ABSTRACT
Pediatric medulloblastomas are the most frequently diagnosed embryonal tumors of the central nervous system. Current therapies cause severe neurological and cognitive side effects including secondary malignancies. Cellular immunotherapy might be key to improve survival and to avoid morbidity. Efficient killing of tumor cells using immunotherapy requires to overcome cancer-associated strategies to evade cytotoxic immune responses. Here, we examined the immune response and immune evasion strategies in pediatric medulloblastomas. Cytotoxic T-cells, infiltrating medulloblastomas with variable activation status, showed no correlation with overall survival of the patients. We found limited numbers of PD1+ T-cells and complete absence of PD-L1 on medulloblastomas. Medulloblastomas downregulated immune recognition molecules MHC-I and CD1 d. Intriguingly, expression of granzyme inhibitors SERPINB1 and SERPINB4 was acquired in 23% and 50% of the tumors, respectively. Concluding, pediatric medulloblastomas exploit multiple immune evasion strategies to overcome immune surveillance. Absence of PD-L1 expression in medulloblastoma suggest limited or no added value for immunotherapy with PD1/PD-L1 blockers.
Introduction
Brain tumors are the second most frequent cancers after hematological malignancies in children. The embryonal tumors of the central nervous system (CNS), i.e. medulloblastoma, atypical teratoid rhabdoid tumor, CNS primitive neuroectodermal tumor (CNS-PNET), are the most frequently diagnosed brain tumors in children in the Netherlands, accounting for 40% of pediatric brain cancers in 2015. Embryonal tumors have an incidence of 4.0 per 1,000,000 children in the Netherlands.Citation1 The survival rate of medulloblastoma patients largely depends on the clinical and molecular features of the tumor varying from >90% 5-years overall survival for patients with a WNT-driven medulloblastoma till <50% for patients with a metastatic Group 3 or SHH-driven tumor with a TP53 mutation.Citation2
Despite molecular phenotyping, therapeutic possibilities are limited to radiation therapy, chemotherapy and surgery,Citation3 causing severe late onset neurological and cognitive side effects including secondary malignancies.Citation4,5 In analogy with other malignancies, immunotherapy might be key to improve survival and to avoid these side effects.Citation6–10
Preclinical data from mouse models of SHH and Group 3 medulloblastomas revealed that SHH tumors have higher percentages of dendritic cells, T-cells and myeloid cells than Group 3 tumors in mice.Citation11 Little is known on immune infiltration of medulloblastomas in humans, although a study with six patients demonstrated that infiltrative myeloid cells are more immunosuppressed and T-cell lineages are less frequent than in other pediatric brain tumors.Citation12
Next to immune cell infiltration, efficient killing of medulloblastomas during immunotherapeutic protocols can only be achieved when mechanisms to evade recognition or killing are overcome. It has been well established that cancers employ multiple mechanisms to evade our immune system, making them less susceptible for immunotherapy.Citation13 Studies in gliomas, medulloblastomas, and CNS-PNETs have shown that certain tumors downregulate MHC-I or lack CD1 d expression in order to evade T cell-mediated immunity and NKT cell recognition, respectively.Citation14–17 Another potential mechanism to evade immune recognition and subsequent cytotoxic killing is expression of intracellular apoptosis inhibitors (e.g. caspase inhibitors) to escape from death receptor-induced apoptosis and granzyme-mediated killing pathways.Citation13 Granzymes are the major tumor killing molecules secreted by cytotoxic cells. In humans, five granzymes (i.e. GrA, GrB, GrH, GrK, and GrM) exist with distinct substrate specificity and only partially overlapping routes of apoptosis induction.Citation18 Certain tumors can express serine protease inhibitors (Serpins) to directly block granzyme activity.Citation19–21 Recently, we have demonstrated that CNS-PNETs can induce expression of SERPINB1 (GrH inhibitor), SERPINB4 (GrM inhibitor), and SERPINB9 (GrB inhibitor).Citation17
