ABSTRACT
Survival of patients with germ-cell cancer (GCC) and primary progression or relapse after cisplatin-based first-line chemotherapy is highly heterogeneous, ranging from close to zero to more than 70%. We investigated β-1,4-Galactosyltransferase-I (B4GALT1) expression levels in peripheral lymphocytes in a cohort of 46 testicular cancer patients. B4GALT1 enhances immune cell crosstalk via glycosylation of surface molecules. A high expression level of B4GALT1 in T-lymphocytes, but not in monocytes, was associated with a lower risk of relapse with a hazard ratio (HR) of 0.66 (95% confidence interval (CI) of HR: 0.45-0.97; p = 0.02) upon multivariate Cox regression analysis. Correspondingly, interleukin 10 (IL10), a cytokine released by cytotoxic T-cells, was likewise significantly elevated in T-lymphocytes of non-relapse GCC patients (HR: 0.3; 95% CI of HR: 0.14-0.65; p = 0.002). Our data indicate that glycosylation and activation of T-lymphocytes may play a pivotal role in disease control in GCC patients with primary progressive or relapsed disease.
Introduction
Testicular cancer occurs in young to middle-aged male patients and can be cured even in metastatic disease, after progression or relapse. The 3-year overall survival (OS) probability of patients with progression or relapse after a first-line cisplatin-based chemotherapy regimen ranges between close to zero and 70% or more.Citation1
Salvage treatment consists either of four cycles of conventional-dose chemotherapy (CDCT) or high-dose chemotherapy (HDCT) with autologous stem cell reinfusion (ASCR)Citation2 followed by residual tumor resection if needed. The score of the International Prognostic Factor Study Group (IPFSG) helps to predict relapse and survival according to tumor marker levels of alpha fetoprotein (AFP) and human chorionic gonadotropin (HCG) as well as clinical parameters such as primary tumor site, response to first-line treatment, progression-free time interval, presence of liver, bone and brain metastases and histology, namely seminoma and non-seminoma. The score ranges from -1 to 3 points, defining very low (-1 point), low (0 points), intermediate (1 point), high (2 points) and very high (3 points) risk categories in respect to progression free survival.Citation3
As T-cell function is important for disease control in cancer patientsCitation4,Citation5 and little is known about mechanisms of disease control in germ cell-cancer (GCC) patients, we sought to retrospectively identify immunomodulatory properties in T-lymphocytes and monocytes derived from samples of stored leukapheresis products. All products had been obtained prior to HDCT by mobilization with chemotherapy plus granulocyte colony-stimulating factor (G-CSF) and frozen for subsequent ASCR. Lymphocytes and monocytes were isolated from thawed samples via flow cytometry based cell sorting.
We particularly focused on β-1,4-Galactosyltransferase-I (B4GALT1) gene expression levels which were reported to be highly relevant for disease control in stage I non-small-cell-lung cancer (NSCLC) patients.Citation6 B4GALT1 is a type II membrane-bound glycoprotein, which exclusively transfers UDP-galactose to different acceptor sugar molecules and is of particular importance for the interaction and adhesion of immune cells,Citation7 either through binding to appropriate glycoside substrates on adjacent cell surfaces or modification of N-acetylglucosamine (GlcNAc) residues of surface molecules such as leucocyte integrins.Citation8,Citation9 The mRNA exists in two isoforms.Citation10,Citation11 In our study we focused on the longer transcript variant coding for a surface membrane protein. B4GALT1 seems to be important for B-cell function and migrationCitation12 and in B-cell malignancies.Citation13 Furthermore, B4GALT1 may represent a novel biomarker in colorectal cancers that is regulated by DNA promotor methylationCitation14 and predicts adverse outcome in non-metastatic clear cell renal cell carcinoma.Citation15
Because selectins and leucocyte integrins are modified by B4GALT1 and play a substantial role in tumor control,Citation6 we additionally measured the protein levels of very late antigen 4 (VLA4; CD49 d), platelet endothelial cell adhesion molecule 1 (PECAM1; CD31) and chemokine receptor 4 (CXCR4; CD184) on peripheral lymphocytes and monocytes in GCC patient samples via flow cytometry but did not find an association with relapse-free survival in our patient cohort. Association of IL10 expression in T-lymphocytes with relapse-free survival and up-regulation of B4GALT1 after lectin stimulation in vitro supports the notion that activated lymphocytes are involved in tumor surveillance in GCC.
