ABSTRACT
Metastatic basal cell cancer (BCC) is an ultra-rare malignancy with no approved therapies beyond Hedgehog inhibitors. We characterized the genomics, tumor mutational burden (TMB), and anti-PD-1 therapy responses in patients with locally advanced or metastatic BCC. Overall, 2,039 diverse cancer samples that had undergone comprehensive genomic profiling (CGP) were reviewed. Eight patients with locally advanced/metastatic BCC were identified (two had two CGP analyses; total, 10 biopsies). Two tumors demonstrated PD-L1 amplification. Seven patients had >1 actionable alteration. The TMB (mutations/mb) (median (range)) was 90 (3-103) for the BCCs versus 4 (1-860) for 1637 cancers other than BCC (P < 0.0001). Median progression-free survival (PFS) for all four patients treated with PD-1 blockade was 10.7 months (range, 3.8 to 17.6+ months); three patients had an objective response. In conclusion, advanced/metastatic BCC often has biological features (high TMB; PD-L1 amplification) predictive of immunotherapy benefit, and patients frequently respond to PD-1 blockade.
Introduction
Basal cell carcinoma is the most common malignancy with approximately 2.8 million cases diagnosed annually in the United States. Rarely, basal cell carcinomas can become locally destructive and/or metastasize.Citation1 The development of metastatic basal cell carcinoma is highly dependent on the primary tumor size. Previous studies have shown that there is an approximately 2% incidence of metastasis for tumors larger than 3 cm in diameter. The incidence increases to 25% for tumors larger than 5 cm in diameter and is 50% for tumors larger than 10 cm in diameter. In addition, a greater than 90% metastasis rate has been observed for tumors that are greater than 25 cm2.Citation2,3
Most basal cell carcinomas harbor mutations in the Hedgehog pathway.Citation4 Currently, the Hedgehog inhibitors vismodegib and sonidegib are approved by the Food and Drug Administration (FDA) for treating locally advanced/metastatic basal cell carcinoma.Citation5,6 However, there are no approved therapies for patients who progress on Hedgehog inhibitors. Furthermore, response rates to Hedgehog inhibitors are only 30–45%, with a median duration of response of approximately six months.Citation5,6
Antibodies blocking programmed death receptor (PD-1)/programmed death receptor – ligand 1 (PD-L1) show efficacy in both solid tumors and lymphoma.Citation7,8 Biomarkers for response include PD-L1 positivity by immunohistochemistry, microsatellite instability, and high tumor mutations burden (TMB).Citation7,8 Interestingly, approximately 90% of basal cell carcinomas are positive for PD-L1 by immunohistochemistry,Citation9 and the intensity of PD-L1 staining on basal cell carcinoma cells increases with the number of prior treatment modalities.Citation9 Furthermore, basal cell carcinoma is one of the most mutated types of human cancer.Citation10 Similar to melanoma, the majority of mutations identified in basal cell carcinoma have an ultraviolet (UV) signature. Therefore, advanced basal cell carcinomas have features (PD-L1 positivity and high TMB) that would predict checkpoint inhibitor response.
There are a few case reports of successful treatment of refractory basal cell carcinoma with anti-PD-1 therapy.Citation1,11,12 Herein, we present the genomic correlates of the largest case series to date of patients with advanced/metastatic basal cell cancer treated with anti-PD-1 therapy.
Results
Patient Characteristics: Nine biopsies from eight patients with locally advanced or metastatic basal cell carcinoma and a calculated TMB were identified (Supplemental Figure 1). Patient characteristics are listed in and Supplemental Table 1. Five patients had locally advanced disease while three patients had metastatic disease.
Table 1. Patient profiles and treatment response.