Interference with the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) immune checkpoint is a pivotal pathway for immune escape by tumors.Citation22,23 Expression of PD-L1 on tumor cells, diminishes T-cell activity towards the tumor. Therefore, blocking this axis with antibodies directed to PD-1 (nivolumab and pembrolizumab) resulted in improved therapy response in non-small cell lung cancer (NSCLC), melanoma, and their corresponding brain metastases.Citation24–26 Recently, Berghoff et al. showed that 88% of adult glioblastomas had PD-L1 expression. Given this high frequency of PD-L1 expression, clinical trials are needed to evaluate the clinical benefit of PD1/PD-L1 blockade in glioblastoma.Citation27 However Nduom et al., found that 2.77% of glioblastoma cells had PD-L1 expression, meaning that 61% of patients had >1% and only 38% of patients had >5% PD-L1 positive tumor cells. Expression of PD-L1 was a significant prognosticator for poor survival.Citation28 Surveying a large set of adult brain cancers revealed that expression of PD-L1 was restricted to grade IV tumors i.e. glioblastoma multiforme (7.8%) and gliosarcoma (25%). Other grade I-III tumors e.g. (anaplastic) astrocytoma, (anaplastic) oligodendroglioma, ependymoma did not express PD-L1, arguing that either brain tumors are not immunogenic or other immune evasion strategies are exploited.Citation29
Expression of CDK5 in medulloblastoma has been shown in mouse models to induce persistent PD-L1 expression, resulting in resistance towards CD4-dependent cytotoxic T-cell activity.Citation30 Therefore, CDK5 might be the pivotal factor in PD1/PD-L1 immune response.Citation30 A recent paper on PD-L1 expression in medulloblastomas showed that PD-L1 positive tumors had less influx of T-cells and those patients had a worse survival. These data suggest that also medulloblastoma patients may benefit from PD1/PD-L1 axis blockage.Citation31 In contrast the study of Aoki et al showed that all four analyzed medulloblastomas had no PD-L1 expression.Citation32
The aim of this study is to survey human pediatric medulloblastomas for tumor-infiltrating lymphocytes (TILs), immune checkpoints, and expression of immune evasion molecules, allowing to facilitate prediction of the tumor response to immunotherapy. However, in contrast to previous reports, we found no activated T-cells or PD-L1 expression in pediatric medulloblastomas, suggesting that the added value of immunotherapy with PD1/PD-L1 blockers in this cancer type is limited.
Materials and methods
Patients
We examined by immunohistochemistry the immune response and immune checkpoints in 26 primary pediatric medulloblastomas operated between 1990–2015 at the University Medical Center Utrecht (Utrecht, The Netherlands). Patient characteristics are shown in . The study material was derived from the archive of the Department of Pathology of the University Medical Center Utrecht, Utrecht, The Netherlands and distributed by the Biobank of the Department of Pathology. The biobank is overseen by the institutional medical ethical review board.
Table 1. Patient characteristics.
Since we are using archival pathology material which does not interfere with patient care and does not involve physical involvement of the patient, no ethical approval is required according to Dutch legislation.Citation33 Use and storage of anonymous or coded left over material for scientific purposes is part of the standard treatment contract with patients and therefore informed consent procedure was not required according to our institutional medical ethical review board.Citation34
Overall survival data were obtained from the Comprehensive Cancer Center of The Netherlands (Integraal Kankercentrum Nederland).
Immunohistochemistry
Immunohistochemistry was carried out on 4 μm thick formalin fixed paraffin embedded consecutive sections as described before by Vermeulen et al.Citation17 Immunohistochemistry for PD-1 (Abcam clone NAT105, ab52587) 1:50 and PD-L1 (Abcam clone 28-8, ab205921) 1:50 was performed using an automated immunostainer (Benchmark Ultra, Ventana, Roche). Immunohistochemistry for myeloperoxidase (MPO) (Leica Biosystems Novocastra clone 59A5) was performed manually. Appropriate positive and negative controls were included in all stainings. For classification, all tumors were restained, reevaluated, and molecularly classified using Illumina DNA methylation arrays as described,Citation35 according to the 2016 edition of the WHO classification of tumors of the central nervous system.
Scoring of immunohistochemistry
Reclassification of cases was performed independently by two experienced neuropathologists (WGMS and WVH). All scoring was done blinded to patient characteristics and results of other staining by three independent observers (JFV, WGMS, WVH). Analysis of the immune influx was performed on whole slides at 20x magnification. Immune influx was corrected for the size of tissue on the slide and the tumor percentage as calculated with the manufacturers algorithm based on digitalized immunochemical slides using a digital slide scanner (Aperio Technologies Inc.). SerpinB1, SerpinB4, SerpinB9, CD1 d, HLA-A and HLA-B expression was scored as present (+) or absent (−), using a cut-off of 5% positivity.