Here we demonstrate for the first time that a high mRNA expression level of B4GALT1 in peripheral blood T-lymphocytes derived from leukapheresis products correlates with a lower risk of relapse in patients with GCC, who had undergone HDCT followed by ASCR. To our knowledge, the prognostic impact of B4GALT1 expression in T-lymphocytes in patients with GCC has not been reported so far.
Methods
Patients and sample preparation
Between May 1999 and January 2012, a total of 73 patients with progressive or relapsed GCC after cisplatin-based first-line chemotherapy were treated at the Department of Hematology, Oncology and Immunology, University Hospital Marburg with single or sequential HDCT and ASCR. Of 46/73 patients, aliquots of leukapheresis products were available and cell counts were sufficiently high for analysis. Among these patients, 27/46 had been treated within a multicenter, randomized phase III clinical trial.Citation16 Upon a patient's written informed consent leukapheresis products were collected after mobilization with chemotherapy and G-CSF, frozen in 10% dimethyl sulfoxide (Carl Roth GmbH, Karlsruhe, Germany) and stored in liquid nitrogen prior to be used as ASCR after HDCT. For quality assurance and research purpose several 1 ml samples of the leukapheresis products were stored separately. Subsequently, all patients received HDCT followed by ASCR. HDCT consisted of three cycles carboplatin (1,500 mg/m2) and etoposide (1,500 mg/m2) per cycle in 22/27 patients. Due to renal impairment 4/27 patients received three HDCT cycles at a reduced dose of carboplatin (1,200 mg/m2) and etoposide (1,200 mg/m2) per cycle as requested by the study protocol.Citation16 1/27 patients was randomized to a single cycle of HDCT with carboplatin (2,200 mg/m2), etoposide (1,800 mg/m2) and cyclophosphamide (6,400 mg/m2).Citation16 Outside of the study protocol, 15/46 patients were treated on an individual basis with paclitaxel (225 mg/m2), cisplatin (100 mg/m2), etoposide (750 mg/m2) and ifosfamide (10 g/m2).Citation17 Exact data about the chemotherapy dosages were unavailable for 4/46 patients. A single HDCT was used in 17/46 patients, two HDCT cycles were used in 2/46 patients, and three HDCT cycles were used in 27/46 patients.
For analysis, leukapheresis aliquots were thawed and immediately resuspended in 5 ml of sodium chloride solution (0.9%) with 5% human serum albumin (HSA) (CSL Behring, Marburg, Germany). All steps were performed on ice. Density centrifugation with Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) at 4°C was performed in order to extract mononuclear cells (MNC). After washing with sodium chloride solution (0.9%) containing 5% HSA, cells were resuspended in 1 ml phosphate buffered saline (PBS). 100 µl of the cell suspension were used for RNA isolation from total MNC with peqGOLD TriFast® (peqlab, Erlangen, Germany) according to the manufacturer's instructions. MNC obtained from buffy coats of healthy donors were treated with 1 μg/ml Concanavalin A (Con A) in RPMI medium for 24 h at 37°C prior to cell sorting.
