)10 and 6, ephrin-B2 (EFNB2), IFN-γ, IL-6, Neuropilin-2 (NPR2), transforming growth factor (TGF)β1, TGFβR1 and TNF-α induced protein (TNFAIP)2(). Genes downregulated by IL-25 in vivo included the anti-angiogenic IFN-γCitation38,Citation39 and CXCL10 (an IFN-γ inducible chemokine)Citation40, perhaps in an effort of the tumor to counteract the massive dampening of pro-angiogenic genes operated by IL-25.
Immunohistochemical analyses of tumor masses from hrIL-25 vs PBS treated mice confirmed the down-regulation of VEGF-C, CXCL6 and ANGPT3 at protein level ( and ). TNFa-IP2 protein was barely expressed in tumors from PBS treated mice, thus making it difficult to assess its down-regulation in tumors from hrIL-25 treated mice (not shown).
In summary, inhibition of angiogenesis, a pivotal component of the in vivo anti-tumor activity of IL-25, was driven by the ability of the cytokine to downregulate the expression of different pro-angiogenic molecules in B-NHL cells.
Discussion
FL, DLBCL and BL originate from the malignant transformation of GC-B cells. The definitive cure for these malignancies is still to be achieved, and a deeper insight into the microenvironment of GC-derived B-NHL may help develop new therapeutic strategies.
We have demonstrated that normal and neoplastic GC B cells expressed IL-17AR, composed of the IL-17RA and IL-17RC chains.Citation5 IL-17AR signaled in both cell types by inducing phosphorylation of NF-kBp65. IL-17A expressed by T cells and mast cells in B-NHL microenvironment promoted growth of lymphoma cells in vitro and in vivo through stimulation of tumor cell proliferation and neoangiogenesis. Thus, the IL-17A/IL-17AR axis may represent a potential therapeutic target in B-NHL.Citation5
Normal GC B cells isolated from tonsil expressed IL-17RB, that heterodimerizes with IL-17RA to form the IL-25R.Citation5 Both IL-17A and IL-25 induced de novo chemotaxis of GC B cells to CXCL12 and CXCL13, two crucial chemokines for B cell trafficking in the GC,Citation37 by down-regulating RGS-16 protein expression and NFkBp65 phosphorylation.Citation37
IL-25 is a Th2-type cytokine endowed with pro-angiogenic activity related to induction of pro-angiogenic molecules as bFGF and VEGF in human endothelial cells and fibroblasts.Citation23–25 Furthermore, we show that hrIL-25 exerts a direct pro-angiogenic activity in the CAM assay. Although these features would be predictive of a tumor-promoting role of IL-25, the literature on this topic is controversial.Citation27–31
B-NHL cells expressed IL-25R while IL-25 was abundantly expressed by the tumor cell themselves and, at a lower extent, non-neoplastic cells infiltrating the B-NHL microenvironment. IL-25 signaled in B-NHL cells through multiple pathways, as witnessed by the upregulation of pNFKB-p65, pSTAT-1 and pJNK, and inhibited the in vitro proliferation of tumor cells.
We next investigated the in vivo effects of IL-25 on the growth of human SU-DHL-4 and Oci-Ly8 DLBCL cells in immunodeficient SCID/NOD mice. Tumors formed in mice treated with hrIL-25 vs PBS were significantly smaller and displayed necrotic-hemorragic areas associated with defective microvascular supply and reduced neoplastic cell proliferation. Expression of different pro-angiogenic genes was found to be down-modulated by IL-25 treatment. These genes included: VEGF-C, that promotes angiogenesis and lymphangiogenesis by stimulating endothelial cell growth and migration;Citation41 VEGF-A, a primary molecule driving expansion of the tumor vascular bed;Citation42 ANGPTL-3, a member of the angiopoietin-like family of secreted factors expressed predominantly in the liver during development;Citation43 ANPEP, responsible for post-secretory processing, involved in intracellular cell signaling and invasion/metastasis of various malignancies,Citation44–46 and endowed with a relevant role in neoangiogenesis;Citation47 CXCL6, a pro-angiogenic gene induced in microvascular endothelial cells after stimulation with pro-inflammatory stimuli;Citation48,Citation49 IL-6, a gene coding for a cytokine with potent pro-angiogenic activity, in part through VEGF induction;Citation50,Citation51 andTNFAIP2, a TNFα-regulated gene expressed in endothelial cells and peripheral blood monocytes that stimulates endothelial capillary tube formation in vitro.Citation52
Immunohistochemical staining of tumors explanted from IL-25 vs PBS treated mice confirmed at protein level the strong down regulation of some of the genes enlisted above, the most prominent being VEGF-C. Moreover, tumors explanted from IL-25-treated mice displayed a strongly reduced pro-angiogenic activity in the CAM assay compared to tumors from PBS-treated mice, reinforcing the evidence for the in vivo anti-angiogenic effects of IL-25.