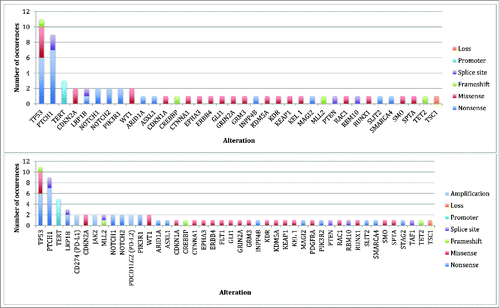
Genomics: The median (range) number of genomic alterations (characterized alterations, excludes variants of unknown significance) found per patient biopsy was 9 (2-19). The most common alterations identified were in the TP53, PTCH1, and TERT genes (). All patients had ≥1 genomic alterations. Amplification of CD274 (PD-L1), PDCD1LG2 (PD-L2), and JAK2 (chromosome 9p24.1 amplification) was identified in two patients. The median (range) number of potentially actionable alterations with either an on- or off -label FDA approved drugs was 5 (0-11); seven patients had ≥1 such potentially actionable alterations (Supplemental Figures 2 and 3). Excluding PTCH1 and SMO alterations, seven patients had one or more actionable alterations with an off-label approved drug (median (range) = 3 (0-9)).
Figure 1. Genomic alterations identified* Top Panel: Total alterations (N = 62) identified by NGS on initial biopsy (N = 8 biopsies). Bottom Panel: Total alterations (N = 77) identified by NGS on initial and subsequent biopsies (N = 10 biopsies). *Some patients had multiple alterations in the same gene (i.e. TP53 and PTCH1).
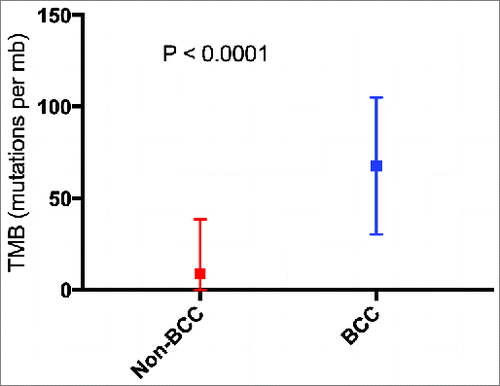
TMB: Nine biopsies from eight patients had TMB calculated. The median TMB (mutations/mb) (range) for nine basal cell carcinoma samples was 90 (3-103) while the median TMB (range) for 1637 samples with cancers other than basal cell carcinoma was 4 (1-860) (P < 0.0001) (Supplemental Table 1 and ). Of the eight patients evaluable for TMB, seven had a high TMB (≥ 20 mutations/megabase); six of these patients had a very high TMB (≥ 50 mutations/megabase).
Figure 2. Mean tumor mutational burden for cancers other than basal cell carcinoma (N = 1,637) vs. basal cell carcinoma (N = 9 biopsies with available data). P value calculated using Mann Whitney U test. Squares represent mean TMB. Bars represent the standard deviation of the mean. Abbreviations: BCC = basal cell carcinoma; mb = megabase; TMB = tumor mutational burden.
Treatment with PD-1 blockade (): Four patients were treated with PD-1 blockade. Three of these patients had had tumor progression on ≥1 Hedgehog inhibitor while one patient received a combination of nivolumab and vismodegib.
Patient 2, with metastatic disease and a chromosome 9p24.1 amplification, has an ongoing near complete response (CR) on nivolumab (17.6+ months duration). Patient 3, also with metastatic disease and a chromosome 9p24.1 amplification, had a partial response (PR) of 4.2 months duration on nivolumab. Patient 5, with locally advanced disease (10 × 11 cm unresectable tumor), has an ongoing CR of 8.1+ months duration on the combination of nivolumab and vismodegib. Patient 8, with metastatic disease, had progressive disease on pembrolizumab after 2.5 months. The median PFS for all four patients treated with PD-1 blockade was 10.7 months. For the 7 patients treated with a Hedgehog inhibitor, the median PFS was 11.1 months (P = 0.91) (Supplemental Figure 4).