Flow cytometric analysis
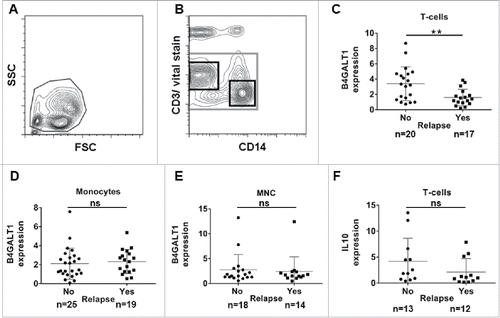
The remaining cells were pelleted and the following antibodies were added: CD3 (UCHT1) conjugated with V450, CD31 (M89D3) with Alexa Fluor® 488, CD184 (12G5) with Phycoerythrin or BD Multitest™ CD3/16/56/45/19 (all BD Biosciences, San José, USA), CD14 (TÜK4) with Peridinin Chlorophyll (PerCP) and CD49 d (MZ18-24A9) with Allophycocyanin (both Miltenyi Biotec, Bergisch Gladbach, Germany). The suspension was incubated for 30 min in the dark at 4°C and cells were suspended in 400 µl of PBS after washing with PBS. Cells and reagents were continuously kept on ice. 1 µl of Sytox Blue Dead Cell Stain® (Molecular Probes, Eugene, USA) was added immediately before measurement with a MoFlo® flow cytometer (Beckman Coulter, Brea, California, USA). For analysis and sorting, exclusion of debris was done with forward scatter versus side scatter gating (a). Employing CD14 versus CD3 gating, vital T-cells (left upper black gate in b) and vital monocytes (lower right black gate in b) were sorted into separate tubes containing cold peqGOLD Trifast® reagent (peqlab, Erlangen, Germany). RNA extraction was performed directly after sorting according to the manufacturer's instructions. RNA samples were stored at -80°C.
Figure 1. Gating strategy applied for cell sorting with further RT-PCR analysis of B4GALT1 and IL10 expression levels in compartments of MNC from leukapheresis products. (A) Forward scatter versus side scatter gating for exclusion of debris. (B) CD14 versus vital stain gating for the exclusion of dead cells (grey gate). CD14 versus CD3 plotting in order to identify CD3-positive T-cells (upper left black gate) and CD14-positive monocytes (lower right black gate). (C) B4GALT1 levels in peripheral blood T-lymphocytes are significantly higher in patients with no relapse compared to those patients with relapse (Mann-Whitney-test, p = 0.008). Neither B4GALT1 levels in (D) monocytes (p = 0.251), and (E) unsorted MNC samples (p = 0.650), nor (F) IL10 expression levels in T-cells (p = 0.178) are significantly different upon Mann-Whitney-testing. Plots show mean and standard deviation, respectively. FSC: forward scatter; MNC: mononuclear cells; ns: not significant; SSC: side scatter.
Real-time PCR
cDNA was obtained employing the Omniscript RT Kit (Qiagen, Hilden, Germany). RT-PCR for the target genes B4GALT1 (primer sequences forward: 5ˈ-tgaccataatgcgtacaggtg-3 ' and reverse: 5ˈ-ccaaaatactgaacataaggtaggc-3ˈ) and IL10 (forward: 5ˈ-agaacctgaagaccctcaggc-3ˈ and reverse: 5ˈ-ccacggccttgctcttgtt-3ˈ; internal control gene: abl forward: 5ˈ-tcatgaaagagatcaaacacccta-3ˈ and reverse 5ˈ-catgaactcagtgatgatatagaacg-3ˈ) was performed in the CFX96 Real-time system® device (Bio-Rad, Munich, Germany) using the SYBR Green® PCR Master Mix assay (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Amplifications included one step at 95°C for 15 min, followed by 45 cycles of 94°C for 15 s, 59°C for 30 s and 72°C for 30 s for B4GALT1 RT-PCR. For IL10 RT-PCR, the annealing temperature was 60°C. For amplification of human interferon gamma (IFNG), the RT2` qPCR Primer Assay for Human IFNG (Qiagen, Hilden, Germany) was used. Primer annealing was done at 58°C and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control gene (forward: 5 '-ctcctccacctttgacgctg-3 ' and reverse: 5 '-accaccctgttgctgtagcc-3 '). RT-PCR data were analyzed using a standard curve and the ΔΔCt-method. Gene specific signals were normalized to internal control signals from the same samples, respectively.
Statistical analyses
Means of the fluorescence signals and cell counts were calculated with FlowJo® 7.6.5 software (FlowJo LLC, Ashland, Oregon, USA) and graphs were created with GraphPad Prism® 5.02 software (GraphPad software Inc., San Diego, California, USA).