IL-25 inhibited in vivo tumor cell proliferation, consistently with the results obtained in vitro with B-NHL cells isolated from invaded lymph nodes. The possibility that part of the anti-proliferative effect of IL-25 in vivo was secondary to the anti-angiogenic activity of the cytokine cannot be excluded.
These results demonstrate for the first time the ability of IL-25 to inhibit neoangiogenesis in two different xenograft models of B-NHL by reprogramming the angiogenic phenotype of these malignancies. The finding that the intrinsic pro-angiogenic activity of IL-25 was overwhelmed by the effects of the cytokine on B-NHL cells may appear surprising since IL-25 was mainly produced by malignant cells that also expressed IL-25R. Thus the question arises why should B-NHL cells be involved in an autocrine/paracrine loop that dampens their growth. A few considerations deserve mention. First, the possibility that human IL-25 did not stimulate efficiently mouse angiogenesis is disproved by the evidence that human and mouse IL-25 display species cross-reactivity.Citation8 Second, the xenograft models tested in this study highlight the direct effects of IL-25 on B-NHL cells, but do not allow to investigate the activity of the cytokine on other cell types present in the tumor microenvironment. Third, tumor cells can upregulate the expression of some anti-angiogenic genes in the course of a prominent pro-angiogenic response. For example, we have previously demonstrated that, in a xenograft model of human neuroblastoma, resistance to anti-angiogenic immunotherapy was characterized by an impressive wave of neo-angiogenesis with thousands fold upregulated expression of numerous pro-angiogenic and few anti-angiogenic genes, such as CXCL9 and CXCL10.Citation53 This latter phenomenon, that is not easily explained, may represent an attempt of cancer cells to modulate their exuberant pro-angiogenic activity. In conclusion, further studies are needed to gain more insight into the functional impact of IL-25 on tumor neoangiogenesis in the frame of the effects of the cytokine on non-malignant cells present in the B-NHL microenvironment.
Material and methods
Patients, cell isolation and cell lines
The present study was approved by the Institutional Review Board of Istituto Giannina Gaslini, Genova, Italy on October 27th, 2005. Nineteen infiltrated lymph nodes from patients with FL (n = 10, 6 males and 4 females, age range: 43–68), DLBCL (n = 6, 3 males and 3 females, age range: 56–69) and BL (n = 3, 2 males and 1 female, age range: 6–16) were obtained after informed consent in accordance with the Declaration of Helsinki.Diagnosis of FL, DLBCL (GC-type), and BL was established according to the criteria of the Revised European-American Classification of Lymphoid Neoplasms.Citation34 From now onwards DLBCL (GC-type) will be referred to as DLBCL. All patients were studied at diagnosis and were untreated.
Lymph node mononuclear cells (MNCs) were isolated with standard procedure,Citation54 and cryopreserved in a freezing solution composed of 50% RPMI 1640 (Sigma Chemical Co., St. Louis, MO), 40% fetal bovine serum (FBS) (Sigma), and 10% DMSO (Sigma). Cells were kept in liquid nitrogen until tested. Then, neoplastic B-NHL cells, expressing either κ or λ immunoglobulin (Ig) light chains, were enriched (>95%) by immunomagnetic positive selection for the clonotypic light chain, as reported.Citation5
The human SU-DHL-4 (DLBCL origin),Citation55 DoHH2 (FL origin),Citation56 Raji and Ramos (BL origin),Citation57 and Oci-Ly8 (considered of DLCBL origin) cell lines were provided 6 months ago by Interlab Cell Line Collection (Istituto Nazionale per la Ricerca sul Cancro, Genova, Italy), that certifies their origin by multiplex short tandem repeat profiling. These cell lines were cultured in RPMI 1640 medium (Sigma) supplemented with 10% FBS (Sigma).