Discussion
We demonstrate that the majority of patients with advanced and/or metastatic basal cell carcinoma have numerous genomic alterations that are potentially targetable by both on- and off-label FDA approved drugs. Importantly, seven of eight patients (84%) had a high TMB, which can be a marker of response to immunotherapy.Citation13 Further, two of the patients had amplification of PD-L1, which is the hallmark of Hodgkin lymphoma, known to be exquisitely responsive to checkpoint inhibitors in Hodgkin lymphoma.Citation14 Finally, we present four patients with locally advanced/metastatic basal cell carcinoma treated with PD-1 blockade (seven of whom had previously failed ≥1 Hedgehog inhibitors, while one patient with an ongoing CR received concomitant vismodegib and nivolumab). The response rate in our cohort was 75% (3 of 4 patients), with a median PFS of 10.7 months (range, 3.8 to 17.6+ months).
TMB, measured by CGP has been shown to correlate with response to PD-1/PD-L1 blockade in patients with melanomaCitation15 and urothelial carcinoma.Citation16 Herein, TMB reveals that advanced basal cell carcinoma has an extremely high mutational burden. Indeed, six patients had a TMB of ≥50 mutations/megabase (≥20 mutations/megabase is considered high). Prior studies have also demonstrated a high TMB and ultraviolet (UV) signature in basal cell carcinoma (Supplemental Table 3).Citation10,17 This observation suggests that, like melanoma, basal cell carcinoma has a marker associated with a high likelihood of responding to checkpoint blockade.
Interestingly, two patients had amplification of chromosome 9p24.1, the region containing PD-L1, PD-L2, and JAK2. Chromosome 9p24.1 copy number alterations are a disease-defining feature in Hodgkin lymphoma, with 97% of patients having 9p24.1 copy number alterations.Citation18 Heavily-pretreated Hodgkin lymphoma demonstrates response rates as high as 87% to PD-1 blockade.Citation14
Patients with advanced/metastatic basal cell carcinoma have a poor prognosis after progressing on Hedgehog inhibitor therapy, with limited therapeutic options. Our data presents a biologic rational and clinical data that support the use of checkpoint blockade with PD-1 inhibitors in these patients. Larger clinical trials are needed to confirm these findings.
Methods
Patient selection: We evaluated 2,039 cancer samples that underwent comprehensive genomic profiling (CGP). Only patients with a diagnosis of locally advanced/metastatic basal cell carcinoma confirmed by a dermatopathologist (PRC) are presented. This study was performed in accordance with UCSD Institutional Review Board guidelines for the PREDICT study (NCT02478931) and for any investigational treatments for which patients gave consent in accordance with the Declaration of Helsinki
Next Generation Sequencing and Assessment of Tumor Mutational Burden (TMB): Formalin-fixed paraffin embedded tumor samples were submitted for CGP to Foundation Medicine (N = 2039 patients) (clinical laboratory improvement amendments (CLIA)-certified lab): the FoundationOne (hybrid-capture-based CGP; 182, 236 or 315 genes, depending on the time period). (http://www.foundationone.com/).Citation19
For TMB (mutations per megabase (mb)), the number of somatic mutations detected on NGS (interrogating 1.2 mb of the genome) were quantified and that value extrapolated to the whole exome using a validated algorithm.Citation16 Alterations likely or known to be bona fide oncogenic drivers and germline polymorphisms were excluded.
Definition of actionable alteration: An alteration was defined as potentially actionable if its protein product is a component of a molecularly defined pathway for which there is at least one available FDA-approved drug that may impact the function of the protein product of the alteration or the immediate downstream effectors of the protein product.
Statistical Analysis and Outcome Evaluation: Mann Whitney U test was used to assess continuous variables. Responses were assessed based on physician notation; physicians used RECIST criteria. Progression-free survival (PFS) was calculated by the method of Kaplan and Meier (P values by log-rank (Mantel-Cox) test). For patients who received multiple treatment regimens, the treatment with the longest PFS was chosen for analysis. Patients were censored at date of last follow up for PFS if they had not progressed. Statistical analyses were performed using Graph-Pad Prism version 7.0 (San Diego, CA, USA).