In a first step prognostic relevance was investigated with univariate Cox proportional hazard models for each biomarker. As for some biomarkers the distributions of the observed values were positively skewed, their values were log-transformed. Additionally, each marker was dichotomized at the median and Kaplan-Meier curves were created for the two groups with low and high marker values. The curves were compared using the log-rank test. In a second step, multivariate Cox proportional hazard models with covariates international prognostic factor score and number of transplantations were fitted for each biomarker significant in univariate analysis (). All these analyses were performed using the statistical analysis software R (www.r-project.org, version 3.2.3). Differences with a probability of p ≤ 0.05 were considered statistically significant and indicated with a*.
Table 1. Difference in relapse-free survival was calculated with the Cox regression model.
Results and discussion
To the current state of knowledge immunomodulatory treatment approaches are not applied in GCC but at least little success has been reported with checkpoint inhibitor treatment in platinum refractory disease state.Citation18 In order to address the issue of immunosurveillance, patients were retrospectively grouped according to their remission status after HDCT. Among all patients, 27/46 (58.7%) remained relapse-free after HDCT. The median follow-up period in this patient cohort was 90 months (range 15 to 168). The remaining 19/46 patients (41.3%) suffered from relapses after HDCT (median 6 months, range 1 to 22).
We applied the IPFSG score to our patient cohort as follows: 2/46 patients underwent HDCT primarily and could not be grouped according to the IPFSG score. Score calculation was also not possible for 4/46 patients because of missing data. 26 of the remaining 40 patients received HDCT at first relapse or upon progression. 5/40 patients (12.5%) with a score of 0 were classified as “good” and 10/40 (25%) with a score of 1 were classified as “intermediate”. 11/40 patients with a score of 2 (high risk) and 3 (very high risk) points were combined in one group and their prognosis was classified as “poor”. In addition, all patients who received HDCT after second or subsequent relapse (14/40) were also classified as “poor” apart from the score calculation, resulting in 25/40 patients in this group (62.5%).Citation19 Patient data are depicted in detail in . Analysis of distributions of T-lymphocytes and monocytes were performed by flow cytometry and revealed no differences in the compartments between the two patient groups with or without relapse after HDCT ( and and Fig. S1a and b). We also determined the expression levels of surface molecules related to cell adhesion on both T-cells and monocytes, but did not find any difference in the expression levels of PECAM-1, VLA-4 and CXCR4 between the patient groups with and without relapse after HDCT, as depicted in Fig. S2. Measuring the basal expression level of B4GALT1 in different peripheral blood lymphocyte populations from healthy donors, we show that B-lymphocytes exhibit higher basal B4GALT1 transcript levels compared to T-lymphocytes or NKT-cells (Fig. S3a). Furthermore, granulocytes responded to G-CSF treatment with an induced expression of B4GALT1 in vitro but lymphocyte subpopulations did not exhibit B4GALT1 alteration after treatment with G-CSF in vivo or in vitro (Fig. S4). After separation of T-lymphocytes and monocytes from MNC by flow cytometry based cell sorting ( and ) we performed RT-PCR in order to determine B4GALT1 mRNA expression levels in those cell-subsets in our cohorts of GCC patients. Within the T-cell population the expression level of B4GALT1 was found to be significantly higher (p = 0.008) in the group of patients without relapse after HDCT compared to the group of patients who experienced a further relapse after HDCT (c) upon Mann Whitney testing. Comparison of non-relapse versus relapse patients by univariate Cox regression analyses showed a hazard ratio (HR) for the risk of relapse of 0.62 (95% confidence interval (CI) of HR: 0.42-0.91, p = 0.02) (). However, we could not find a significant difference in B4GALT1 expression levels within the monocyte population (d and ) or total MNC (e and ). Thus, only B4GALT1 mRNA expression in T-lymphocytes was significantly associated with durable disease remission.
Table 2. Patients' characteristics.
