ABSTRACT
Versican promotes experimental tumor growth through cell- and non cell-autonomous mechanisms. Its role in mesothelioma progression has not been investigated so far. In this study we investigated the impact of tumor-derived versican in mesothelioma progression and the underlying mechanism of its action. For this purpose, versican-silenced or control ΑΕ17 and ΑΒ1 murine mesothelioma cells were intrapleuraly injected into syngeneic mice, in order to create pleural mesotheliomas and pleural effusions. Intratumoral and pleural immune subsets were assessed using flow cytometry. Mesothelioma cells were co-cultured with syngeneic macrophages to examine versican’s impact on their interaction and endothelial cells to assess the effect of versican in endothelial permeability. Versican expression was assessed in human mesotheliomas and mesothelioma-related pleural effusions and benign pleural tissue and effusions. We observed that, versican silencing reduced mesothelioma mass and pleural fluid volume by affecting tumor cell proliferation and apoptosis in vivo, while tumor cell growth remained intact in vitro, and limited pleural vascular permeability. Mice harboring versican-deficient tumors presented fewer tumor/pleural macrophages and neutrophils, and fewer pleural T-regulatory cells, compared to the control animals. Macrophages co-cultured with versican-deficient mesothelioma cells were polarized towards M1 anti-tumor phenotype and demonstrated increased tumor cell phagocytic capacity, compared to macrophages co-cultured with control tumor cells. In co-culture, endothelial monolayer permeability was less effectively stimulated by versican-deficient cells than control cells. Versican was over-expressed in human mesothelioma tissue and mesothelioma-associated effusion. In conclusion, tumor cell-derived versican stimulates mesothelioma progression by shaping a tumor friendly inflammatory milieu, mainly by blunting macrophage anti-tumor activities.
Introduction
Malignant Pleural Mesothelioma (MPM) is an aggressive tumor of the pleural cavity mainly resulting from asbestos exposure.Citation1 Since production and use of asbestos are still permitted in many countries (among them some of the most populous), a global epidemic of the disease is likely in the next few decades.Citation2 The problem is getting even more complicated, if one considers, that the currently used therapies provide only marginal clinical benefit.Citation3 Therefore, unraveling the mechanisms of MPM progression is believed to reinforce the development of novel, more effective and safe treatment modalities.
Versican (PG-M, encoded by CSPG2 gene), a large chondroitin sulfate proteoglycan mainly resting on extracellular matrix (ECM),Citation4 plays a fundamental role in the development of cardiovascular Citation5 and central nervous Citation6 system. It is overexpressed by solid tumors Citation7 and it has been shown to promote tumor growth by enhancing cancer cell proliferation and angiogenesis in experimental astrocytoma,Citation8 or by stimulating macrophages in experimental glioma Citation9 and metastatic lung adenocarcinoma.Citation10 ADAMTs 1/4/5/9/15/20 proteases cleave versican Citation11 and detach it from the ECM, and thus produce the DPEAEE fragment,Citation12 also known as versikine, which leads to CD8 + T-lymphocyte activation Citation13 and angiogenesis.Citation14 However, the role of versican in MPM progression has not been investigated so far. We here hypothesized that versican would promote mesothelioma progression mainly by preventing tumor cell apoptosis and by shaping a tumor-friendly microenvironment.
Results
Versican promotes mesothelioma growth and the formation of malignant pleural effusion (MPE) in vivo
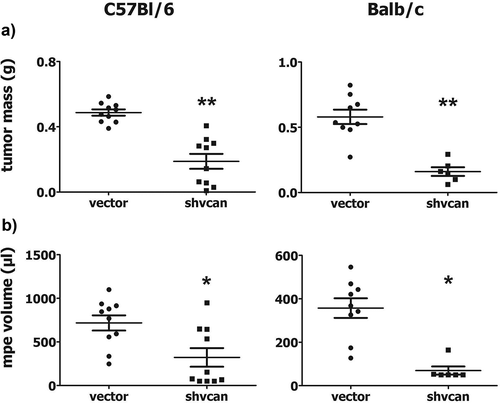
AE17 and AB1 versican-deficient (shvcan) clones (expressing less than 10% of versikine and versican core protein compared to vector cells) (Fig. S1A,B) did not differ from vector-transfected AE17 and AB1 cells (vector) as for their viability (Fig. S1C) and proliferation rate (Fig S1D), which were determined by MTS assay and flow cytometry respectively. AE17 and AB1 vector or shvcan cells were injected into the pleural cavity of syngeneic C57Bl/6 and Balb/c mice respectively, in order to create pleural mesotheliomas. Mice bearing versican-deficient tumors were characterized by decreased tumor burden ()) and MPE volume ()) compared to control animals. Shvcan tumors expressed significantly less versikine (Fig. S2A) and versican core protein (Fig. S2B) compared to control ones, reflecting the in vitro pattern of versican expression by mesothelioma clones. The latter finding verifies that silencing of tumor cell-derived versican was maintained in vivo and suggests that most of versican protein contained in mesothelioma tissue is of tumor cell origin.