Abbreviations
| CR | = | complete response |
| FDA | = | Food and Drug Administration |
| f | = | female |
| m | = | male |
| mb | = | megabase |
| N/A | = | not available |
| PD | = | progressive disease |
| PFS | = | progression free survival |
| PR | = | partial response |
| TMB | = | tumor mutational burden |
Conflict of interest
Dr. Frampton, Dr. Stephens, and Dr. Miller are employees and equity holders of Foundation Medicine. Dr. Kurzrock receives research funding from Genentech, Merck, Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant, as well as consultant fees from X Biotech, and Actuate Therapeutics and has an ownership interest in Curematch Inc.
Supplemental_Tables_and_figures.docx
Download MS Word (148.4 KB)Additional information
Funding
References
- Ikeda S, Goodman AM, Cohen PR, Jensen TJ, Ellison CK, Frampton G, Miller V, Patel SP, Kurzrock R. Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. Npj Genomic Med. 2016;1:16037. doi:10.1038/npjgenmed.2016.37.
- Uzquiano MC, Prieto VG, Nash JW, Ivan DS, Gong Y, Lazar AJ, Diwan AH. Metastatic basal cell carcinoma exhibits reduced actin expression. Mod Pathol. 2008;21(5):540–3. doi:10.1038/modpathol.3801051. PMID:18223552.
- Vu A, Laub D. Metastatic Basal Cell Carcinoma: A Case Report and Review of the Literature. Eplasty. 2011;11,5(Suppl 1): S26–S29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3088016/. Accessed November 4, 2017. PMID:21559224
- Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–54. doi:10.1038/nrc2503. PMID:18813320.
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. N Engl J Med. 2012;366(23):2171–9. doi:10.1056/NEJMoa1113713. PMID:22670903.
- Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kudchadkar R, Trefzer U, Gogov S, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–28. doi:10.1016/S1470-2045(15)70100-2. PMID:25981810.
- Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–20. doi:10.1038/nrclinonc.2016.168. PMID:27805626
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–56. doi:10.1158/1535-7163.MCT-14-0983. PMID:25695955.
- Chang J, Zhu GA, Cheung C, Li S, Kim J, Chang ALS. Association Between Programmed Death Ligand 1 Expression in Patients With Basal Cell Carcinomas and the Number of Treatment Modalities. JAMA Dermatol. 2017;153(4):285–90. doi:10.1001/jamadermatol.2016.5062.
- Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134(1):213–20. doi:10.1038/jid.2013.276. PMID:23774526.
- Lipson EJ, Lilo MT, Ogurtsova A, Esandrio J, Xu H, Brothers P, Schollenberger M, Sharfman WH, Taube JM. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer. 2017;176(2):498–502. doi:10.1186/s40425-017-0228-3. PMID:28344809
- Winkler JK, Schneiderbauer R, Bender C, Sedlaczek O, Fröhling S, Penzel R, Enk A, Hassel JC. Anti-programmed cell death-1 therapy in nonmelanoma skin cancer. Br J Dermatol. 2017;176(2):498–502. doi:10.1111/bjd.14664. PMID:27061826.
- Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598–608. doi:10.1158/1535-7163.MCT-17-0386. PMID:28835386
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N Engl J Med. 2015;372(4):311–9. doi:10.1056/NEJMoa1411087. PMID:25482239.
- Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res. 2016;4(11):959–67. doi:10.1158/2326-6066.CIR-16-0143.
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet Lond Engl. 2016;387(10031):1909–20. doi:10.1016/S0140-6736(16)00561-4.
- Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, Seplyarskiy VB, Sharpe HJ1, McKee T, Letourneau A, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48(4):398–406. doi:10.1038/ng.3525. PMID:26950094.