Antibodies and flow cytometry
The following antibodies were used for flow cytometry experiments: Phycoerythrin (PE)-conjugated anti IL-17RA, PE anti IL-17RB (R&D System Inc.); PE or Fluorescein Isothiocyanate (FITC)-conjugated anti-κ and anti-λ Ig light chains (R&D System); FITC-anti human Ki67 (DAKO), anti-PCNA, anti-IL-25 (R&D System Inc). Cells were stained with fluorochrome conjugated antibodies or with isotype and fluorochrome matched control antibodies. For intracellular experiments cells were fixed and permeabilized using cytofix/cytoperm kit (Becton-Dickinson), in accordance to manufacturer's instructions. Cells were run on a Gallios Instrument (Beckman Coulter) and data were analyzed using the Kaluza software (Beckman Coulter). Data were expressed as percentage of positive cells.
Immunohistochemistry on human tissue samples
For immunohistochemical analysis tissue samples from 5 lymph nodes involved by FL were collected from the archives of the Human Pathology Section, University of Palermo. IL-25 and IL-17RB protein expression was evaluated using the primary polyclonal antibody goat anti-human IL-25 (Millipore, Massachusetts, USA) and the primary polyclonal antibody rabbit anti-human IL-17RB (Sigma). The formalin-fixed and paraffin embedded tissue sections of four-micrometer-thick were deparaffinized and rehydrated to water. High temperature antigen unmasking was performed using Tris-HCl/ EDTA pH 9.0 or pH6 citrate buffer (Novocastra, Newcastle, UK) in a PT Link pre-treatment module (Dako, Denmark) at 98°C for 30 minutes.
Subsequently, the sections were brought to room temperature and washed in PBS. After neutralization of the endogenous peroxidase activity using 3% H2O2 for 10 min, the sections were incubated with protein block (Novocastra) for 10 minutes. The slides were then incubated overnight with the primary polyclonal antibody goat anti-human IL-25 (dilution 1:50) and primary polyclonal antibody rabbit anti-Human IL-17RB (dilution 1:20) at the temperature of 4°C. Staining was revealed by LSAB kit and DAB (3,3'-Diaminobenzidine, Novocastra) substrate-chromogen. After counterstaining with Harris hematoxylin (Novocastra), all the sections were analyzed under an AXIO Scope A1 optical microscope (ZEISS, Germany) and microphotographs were collected through an Axiocam 503 Color digital camera (Zeiss) using the Zen2 software.
Cell signaling
The phosphorylation of NF-kBp65 (Ser536), STAT-1 and JNK (Cell Signaling Technology, Danvers, MA USA) was investigated by flow cytometry as reported.Citation5 Briefly, cells were treated for 0–1-5–10-30–60 min with or without (medium) 50ng/ml rhIL-125 (R and D System, Minneapolis, USA) and stained with an Alexa 488-conjugated anti-phosphorylated and unphosphorylated NF-kBp65, STAT-1 or JNK mAbs (Cell Signaling Technology), according to the instructions of the manufacturer. Cells were washed and run on Gallios cytometer. Data were analyzed using the Kaluza software.
Cell proliferation, apoptosis and chemotaxis
Lymphoma B cells were cultured in RPMI for 24–72 h with or without different concentrations of rhIL-25 (50ng/ml) and tested for proliferation. Tumor cell proliferation was investigated: i) by intracellular flow cytometric detection of PCNA; ii) by overnight pulse with tritiated thymidine (3H-TdR) of cells. 100 ng/ml rhCD40L (Immunotools, Friesoythe, Germany) was tested as positive control. The results of the latter assay were obtained by comparing cpm of cytokine-treated vs cpm of untreated cells. Apoptosis was assessed by the rhAnnexin V/FITC kit (Bender Medsystem) and analyzed by flow cytometry. Apoptotic cells were identified as Annexin V+ cells. Chemotaxis was investigated using 5 µm pore-size transwell plates (Costar) as reported.Citation58 Five × 105 normal or neoplastic cells were dispensed in the upper chamber, and increasing concentrations of rhIL-25 (100–300-600ng/ml) or medium were added to the lower chamber.Citation58 Plates were incubated 2 h at 37°C. Migrated cells were collected and counted.