Statistics
Statistical analysis was performed using IBM SPSS version 23 (SPSS Inc.). Data are depicted as median and inter quartile range (IQR) or as mean and 95% confidence interval (95% CI) when stated, taking all patients into account. Differences in number of TILs per histological subtype were analyzed using a Kruskal-Wallis test and correlations using Pearson correlation. Overall survival (OS) was used as outcome endpoint and defined as the time between date of surgery and death. Censored patients were confirmed alive at time of censoring. Thus, there was no loss to follow-up. Survival rates were plotted according to the Kaplan-Meier method and associations were analyzed using the log-rank test. High and low number of TILs were stratified after median split. Statistical significance was set at p < 0.05.
Results
Distribution and characterization of tumor infiltrating lymphocytes in medulloblastoma
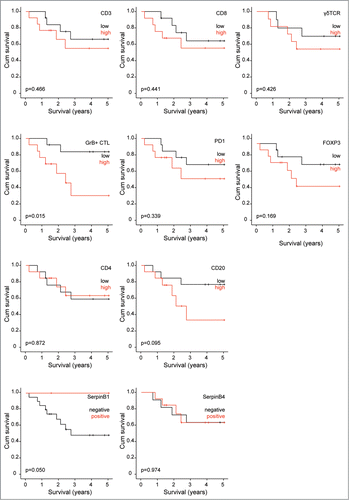
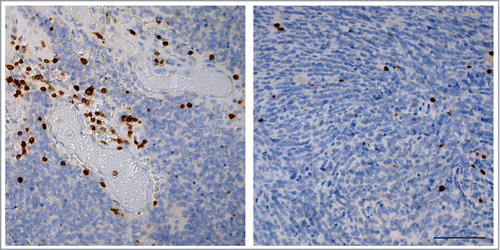
Infiltration of CD3+ T-lymphocytes in human pediatric medulloblastoma occurred in different patterns, most frequently intratumoral infiltration coinciding with perivascular infiltration (). Peritumoral infiltration as observed in brain metastases was not seen in our samples. The number of tumor-infiltrating lymphocytes (TILs) in pediatric medulloblastoma was limited and highly variable between tumors i.e. 23.5 (26.2) [median (IQR)] CD3+ T-cells and 1.56 (2.8) CD20+ B-cells per 2mmCitation2 tumor tissue, but the number of infiltrating T-cells and B-cell was highly correlated (ρ = 0.748, p<0.001). These data show that there are 10 times more T-cells than B-cells in the tumor tissue, suggesting selective infiltration of medulloblastomas by TILs. The number of TILs did not differ between histological subtypes (CD3+ T-cells p = 0.247; CD20+ B-cells p = 0.668), nor between molecular subtypes (CD3+ T-cells p = 1.0; CD20+ B-cells p = 1.0) of medulloblastoma.
Figure 1. Distribution of tumor infiltrating lymphocytes in pediatric medulloblastoma. Distribution of CD3+ T-cells in pediatric medulloblastoma resembles two distinct patterns i.e. perivascular (left panel) and intratumoral (right panel) that often coincide. Scale bar equals 100 μm.
To further characterize the composition of TILs, we analyzed T-cell subspecies. CD8+ T-cells were the main contributing T-cell population with 52% (47-58%) [mean (95% CI)] followed by CD4+ T-cells 35% (28-43%) and FOXP3+ regulatory T-cells (Treg) 2.5% (1.3-3.8%) to the total number of TILs. Furthermore we found 1.70 (1.45) [median (IQR)] times more CD8+ than CD4+ T-cells in the tumors. The influx of these T-cell subsets was correlated to the number of CD3+ T-cells (CD8+ ρ = 0.954, p<0.001; CD4+ ρ = 0.841, p<0.001; Treg ρ = 0.422, p = 0.032) as expected. Next, we studied the influx of γδ-T-cells, an additional cytotoxic lymphocyte subset.Citation36,37 We detected low numbers of γδ-T-cells in our cohort of pediatric medulloblastoma [0.05 (0.11)] per 2mmCitation2 tumor tissue. Recruitment of NK cells was limited to single NK cells, which is in line with results in other CNS tumors.Citation38,39 Furthermore, the influx of neutrophilic granulocytes was highly variable between tumors, i.e. 17.5 (19.5) per 2mmCitation2 tumor tissue and was not correlated to infiltrating T-cells.