- Roemer MGM, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016;34(23):2690–97. doi:10.1200/JCO.2016.66.4482. PMID:27069084.
- Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. doi:10.1038/nbt.2696. PMID:24142049.
- Armand P, Shipp M, Ribrag V, et al. PD-1 Blockade with Pembrolizumab in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Safety, Efficacy, and Biomarker Assessment (KEYNOTE-013) [abstract]. American Society of Hematology Annual Meeting 2016;34(31):3733–39.
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6(4):353–67. doi:10.1158/2159-8290.CD-15-0894. PMID:26658964.
- Samadder N, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz W, Jones D, Tavtigian SV, Done MW, Berry T, et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: A randomized clinical trial. JAMA. 2016;315(12):1266–75. doi:10.1001/jama.2016.2522. PMID:27002448.
- Marquez-Medina D, Popat S. Afatinib: a second-generation EGF receptor and ErbB tyrosine kinase inhibitor for the treatment of advanced non-small-cell lung cancer. Future Oncol Lond Engl. 2015;11(18):2525–40. doi:10.2217/fon.15.183..
- Furumoto Y, Gadina M. The arrival of JAK inhibitors: advancing the treatment of immune and hematologic disorders. BioDrugs Clin Immunother Biopharm Gene Ther. 2013;27(5):431–8. doi:10.1007/s40259-013-0040-7..
- Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–81. doi:10.1016/S1470-2045(12)70270-X. PMID:22805291.
- Loaiza-Bonilla A, Jensen CE, Shroff S, Furth E, Bonilla-Reyes PA, Deik AF, Morrissette J. KDR Mutation as a Novel Predictive Biomarker of Exceptional Response to Regorafenib in Metastatic Colorectal Cancer. Cureus. 8(2). doi:10.7759/cureus.478..
- Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn Pathol. 2006;23(2):91–102. doi:10.1053/j.semdp.2006.08.006. PMID:17193822.
- Weigelt B, Warne PH, Lambros MB, Reis-Filho JS, Downward J. PI3 K pathway dependencies in endometrioid endometrial cancer cell lines. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(13):3533–44. doi:10.1158/1078-0432.CCR-12-3815..
- Janku F, Hong DS, Fu S, Piha-Paul SA, Naing A, Falchook GS, Tsimberidou AM, Stepanek VM, Moulder SL, Lee JJ, et al. Assessing PIK3CA and PTEN in early-phase trials with PI3 K/AKT/mTOR inhibitors. Cell Rep. 2014;6(2):377–87. doi:10.1016/j.celrep.2013.12.035. PMID:24440717.
- Said R, Hong DS, Warneke CL, Lee JJ, Wheler JJ, Janku F, Naing A, Falchook GS, Fu S, Piha-Paul S, et al. P53 Mutations in Advanced Cancers: Clinical Characteristics, Outcomes, and Correlation between Progression-Free Survival and Bevacizumab-Containing Therapy. Oncotarget. 2013;4(5):705–14. doi:10.18632/oncotarget.974. PMID:23670029.
- Schwaederle M, Vladimir L, Validire P, Hansson J, Lacroix L, Soria JC, Pawitan Y, Kurzrock R. VEGF-A Expression Correlates with TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Anti-Angiogenesis Therapy. Cancer Res. 2015;75(7):1187–90. doi:10.1158/0008-5472.CAN-14-2305.
- Koehler K, Liebner D, Chen JL. TP53 mutational status is predictive of pazopanib response in advanced sarcomas. Ann Oncol. 2016;27(3):539–43. doi:10.1093/annonc/mdv598.
- Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, Pirun M, Sander C, Socci ND, Ostrovnaya I, et al. Genome Sequencing Identifies a Basis for Everolimus Sensitivity. Science. 2012;338(6104):221–221. doi:10.1126/science.1226344. PMID:22923433.