In vivo studies
All procedures involving animals were performed in accordance with national and international current regulations (D.l.vo 27/01/1992, n.116, European Economic Community Council Directive 86/609, OJL 358, December 1, 1987). Four-six week old SCID-NOD mice (Harlan Laboratories, Indianapolis) were housed under specific pathogen-free conditions. Twenty four animals were injected s.c. in the left flank with 5 × 106 SU-DHL-4 cells and separated in two groups: one group was treated with 3 weekly doses of rhIL-25 (1 μg/mouse per dose)Citation28 starting one day after injection of tumor cells; the second group was treated with PBS, as control. The same protocol was applied to thirty SCID/NOD mice injected s.c. in the left flank with 2.5 × 106 Oci-Ly8 cells. Twenty days after tumor cell inoculation, mice were killed since signs of poor health such as enlarging tumor masses and presence of ruffled fur became evident and autopsies were carried out. Tumor masses were explanted, measured as describedCitation59 and used for following ex-vivo analyses.
Morphologic and immunohistochemical analyses ex-vivo
Morphological and immunohistochemical analyses were performed on tumor masses explanted from mice, as described.Citation59,Citation60 Briefly, formalin-fixed, paraffin-embedded sections were incubated for 30 min with anti-Mac-1 (Abcam, Cambridge, UK),Gr-1 (Ly-6G/Ly-6C, clone RB6/8C5, BioLegend, San Diego, CA, anti-TNFa-IP2 (clone F-6, Santa Cruz Biotechnology, Dallas, Texas. USA), anti-VEGF-C (Invitrogen, Camarillo, CA), anti-CXCL6/GCP-2) PeproTech, Rocky Hill, NJ, USA), anti-ANGPT3 (clone 3B7, Abnova, Taipei City, Taiwan), anti-mouse CD31 (clone SZ31; Dianova). CD31 positive vessels were counted in 8 randomly chosen fields under a microscope × 400 field ( × 40 objective and × 10 ocular lens; 0.180 mm2 per field). The rates of apoptotic cells were determined by counting the number of TUNEL positive cells/number of total cells in the viable neoplastic tissue under a microscope × 600 field ( × 60 objective and × 10 ocular lens; 0.120 mm2 per field). Automated analyses of cytokine expression were performed as reported (Sorrentino et al. J Pathol 2006; 209: 400–410) by light microscopy on single immunostained sections with Qwin image analysis software (version 2.7), which has the following highly reproducible steps: (1) image acquisition; (2) conversion of RGB image (true colour) to binary image (black and white); (3) filtering to remove noise; (4) measurement of positively stained area.
Angiogenesis PCR-Array
RNA was extracted from tumors removed from hrIL-25 treated or control mice using Qiagen (Hilden) kit, reverse transcribed by the RTCitation2 First Strand cDNA Synthesis kit (SABioscience, Frederick, MD, USA), processed using human Angiogenesis RTCitation2 PCR Array (SABioscience) and data analysis were performed as described.Citation59 This kit contains primers allowing PCR amplification of mRNA from 84 human genes involved in regulation of angiogenesis.PCR was performed on ABI Prism 7700 Sequence Detector (Applied Biosystems, California, USA).
Chick Embryo Chorioallantoic Membrane Assay
CAM assay was carried out as previously reportedCitation61 to investigate the angiogenic activity of IL-25 and of explanted tumor masses xenografts alone or with rhIL-25. Fertilized White Leghorn chicken eggs were incubated at 37°C in constant humidity. On day 3 of incubation, a square window was cut in the shell of each egg, and 2–3 mL of albumen was removed to allow detachment of the developing CAM. The window was sealed with a glass coverslip, and the eggs were returned to the incubator. On day 8, eggs were treated with 1 mm3 sterilized gelatin sponges (Gelfoam Upjohn, Michigan, USA) placed on top of the growing CAM, as previously describedCitation61 and loaded with: 1 μl of PBS as negative control; 1 μl of PBS containing 200 ng of recombinant VEGF-A (R & System, Abington, UK) as positive control; 1 μl of PBS containing hrIL-25. For experiments with explanted tumor masses, on day 8 of incubation, the coverslips were removed and the growing CAMs (10 eggs per group) were treated with either 1–2-mmCitation3 fragments of tumor xenografts alone or with rhIL-25. The coverslips were replaced after these treatments, and the CAMs were examined daily until day 12 of incubation, when they were photographed in ovo using a stereomicroscope equipped with a camera and image analysis system (Olympus Italia, Milano, Italy). Blood vessels entering the sponges within the focal plane of the CAM were counted by two observers in a double blind fashion at a magnification of 50x.