Immune evasion strategies and PD-1/PD-L1 axis in medulloblastoma
The activity of cytotoxic T-lymphocytes (CTLs) is one of the prerequisites for cytotoxic killing of tumor cells. Therefore, we examined the activation status of CTLs by Granzyme B (GrB) positivity. The GrB-positive CTL fraction was low 3.9% (6.8%), while some cases had up to 35% GrB-positive CTLs. Given the low CTL activation, we questioned whether medulloblastomas avoid immune recognition by downregulation of MHC-I (HLA-A and HLA-B) and/or CD1 d in order to escape CTL, NKT, and/or (CD1 d-restricted) γδ-T-cell recognition.Citation40 Whereas blood vessels, and TILs readily express these HLA molecules and CD1 d as expected, we found that HLA-A, HLA-B, and CD1 d expression was negative in all our pediatric medulloblastomas ().
Figure 2. Expression of immune checkpoints and evasion markers in pediatric medulloblastoma. A) Immunohistochemical staining of immune (evasion) markers HLA-A, HLA-B and CD1 d in one case of pediatric medulloblastoma demonstrating that expression of all these markers is absent compared to endothelium and TILs. B) Examples of SerpinB1, SerpinB4 expression in pediatric medulloblastoma. SerpinB9 expression was not detected in pediatric medulloblastoma. C) Expression PD-L1 was not detected in pediatric medulloblastoma by immunohistochemistry. PD-1 positive TILs (arrowheads) are predominantly present at the peripheral zone of the tumor. Positive controls: placenta (PD-L1) and tonsil (PD-1). Scale bar equals 100 μm.
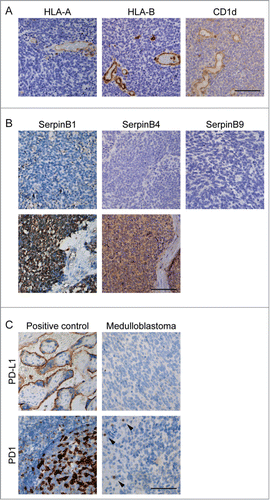
Expression of intracellular apoptosis inhibitors (e.g. caspase inhibitors) to escape from death receptor-induced apoptosis and granzyme-mediated killing pathways are alternative immune evasion pathways employed in multiple cancers.Citation13 Granzymes are the major tumor killing molecules secreted by cytotoxic cells with partially overlapping routes of apoptosis induction.Citation18 Tumors can express Serpins to directly block granzyme activity. We found that six out of 26 tumors acquired expression of SerpinB1 (23%) and 13 out of 26 tumors SerpinB4 (50%). In contrast to CNS-PNETs,Citation17 we did not find expression of SerpinB9 in pediatric medulloblastoma (). Expression of SerpinB1 and/or SerpinB4 was not correlated to influx of TILs. We found no correlation between SerpinB1 and SerpinB4 expression and (molecular) subtypes of medulloblastoma. Interestingly, besides these serpins, expression of downstream inhibitors of apoptotic pathways i.e. Survivin (caspase-3 inhibitor) was present in all tumors and in 12 tumors (46.2%) Bcl-2 (cytochrome c release inhibitor) was expressed. Moreover, expression of Bcl-2 was correlated to SerpinB4 expression (ρ = 0.418, p = 0.042) but not to SerpinB1 (ρ = 0.225, p = 0.268).
In addition to immune escape by anti-apoptotic pathways, we wondered whether these CTLs are functionally impaired by e.g. the PD-1/PD-L1 immune checkpoint. CD8+ T-cells are known to express PD-1 and upon PD-L1 expression by tumor cells, this results in functionally impaired TILs.Citation41,42 First, we examined PD-1 expression and found that PD-1 positive TILs had predominantly a perivascular localization pattern (). The fraction of PD-1+ TILs [4.6% (6.4%)] was low and correlated to GrB-positive CTLs (ρ = 0.646, p<0.001), although it was not correlated to total number of CTLs (ρ = −0.209, p = 0.305). The influx of PD1+ TILs was not correlated to the (molecular) subtype of medulloblastoma. Furthermore, we found that none of the analyzed pediatric medulloblastomas expressed PD-L1 (), suggesting that the PD-1/PD-L1 axis likely is not of great importance in pediatric medulloblastoma and thereby limiting the therapeutic potential of PD-1 blockers.