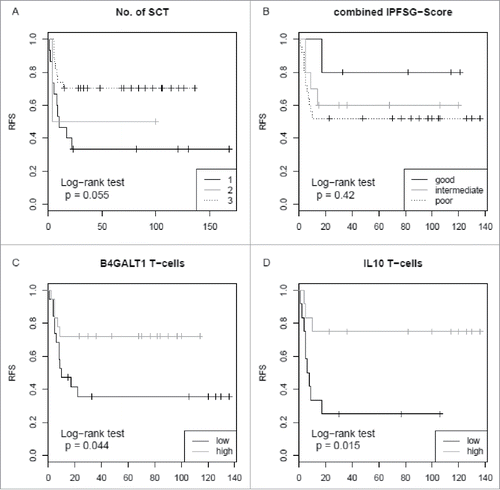
Figure 2. Relapse-free survival rates in 46 relapsed germ cell cancer patients with respect to the number of HDCT and ASCR, the international prognostic factor score, B4GALT1 and IL10 expression levels. (A) RFS is not significantly different in patients with one (black line), two (grey line) or three (dotted line) SCT cycles (log-rank test, p = 0.055). (B) Analysis of patients’ RFS according to the IPFSG categories. Patients with a good prognosis score (i.e. 0; black line) were compared to those with an intermediate (i.e. 1; grey line) and poor prognosis score (i.e. 2–3 and 2nd relapse; dotted line). RFS is not significantly different between these groups (log-rank test, p = 0.420). (C) RFS is significantly higher in patients with high T-cell B4GALT1 mRNA expression levels (grey line) compared to those with low levels (black line) (log-rank test, p = 0.044). High and low refer to values above and below the median value, respectively. (D) RFS is significantly higher in patients with high IL10 mRNA expression levels (grey line) compared to those with low levels (black line) (log-rank test, p = 0.015). IPFSG: International Prognostic Factor Study Group; RFS: relapse-free survival; SCT: stem cell transplantation.
In order to substantiate our hypothesis that activated peripheral T-cells may be involved in disease control of GCC, we performed lectin stimulation of MNC with Concanavalin A (Con A) in vitro and found up-regulation of B4GALT1 mRNA particularly in the CD4+ T-lymphocyte fraction (Fig. S3b). Furthermore, we determined the mRNA expression levels of IL10 as a marker for T-cell activation via qPCR analysis in our samples. Upon univariate Cox regression analysis we found IL10 to be significantly higher expressed in T-lymphocytes from leukapheresis products of patients without relapse after HDCT in comparison to the relapsed group (HR: 0.62; 95% CI of HR: 0.39-1.0; p = 0.05) (). No difference in IL10 expression was detectable in monocytes or total MNC between patients with or without relapse after HDCT (). A subgroup of patients was also evaluated for expression of interferon gamma (IFNG) in CD4+ and CD8+ cells. Although up-regulation of IFNG in lymphocytes of healthy donors was observed after in vitro Con A stimulation we found no difference in IFNG expression between the relapsed patients and those with persistent remission (Fig. S5).
For further corroboration we performed a multivariate Cox regression analysis with regard to the number of cycles of high-dose chemotherapy in single or sequential HDCT and to the prognostic score of good versus poor prognosis.Citation20 Among patients with a high B4GALT1 expression in T-lymphocytes the HR for relapse-free survival (RFS) was 0.66 (95% CI of HR: 0.45-0.97; p = 0.03). IL10 expression in T-lymphocytes also proved to be an independent prognostic parameter as calculated by the multivariate Cox model with an HR of 0.3 (95% CI of HR: 0.14-0.65; p = 0.002). The corresponding graphs for log-rank testing are depicted in . Applying the prognostic score to our cohort resulted in an improved RFS between the good, intermediate or poor prognosis groups, but this did not reach statistical significance, possibly due to low patient numbers.
Next, we sorted lymphocyte subpopulations from a second independent cohort of 19 GCC patients, dividing CD4+ and CD8+ cells. As in healthy individuals we observed a twofold higher B4GALT1 gene expression in B-lymphocytes compared to T-lymphocytes (data not shown) and found a difference of B4GALT1 expression levels between relapse and non-relapse GCC patients in CD8+ cells but not in CD4+ cells (Fig. S6a and b). A trend toward better survival of the group with high B4GALT1 expression in CD8+ cells was observed also in this second patient cohort, but log-rank testing did not give statistical significance for RFS (p = 0.13) or overall survival (p = 0.08), possibly due to the relatively small number of samples (Fig. S6c and d).