Statistical analysis
For CAM assays, means ± Standard Deviation (SD) were evaluated for all the parameters and the statistical significance of the differences between the counts of vessels number was determined by Student's t-test for unpaired data. Differences in tumor volume, proliferation and apoptotic index, pro-angiogenic molecule expression and cell signaling were calculated using Mann-Whitney test comparing two independent samples, with 99% confidence interval (GraphPad Prism 3). All statistical tests were two tailed. A P value lower than 0.05 was considered statistically significant.
Abbreviations
| B-NHL | = | B cell non-Hodgkin lymphomas |
| BL | = | Burkitt lymphoma |
| DLBCL | = | diffuse large B-cell lymphoma |
| FL | = | follicular lymphoma |
| GC | = | germinal center |
| IL | = | Interleukin |
| MRFI | = | Mean Relative Fluorescence Intensity |
| p | = | phosphorylated |
| R | = | receptor |
supp_data.zip
Download Zip (74.7 KB)Acknowledgments
This work was supported by grants from Associazione Italiana Ricerca Cancro (A.I.R.C.), Milano, Italy to V.P. (N°13003), from Cinque per mille IRPEF-Finanziamento Ricerca Sanitaria and from Ricerca Corrente Ministeriale, by grants from the Italian Ministry of Health, Ricerca Finalizzata (RF-2013–02357552 to E.D.C.) and by a grant from Associazione “Il Sorriso di Antonio” (Corato, Italy) to D.R. E.F. was supported by a Fondazione Umberto Veronesi fellowship.
Additional information
Funding
References
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi:10.1038/nm.3394. PMID:24202395.
- Fowler NH, Cheah CY, Gascoyne RD, Gribben J, Neelapu SS, Ghia P, Bollard C, Ansell S, Curran M, Wilson WH, et al. Role of the tumor microenvironment in mature B-cell lymphoid malignancies. Haematologica. 2016;101:531–40. doi:10.3324/haematol.2015.139493. PMID:27132279.
- Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi:10.1146/annurev-immunol-020711-075027. PMID:22224767.
- Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14:517–34. doi:10.1038/nrc3774. PMID:25008267.
- Ferretti E, Di Carlo E, Ognio E, Guarnotta C, Bertoni F, Corcione A, Prigione I, Fraternali-Orcioni G, Ribatti D, Ravetti JL, et al. Interleukin-17A promotes the growth of human germinal center derived non-Hodgkin B cell lymphoma. Oncoimmunology. 2015;4:e1030560. doi:10.1080/2162402X.2015.1030560. PMID:26451300.
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi:10.1016/j.immuni.2008.03.004. PMID:18400188.
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi:10.1016/j.immuni.2008.11.009. PMID:19144317.
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi:10.4049/jimmunol.169.1.443. PMID:12077275.
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi:10.1084/jem.20051615. PMID:16606668.
- Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi:10.1084/jem.20061675. PMID:17562814.
- Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–6. doi:10.1182/blood-2002–09-2817. PMID:12511410.
- Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, Rhim TY, Park CS. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–6. doi:10.1165/rcmb.2005–0003OC. PMID:15961726.
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi:10.1016/S1074–7613(01)00243–6. PMID:11754819.
- Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–40. doi:10.1182/blood-2002–01-0012. PMID:12239140.
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–7. doi:10.1016/j.cyto.2008.07.017. PMID:18701318.
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi:10.1084/jem.20070406. PMID:17635955.
- Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, Bulek K, Do JS, Aronica M, McKenzie AN, et al. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(-)c-Kit(+) innate cell population. Immunity. 2012;36:821–33. doi:10.1016/j.immuni.2012.03.021. PMID:22608496.
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol 1995;155:5483–6. PMID:7499828.
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–76..
- Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–310. doi:10.4049/jimmunol.181.6.4299. PMID:18768888.
- Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–72. doi:10.1073/pnas.1315854111. PMID:24344271.
- Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, Lloyd CM. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68:82–90. doi:10.1136/thoraxjnl-2012–202003. PMID:23093652.
- Yao X, Wang W, Li Y, Huang P, Zhang Q, Wang J, Wang W, Lv Z, An Y, Qin J, Corrigan CJ, et al. IL-25 induces airways angiogenesis and expression of multiple angiogenic factors in a murine asthma model. Respir Res. 2015;16:39. doi:10.1186/s12931–015-0197–3. PMID:25889697.
- Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, Lv Z, Fan Y, An Y, Wang YH, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci U S A. 2011;108:1579–84. doi:10.1073/pnas.1014241108. PMID:21205894.
- Wang W, Fan YQ, Lv Z, Yao XJ, Wang W, Huang KW, Meng Q, Fang CL, Lee TH, Corrigan CJ, et al. Interleukin-25 promotes basic fibroblast growth factor expression by human endothelial cells through interaction with IL-17RB, but not IL-17RA. Clin Exp Allergy. 2012;42:1604–14. doi:10.1111/j.1365–2222.2012.04062.x. PMID:23106660.
- Lavocat F, Ndongo-Thiam N, Miossec P. Interleukin-25 Produced by Synoviocytes Has Anti-inflammatory Effects by Acting As a Receptor Antagonist for Interleukin-17A Function. Front Immunol. 2017;8:647. doi:10.3389/fimmu.2017.00647. PMID:28620392.
- Furuta S, Jeng YM, Zhou L, Huang L, Kuhn I, Bissell MJ, Lee WH. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci Transl Med. 2011;3:78ra31. doi:10.1126/scitranslmed.3001374..
- Benatar T, Cao MY, Lee Y, Lightfoot J, Feng N, Gu X, Lee V, Jin H, Wang M, Wright JA, et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol Immunother. 2010;59:805–17. doi:10.1007/s00262–009-0802–8. PMID:20012860.
- Huang CK, Yang CY, Jeng YM, Chen CL, Wu HH, Chang YC, Ma C, Kuo WH, Chang KJ, Shew JY, et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene. 2014;33:2968–77. doi:10.1038/onc.2013.268. PMID:23851503.
- Jiang Z, Chen J, Du X, Cheng H, Wang X, Dong C. IL-25 blockade inhibits metastasis in breast cancer. Protein Cell. 2017;8:191–201. doi:10.1007/s13238–016-0345–7. PMID:27909985.
- Luo Y, Yang Z, Su L, Shan J, Xu H, Xu Y, Liu L, Zhu W, Chen X, Liu C, et al. Non-CSCs nourish CSCs through interleukin-17E-mediated activation of NF-kappaB and JAK/STAT3 signaling in human hepatocellular carcinoma. Cancer Lett. 2016;375:390–9. doi:10.1016/j.canlet.2016.03.012. PMID:27000993.
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi:10.1016/j.immuni.2007.07.009. PMID:17723214.
- Carbone A, Gloghini A, Cabras A, Elia G. The Germinal centre-derived lymphomas seen through their cellular microenvironment. Br J Haematol. 2009;145:468–80. doi:10.1111/j.1365–2141.2009.07651.x. PMID:19344401.
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–32. doi:10.1182/blood-2011–01-293050. PMID:21300984.
- Wong CK, Cheung PF, Ip WK, Lam CW. Interleukin-25-induced chemokines and interleukin-6 release from eosinophils is mediated by p38 mitogen-activated protein kinase, c-Jun N-terminal kinase, and nuclear factor-kappaB. Am J Respir Cell Mol Biol. 2005;33:186–94. doi:10.1165/rcmb.2005–0034OC. PMID:15860795.
- Johnson PW, Watt SM, Betts DR, Davies D, Jordan S, Norton AJ, Lister TA. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system. Blood 1993;82:1848–57. PMID:7691240.
- Ferretti E, Ponzoni M, Doglioni C, Pistoia V. IL-17 superfamily cytokines modulate normal germinal center B cell migration. J Leukoc Biol. 2016;100:913–8. doi:10.1189/jlb.1VMR0216–096RR. PMID:27566830.
- Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–86. doi:10.1016/S1074–7613(00)80218–6. PMID:10894167.
- Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–100. PMID:12874012.
- Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163:5686–92. PMID:10553099.
- Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1998;95:14389–94. doi:10.1073/pnas.95.24.14389. PMID:9826710.
- Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27. doi:10.1111/joim.12019. PMID:23216836.
- Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, Walker KM, Chen LH, Wattler S, Nehls M, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics 1999;62:477–82. doi:10.1006/geno.1999.6041. PMID:10644446.
- Menrad A, Speicher D, Wacker J, Herlyn M. Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res. 1993;53:1450–5. PMID:8095183.