TILs are not associated with patient survival
Depending on the molecular characteristics patients have a prognostic beneficial or worse phenotype of pediatric medulloblastomas. Potentially immunotherapeutic strategies can contribute to better survival. For that reason we analyzed our patient cohort regarding the association between overall survival and TILs. We stratified for low and high expression of each factor. In our cohort we did not find an association between TILs and patients survival (), although there is a trend towards beneficial outcome for patients with low TILs. However, we found that patients with high numbers of GrB+ CTLs have worse survival than patients with low numbers of GrB+ CTLs (p = 0.015). High expression of anti-apoptotic molecules i.e. Bcl-2 and Survivin did not contribute to survival (data not shown). Overall survival of patients was not dependent on the expression of SerpinB4 (p = 0.974). In contrast to oropharynx squamous cell carcinomas, high expression of SerpinB1 in pediatric medulloblastoma was associated with better survival (p = 0.050).
Discussion
Pediatric medulloblastomas are the most frequently diagnosed embryonal tumors of the CNS in children. The prognosis is mainly dependent on the molecular subtype of the tumor, although therapeutic strategies are limited to conventional radiation therapy, chemotherapy, and surgery. Because of severe neurological side effects, additional strategies such as immunotherapy are being investigated. This study investigates the composition and prognostic value of TILs in pediatric medulloblastoma and the immune evasion strategies exploited by these tumors. We show that the main TIL subsets are CD3+, CD8+ T-cells which have predominantly a perivascular and intratumoral infiltration pattern. The CTLs are barely activated given the low percentage of GrB and PD1 positive cells. It has been hypothesized that pediatric and in particular embryonic tumors are not immunogenic, and therefore immunotherapeutic interventions have a limited success rate compared to e.g. metastases of NSCLC or melanoma.Citation43 A recent study compared several pediatric tumors, which revealed that glioblastoma, neuroblastoma as well as the embryonic Atypical teratoid/rhabdoid tumor have increased expression of PD-L1 and increased amounts of TILs.Citation44 Together this might give a direction why the influx of TILs does not influence the overall survival of medulloblastoma patients in contrast to other tumor types.Citation28,45 One of the mechanisms for immune modulation is the lack of expression of MHC-I and CD1 d which is the case in all tumors. Despite the lack of MHC-I in neuroblastoma,Citation46 still influx of TILs has been observed.Citation44 Since downregulation of MHC-I results in activation of NK cells, therapeutic strategies exploiting NK cells might be of clinical significance. So far no clinical data is available on the effectiveness of NK cell based therapies in medulloblastoma. In order to engage NK cells to kill medulloblastoma cells, expression of ligand for the activation receptors might be required.Citation47 Expression of ligands for NKG2D (NK cell activation receptor) are elevated in 50% of medulloblastomas.Citation48 In line with this, several patients have been intrathecally treated with LAK cells, showing a remission of the medulloblastoma.Citation49–51 Recently Castriconi et al., showed for the first time that NK cells can kill medulloblastoma cells in vitro, which opens the way to study the potential of NK cell-based immunotherapy in medulloblastoma.Citation52 We showed that half of the tumors expressed SerpinB4 and 23% SerpinB1 to potentially resist granzyme induced apoptosis. Expression of SerpinB1 but not SerpinB4 had a beneficial effect on survival. High expression of SerpinB1 has been linked to increased sensitivity towards cisplatin-based chemotherapy and increased survival in melanoma.Citation53 Since cisplatin is a standard chemotherapeutic agent in treatment protocol of pediatric medulloblastoma, the improved survival might be related to increased cisplatin sensitivity, rather than to a SerpinB1 dependent immunomodulatory effect.