Thus, our data clearly support the notion that B4GALT1 expression levels in T-lymphocytes denote a novel biomarker for the prognosis of GCC patients undergoing salvage HDCT and that activated T-lymphocytes may be involved in tumor control in such patients.
Immunohistochemistry (IHC) staining revealed moderate infiltration of B4GALT1 expressing T-cells in the tumor tissue. Teratocarcinoma cells expressed more B4GALT1 protein than cells of pure seminomas (Fig. S7 and Table S1). Given the up-regulation of epidermal growth factor receptor (EGFR) signaling pathways in GCCCitation21 and B4GALT1 mediated inhibition of EGFR signaling in hepatocellular carcinoma,Citation22 it could be assumed hypothetically that B4GALT1 expression renders lymphocytes potent to exert immunosurveillance, perhaps via interference with EGFR dimerization or phosphorylation.
We conclude that B4GALT1 expression levels in T-lymphocytes from leukapheresis products of patients with GCC scheduled for salvage HDCT and ASCR seem to be relevant for tumor control and may represent a novel candidate biomarker in such patients. As our study was performed retrospectively and in relapse patients scheduled for salvage HDCT, who had already undergone chemotherapy, expression of B4GALT1 should be evaluated prospectively and may include also patients at initial diagnosis, especially in order to see if the preceding chemotherapy and stimulation with G-CSF have altered the B4GALT1 expression levels. Moreover, strategies of T-cell modulation particularly in patients with low B4GALT1 expression levels in T-lymphocytes may be worthwhile to be studied in further clinical trials.
Disclosure of potential conflicts of interest
The authors declare no competing interests.
supp_data_1423169.ppt
Download MS Power Point (1.6 MB)Acknowledgments
The authors thank Ramona Vietzke for assistance in patient data management and Cordula Loechelt for kind help with sample preparations. Kathleen Stabla gave advice for performing the RT-PCR experiments. We thank Viktoria Wischmann for performance of the IHC staining. In addition we gratefully thank Gavin Giel from the Flow Cytometry Core Facility Marburg for his assistance with the flow cytometry services.
Additional information
Funding
References
- Lorch A, Neubauer A, Hackenthal M, Dieing A, Hartmann JT, Rick O, Bokemeyer C, Beyer J. High-dose chemotherapy (HDCT) as second-salvage treatment in patients with multiple relapsed or refractory germ-cell tumors. Ann Oncol. 2010;21(4):820–5. doi:10.1093/annonc/mdp366.
- Hanna NH, Einhorn LH. Testicular cancer–discoveries and updates. N Engl J Med. 2014;371(21):2005–16. doi:10.1056/NEJMra1407550.
- International Prognostic Factors Study G, Lorch A, Beyer J, Bascoul-Mollevi C, Kramar A, Einhorn LH, Necchi A, Massard C, De Giorgi U, Flechon A, et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. 2010;28(33):4906–11. doi:10.1200/JCO.2009.26.8128.
- Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, Eckel-Passow JE, Dueck AC, Tenner KS, Jen J, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33(7):701–8. doi:10.1200/JCO.2014.57.6298.
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–92. doi:10.1172/JCI67428.
- Lu Y, Wang L, Liu P, Yang P, You M. Gene-expression signature predicts postoperative recurrence in stage I non-small cell lung cancer patients. PLoS One. 2012;7(1):e30880. doi:10.1371/journal.pone.0030880.
- Cheng X, Wang X, Han Y, Wu Y. The expression and function of beta-1,4-galactosyltransferase-I in dendritic cells. Cell Immunol. 2010;266(1):32–9. doi:10.1016/j.cellimm.2010.08.008.
- Han Y, Zhou X, Ji Y, Shen A, Sun X, Hu Y, Wu Q, Wang X. Expression of beta-1,4-galactosyltransferase-I affects cellular adhesion in human peripheral blood CD4+ T cells. Cell Immunol. 2010;262(1):11–7. doi:10.1016/j.cellimm.2009.08.004.
- Furukawa K, Sato T. Beta-1,4-galactosylation of N-glycans is a complex process. Biochim Biophys Acta. 1999;1473(1):54–66. doi:10.1016/S0304-4165(99)00169-5.