- Ishii K, Usui S, Sugimura Y, Yoshida S, Hioki T, Tatematsu M, Yamamoto H, Hirano K. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int J Cancer. 2001;92:49–54. doi:10.1002/1097–0215(200102)9999:9999%3c::AID-IJC1161%3e3.0.CO;2-S. PMID:11279605.
- Hashida H, Takabayashi A, Kanai M, Adachi M, Kondo K, Kohno N, Yamaoka Y, Miyake M. Aminopeptidase N is involved in cell motility and angiogenesis: its clinical significance in human colon cancer. Gastroenterology. 2002;122:376–86. doi:10.1053/gast.2002.31095. PMID:11832452.
- Petrovic N, Schacke W, Gahagan JR, O'Conor CA, Winnicka B, Conway RE, Mina-Osorio P, Shapiro LH. CD13/APN regulates endothelial invasion and filopodia formation. Blood. 2007;110:142–50. doi:10.1182/blood-2006–02-002931. PMID:17363739.
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–9. doi:10.1016/j.it.2004.02.006. PMID:15039047.
- Gijsbers K, Gouwy M, Struyf S, Wuyts A, Proost P, Opdenakker G, Penninckx F, Ectors N, Geboes K, Van Damme J. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;303:331–42. doi:10.1016/j.yexcr.2004.09.027. PMID:15652347.
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. doi:10.1038/sj.onc.1206226. PMID:12629515.
- Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–95. doi:10.3892/or.2016.4544. PMID:26750536.
- Sarma V, Wolf FW, Marks RM, Shows TB, Dixit VM. Cloning of a novel tumor necrosis factor-alpha-inducible primary response gene that is differentially expressed in development and capillary tube-like formation in vitro. J Immunol. 1992;148:3302–12. PMID:1374453.
- Pezzolo A, Marimpietri D, Raffaghello L, Cocco C, Pistorio A, Gambini C, Cilli M, Horenstein A, Malavasi F, Pistoia V. Failure of anti tumor-derived endothelial cell immunotherapy depends on augmentation of tumor hypoxia. Oncotarget. 2014;5:10368–81. doi:10.18632/oncotarget.2015. PMID:25362644.
- Corcione A, Ottonello L, Tortolina G, Facchetti P, Airoldi I, Guglielmino R, Dadati P, Truini M, Sozzani S, Dallegri F, et al. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. J Natl Cancer Inst. 2000;92:628–35. doi:10.1093/jnci/92.8.628. PMID:10772680.
- Epstein AL, Kaplan HS. Feeder layer and nutritional requirements for the establishment and cloning of human malignant lymphoma cell lines. Cancer Res. 1979;39:1748–59. PMID:371794.
- Kluin-Nelemans HC, Limpens J, Meerabux J, Beverstock GC, Jansen JH, de Jong D, Kluin PM. A new non-Hodgkin's B-cell line (DoHH2) with a chromosomal translocation t(14;18)(q32;q21). Leukemia. 1991;5:221–4. PMID:1849602.
- Benjamin D, Magrath IT, Maguire R, Janus C, Todd HD, Parsons RG. Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt's and non-Burkitt's type. J Immunol 1982;129:1336–42. PMID:6286763.
- Corcione A, Ferretti E, Bertolotto M, Fais F, Raffaghello L, Gregorio A, et al. CX3CR1 is expressed by human B lymphocytes and mediates [corrected] CX3CL1 driven chemotaxis of tonsil centrocytes. PLoS One. 2009;4:e8485. doi:10.1371/journal.pone.0008485. PMID:20041188.
- Ferretti E, Di Carlo E, Cocco C, Ribatti D, Sorrentino C, Ognio E, Montagna D, Pistoia V, Airoldi I. Direct inhibition of human acute myeloid leukemia cell growth by IL-12. Immunol Lett. 2010;133:99–105. doi:10.1016/j.imlet.2010.08.002. PMID:20705102.
- Ferretti E, Montagna D, Di Carlo E, Cocco C, Ribatti D, Ognio E, Sorrentino C, Lisini D, Bertaina A, Locatelli F, et al. Absence of IL-12Rbeta2 in CD33(+)CD38(+) pediatric acute myeloid leukemia cells favours progression in NOD/SCID/IL2RgammaC-deficient mice. Leukemia. 2012;26:225–35. doi:10.1038/leu.2011.213. PMID:21844875.
- Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85–91. doi:10.1038/nprot.2006.13. PMID:17406216.