The PD-1/PD-L1 immune checkpoint is a pivotal pathway for immune escape by tumors, since expression of PD-L1 on tumor cells, diminishes T-cell activity towards the tumor.Citation54,55 Therefore, interference in this checkpoint constitutes a promising form of immunotherapy in many tumor types.Citation56–58 For instance, clinical studies with GBM patients revealed that the majority of patients had tumor cells expressing some PD-L1 of which 38% had >5% PD-L1 positive tumor cells, and activation of the PD1/PD-L1 axis was associated with poor prognosis.Citation28 In preclinical studies, CD8+ T-cells are enriched in murine medulloblastoma models and those were more frequently PD1 positive, and administration of PD1 blocking antibodies indeed have a beneficial survival effect in mice.Citation11
In contrast to the very recent study of Murata et al.,Citation31 that describes worst survival of the patients with high PD-L1 expression, we could not detect PD-L1 expression in any of the human pediatric medulloblastoma tumor samples. Our finding is in line with recent studies, which demonstrated that pediatric tumors in general are not likely to exploit the PD1/PD-L1 axis.Citation32,44 The discrepancy between our study and Murata et al might be due to differences in staining procedure and used antibodies. The Murata study used a polyclonal antibody for IHC staining and scored intensity of the staining regardless of the staining pattern, while the described staining for PD-L1 is supposed to be membranous. For clinical decision making only clone 28-8 (monoclonal antibody used in this study) has been validated for assessing response towards NivolumabCitation59 and 22C3, SP142, SP263 towards Pembrolizumab, Atezolizumab and Durvalumab respectively.Citation60 Therefore assessing potential beneficial response towards PD-L1 targeted immunotherapy should be performed using the clinically validated antibodies. Therefore to our opinion this study strongly suggests that the therapeutic potential of immunotherapy with PD1/PD-L1 axis blockers seems limited in pediatric medulloblastomas. Further standardized studies are required to examine the PD-L1 expression in this type of cancer.
Competing interests
The authors have declared that no competing interests exist.
Author contribution
JFV, EJMA, MKJ, RGB, WvH, WGMS, JVH, MK, and RB performed the experiments. JFV, WvH, WGMS, JVH, PF, MK, and NB provided the study material, analyzed and interpreted tumor pathology. JFV and NB designed the experiments and wrote the manuscript. All authors critically reviewed the report and approved the final version of the report for submission. The corresponding author (NB) had access to the primary data, took responsibility for accuracy and completeness of data reporting, and had final responsibility for the decision to submit for publication.
Funding
This work was supported by a research grant from Cancer Foundation Koppie-Au. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Nederlandse Kankerregistratie beheerd door IKNL ©. Cijfers over kanker (text in Dutch) http://www.cijfersoverkanker.nl/kinderen-en-kanker-55.html [accessed April 26, 2016].
- Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131:821–31. doi:10.1007/s00401-016-1569-6. PMID:27040285.
- Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65:1419–24. doi:10.1001/archneur.65.11.1419. PMID:19001159.
- Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol. 2013;15:97–103. doi:10.1093/neuonc/nos267. PMID:23099653.
- Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, Leisenring WM, Meacham LR, Mertens AC, Mulrooney DA, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–55. doi:10.1200/JCO.2008.21.1953. PMID:19364955.
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi:10.1038/nrc3245. PMID:22419253.
- Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–15. doi:10.1038/nm.3541. PMID:24793239.
- Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305. doi:10.1200/JCO.2006.09.9861. PMID:17538176.
- Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell. 2016;29:508–22. doi:10.1016/j.ccell.2016.03.002. PMID:27050100.
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi:10.1038/nature12626. PMID:24048067.
- Pham CD, Flores C, Yang C, Pinheiro EM, Yearley JH, Sayour EJ, Pei Y, Moore C, McLendon RE, Huang J, et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin Cancer Res. 2016;22:582–95. doi:10.1158/1078-0432.CCR-15-0713. PMID:26405194.
- Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol. 2013;191:4880–8. doi:10.4049/jimmunol.1301966. PMID:24078694.
- Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–20. PMID:12050175.
- Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garre ML, Cama A, Basso G, Ferrone S, Gambini C, et al. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67:5471–8. doi:10.1158/0008-5472.CAN-06-4735. PMID:17545629.
- Gerosa MA, Amadori G, Semenzato P, Gasparotto G, Carteri A. Immunobiology of primary CNS tumors in infancy and childhood. B- and T-cell dependent immunity and cytotoxicity and cell kinetic evaluation. Childs Brain. 1980;6:92–102. PMID:6965475.