- Hill RL, Brew K, Vanaman TC, Trayer IP, Mattock P. The structure, function, and evolution of alpha-lactalbumin. Brookhaven Symp Biol. 1968;21(1):139–54.
- Russo RN, Shaper NL, Shaper JH. Bovine beta 1—-4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long forms of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990;265(6):3324–31.
- Zhao J, Gao Y, Cheng C, Yan M, Wang J. Upregulation of beta-1,4-galactosyltransferase I in rat spinal cord with experimental autoimmune encephalomyelitis. J Mol Neurosci. 2013;49(3):437–45. doi:10.1007/s12031-012-9824-3.
- Mittermayr S, Le GN, Clarke C, Millan Martin S, Larkin AM, O'Gorman P, Bones J. Polyclonal Immunoglobulin G N-Glycosylation in the Pathogenesis of Plasma Cell Disorders. J Proteome Res. 2017;16(2):748–62. doi:10.1021/acs.jproteome.6b00768.
- Poeta ML, Massi E, Parrella P, Pellegrini P, De Robertis M, Copetti M, Rabitti C, Perrone G, Muda AO, Molinari F, et al. Aberrant promoter methylation of beta-1,4 galactosyltransferase 1 as potential cancer-specific biomarker of colorectal tumors. Genes Chromosomes Cancer. 2012;51(12):1133–43. doi:10.1002/gcc.21998.
- Xie H, Zhu Y, An H, Wang H, Zhu Y, Fu H, Wang Z, Fu Q, Xu J, Ye D. Increased B4GALT1 expression associates with adverse outcome in patients with non-metastatic clear cell renal cell carcinoma. Oncotarget. 2016;7(22):32723–30. doi:10.18632/oncotarget.8737.
- Lorch A, Kollmannsberger C, Hartmann JT, Metzner B, Schmidt-Wolf IGH, Berdel WE, Weissinger F, Schleicher J, Egerer G, Haas A et al. Single versus sequential high-dose chemotherapyin patients with relapsed or refractory germ cell tumors: A prospective randomized multicenter trial of the German Testicular Cancer Study Group. J Clin Oncol. 2007;25(19):2778–2784. doi:10.1200/JCO.2006.09.2148.
- Hartmann JT, Gauler T, Metzner B, Gerl A, Casper J, Rick O, Horger M, Schleicher J, Derigs G, Mayer-Steinacker R. Phase I/II study of sequential dose-intensified ifosfamide, cisplatin, and etoposideplus paclitaxel as induction chemotherapy for poor prognosis germ cell tumors by the German Testicular Cancer Study Group. J Clin Oncol. 2007;25(36):5742–5747. doi:10.1200/JCO.2007.11.9099.
- Zschabitz S, Lasitschka F, Hadaschik B, Hofheinz RD, Jentsch-Ullrich K, Gruner M, Jager D, Grullich C. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer. 2017;76:1–7. doi:10.1016/j.ejca.2017.01.033.
- Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med. 2007;357(4):340–8. doi:10.1056/NEJMoa067749.
- Lorch A, Kleinhans A, Kramar A, Kollmannsberger CK, Hartmann JT, Bokemeyer C, Rick O, Beyer J. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: long-term results of a prospective randomized trial. J Clin Oncol. 2012;30(8):800–5. doi:10.1200/JCO.2011.38.6391.
- Miyai K, Yamamoto S, Asano T, Tamai S, Matsubara O, Tsuda H. Protein overexpression and gene amplification of epidermal growth factor receptor in adult testicular germ cell tumors: potential role in tumor progression. Cancer Sci. 2010;101(9):1970–6. doi:10.1111/j.1349-7006.2010.01638.x.
- Tang W, Weng S, Zhang S, Wu W, Dong L, Shen X, Zhang S, Gu J, Xue R. Direct interaction between surface beta1,4-galactosyltransferase 1 and epidermal growth factor receptor (EGFR) inhibits EGFR activation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2013;434(3):449–54. doi:10.1016/j.bbrc.2013.03.094.