- Liu D, Song L, Brawley VS, Robison N, Wei J, Gao X, Tian G, Margol A, Ahmed N, Asgharzadeh S, et al. Medulloblastoma expresses CD1 d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149:55–64. doi:10.1016/j.clim.2013.06.005. PMID:23891738.
- Vermeulen JF, van Hecke W, Spliet WG, Villacorta Hidalgo J, Fisch P, Broekhuizen R, Bovenschen N. Pediatric Primitive Neuroectodermal Tumors of the Central Nervous System Differentially Express Granzyme Inhibitors. PLoS One. 2016;11:e0151465. doi:10.1371/journal.pone.0151465. PMID:26963506.
- Bovenschen N, Kummer JA. Orphan granzymes find a home. Immunol Rev. 2010;235:117–27. doi:10.1111/j.0105-2896.2010.00889.x. PMID:20536559.
- Sapoznik S, Hammer O, Ortenberg R, Besser MJ, Ben-Moshe T, Schachter J, Markel G. Novel anti-melanoma immunotherapies: disarming tumor escape mechanisms. Clin Dev Immunol. 2012;2012:818214. doi:10.1155/2012/818214. PMID:22778766.
- van Houdt IS, Oudejans JJ, van den Eertwegh AJ, Baars A, Vos W, Bladergroen BA, Rimoldi D, Muris JJ, Hooijberg E, Gundy CM, et al. Expression of the apoptosis inhibitor protease inhibitor 9 predicts clinical outcome in vaccinated patients with stage III and IV melanoma. Clin Cancer Res. 2005;11:6400–7. doi:10.1158/1078-0432.CCR-05-0306. PMID:16144945.
- van Kempen PM, Noorlag R, Swartz JE, Bovenschen N, Braunius WW, Vermeulen JF, Van Cann EM, Grolman W, Willems SM. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol Immunother. 2016;65:575–85. doi:10.1007/s00262-016-1819-4. PMID:26993499.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi:10.1038/nrc3239. PMID:22437870.
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi:10.1038/nm1517. PMID:17159987.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi:10.1056/NEJMoa1200694. PMID:22658128.
- Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi:10.1016/S1470-2045(16)30053-5. PMID:27267608.
- Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6:40836–49. doi:10.18632/oncotarget.5696. PMID:26517811.
- Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75. doi:10.1093/neuonc/nou307. PMID:25355681.
- Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18:195–205. doi:10.1093/neuonc/nov172. PMID:26323609.
- Garber ST, Hashimoto Y, Weathers SP, Xiu J, Gatalica Z, Verhaak RG, Zhou S, Fuller GN, Khasraw M, de Groot J, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. 2016;18:1357–66. doi:10.1093/neuonc/now132. PMID:27370400.
- Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353:399–403. doi:10.1126/science.aae0477. PMID:27463676.
- Murata D, Mineharu Y, Arakawa Y, Liu B, Tanji M, Yamaguchi M, Fujimoto KI, Fukui N, Terada Y, Yokogawa R, et al. High programmed cell death 1 ligand-1 expression: association with CD8+ T-cell infiltration and poor prognosis in human medulloblastoma. J Neurosurg. 2017;5:1–7. doi:10.3171/2016.11.JNS16991. PMID:28474991.
- Aoki T, Hino M, Koh K, Kyushiki M, Kishimoto H, Arakawa Y, Hanada R, Kawashima H, Kurihara J, Shimojo N, et al. Low Frequency of Programmed Death Ligand 1 Expression in Pediatric Cancers. Pediatr Blood Cancer. 2016;63:1461–4. doi:10.1002/pbc.26018. PMID:27135656.
- CCMO website:. Central Committee on Research involving Human Subjects (Centrale Commissie Mensgebonden Onderzoek) (text in Dutch): http://www.ccmo.nl/nl/uw-onderzoek-wmo-plichtig-of-niet [accessed September 2, 2017].
- van Diest PJ. No consent should be needed for using leftover body material for scientific purposes. For. BMJ. 2002;325:648–51. doi:10.1136/bmj.325.7365.648.
- Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, Cavalli FM, Ramaswamy V, Zapatka M, Reifenberger G, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125:913–6. doi:10.1007/s00401-013-1126-5. PMID:23670100.
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi:10.1126/science.1110267. PMID:15933162.
- Chien YH, Meyer C, Bonneville M. Gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–55. doi:10.1146/annurev-immunol-032713-120216. PMID:24387714.
- Kmiecik J, Zimmer J, Chekenya M. Natural killer cells in intracranial neoplasms: presence and therapeutic efficacy against brain tumours. J Neurooncol. 2014;116:1–9. doi:10.1007/s11060-013-1265-5. PMID:24085644.
- Poli A, Kmiecik J, Domingues O, Hentges F, Blery M, Chekenya M, Boucraut J, Zimmer J. NK cells in central nervous system disorders. J Immunol. 2013;190:5355–62. doi:10.4049/jimmunol.1203401. PMID:23687193.
- Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, et al. CD1 d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 2013;14:1137–45. doi:10.1038/ni.2713. PMID:24076636.
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi:10.1182/blood-2008-12-195792. PMID:19423728.
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10. doi:10.1182/blood-2010-10-310425. PMID:21385853.
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi:10.1038/nature12477. PMID:23945592.
- Majzner RG, Simon JS, Grosso JF, Martinez D, Pawel BR, Santi M, Merchant MS, Geoerger B, Hezam I, Marty V, et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer. 2017;123:3807–15. doi:10.1002/cncr.30724. PMID:28608950.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi:10.1038/nature13954. PMID:25428505.
- Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, Ernestus K, Berthold F. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–6. doi:10.1007/s00262-004-0603-z. PMID:15449039.
- Perez-Martinez A, Fernandez L, Diaz MA. The therapeutic potential of natural killer cells to target medulloblastoma. Expert Rev Anticancer Ther. 2016;16:573–6. doi:10.1080/14737140.2016.1184978. PMID:27144504.
- Fernandez L, Portugal R, Valentin J, Martin R, Maxwell H, Gonzalez-Vicent M, Diaz MA, de Prada I, Perez-Martinez A. In vitro Natural Killer Cell Immunotherapy for Medulloblastoma. Front Oncol. 2013;3:94. doi:10.3389/fonc.2013.00094. PMID:23626949.
- Silvani A, Salmaggi A, Parmiani G, Boiardi A. Successful adoptive immunotherapy with lymphokine-activated killer cells in the treatment of medulloblastoma disseminated via cerebrospinal fluid: case report. Neurosurgery. 1994;34:1078–80 ; discussion 80–1. doi:10.1227/00006123-199406000-00021. PMID:8084395.
- Okamoto Y, Shimizu K, Tamura K, Miyao Y, Yamada M, Matsui Y, Tsuda N, Takimoto H, Hayakawa T, Mogami H. An adoptive immunotherapy of patients with medulloblastoma by lymphokine-activated killer cells (LAK). Acta Neurochir (Wien). 1988;94:47–52. doi:10.1007/BF01406615. PMID:3177046.
- Shimizu K, Tamura K, Yamada M, Okamoto Y, Miyao Y, Park K, Matsui Y, Hayakawa T, Takimoto H, Mogami H. [Adoptive immunotherapy in patients with medulloblastoma by LAK cells]. No To Shinkei. 1989;41:991–5. PMID:2605046.
- Castriconi R, Dondero A, Negri F, Bellora F, Nozza P, Carnemolla B, Raso A, Moretta L, Moretta A, Bottino C. Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur J Immunol. 2007;37:3190–6. doi:10.1002/eji.200737546. PMID:17918205.
- Willmes C, Kumar R, Becker JC, Fried I, Rachakonda PS, Poppe LM, Hesbacher S, Schadendorf D, Sucker A, Schrama D, et al. SERPINB1 expression is predictive for sensitivity and outcome of cisplatin-based chemotherapy in melanoma. Oncotarget. 2016;7:10117–32. doi:10.18632/oncotarget.6956. PMID:26799424.
- Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7 H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–5. doi:10.1158/0008-5472.CAN-03-3259. PMID:14871849.
- Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171–86. doi:10.18632/oncotarget.13895. PMID:27974689.
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi:10.1056/NEJMoa1504627. PMID:26028407.
- Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–67. doi:10.1056/NEJMoa1602252. PMID:27718784.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi:10.1056/NEJMoa1504030. PMID:26027431.
- Diggs LP, Hsueh EC. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res. 2017;5:12. doi:10.1186/s40364-017-0093-8. PMID:28331612.
- Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–22. doi:10.1016/j.jtho.2016.11.2228. PMID:27913228.