Figure 1. Tumor-derived versican enhances experimental mesothelioma progression.
C57Bl/6 and Balb/c mice were euthanized 14 days upon intrapleural injection of control (vector) or versican-deficient (shvcan) AE17 and AB1 mesothelioma cells, respectively. Tumor mass (a) and Malignant Pleural Effusion (MPE) (b) were collected and quantified, *p < 0.05, **p < 0.001 compared to vector. Data are presented as mean ± standard error of mean (sem).
Versican enhances tumor cell proliferation, limits tumor cell apoptosis and provokes vascular hyperpermeability
In an effort to unveil the underlying mechanisms of mesothelioma-promoting effects of versican, we focused on the effect of versican silencing in tumor cell proliferation and apoptosis, as well as tumor angiogenesis. Versican-deficient mesotheliomas exhibited decreased tumor cell proliferation (), Fig. S3A) and increased tumor cell apoptosis (, Fig. S3B), as it was revealed by immunohistochemistry. Using anti-CD31 immunofluorescence staining we demonstrated that tumor angiogenesis [assesed by microvascular density (Fig. S4) and vessel/tumor area (data not shown)] was not affected.
Figure 2. Tumor-derived versican promotes cancer cells’ proliferation and impedes tumor cells’ apoptosis in vivo.
Tumor-cell proliferation [proliferating cell nuclear antigen antibody (anti-PCNA) immunostaining] and apoptosis [deoxynucleotidyl nick end-labeling (TUNEL) assay] were evaluated in pleural tumor sections. Number of PCNA(+), proliferating tumor cells per high power field (hpf) (a) and number of TUNEL (+) apoptotic tumor cells per hpf (b) of C57Bl/6 and Balb/c mice with mesothelioma respectively, *p < 0.05, **p < 0.01 compared to vector. Data are presented as mean ± standard error of mean (sem).
![Figure 2. Tumor-derived versican promotes cancer cells’ proliferation and impedes tumor cells’ apoptosis in vivo.Tumor-cell proliferation [proliferating cell nuclear antigen antibody (anti-PCNA) immunostaining] and apoptosis [deoxynucleotidyl nick end-labeling (TUNEL) assay] were evaluated in pleural tumor sections. Number of PCNA(+), proliferating tumor cells per high power field (hpf) (a) and number of TUNEL (+) apoptotic tumor cells per hpf (b) of C57Bl/6 and Balb/c mice with mesothelioma respectively, *p < 0.05, **p < 0.01 compared to vector. Data are presented as mean ± standard error of mean (sem).](/cms/asset/831b01c8-9f60-4637-8358-ad9785c76c44/koni_a_1537427_f0002_b.gif)
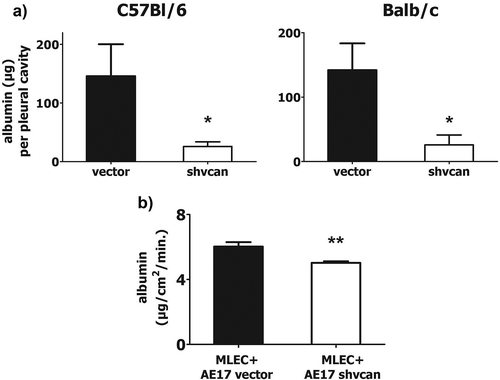
In order to assess whether versican silencing had any impact on pleural vascular permeability, a major determinant of MPE formation,Citation15 albumin-binding Evans Blue dye was injected intravenously before sacrifice and its pleural fluid and serum levels were measured. We observed significantly lower pleural vascular permeability () in mice harboring versican-deficient mesotheliomas. Serum levels of Evans Blue did not differ between groups (data not shown). To further validate this observation, we conducted in vitro co-culture experiments using AE17 cells and syngeneic murine lung endothelial cells in order to explore whether mesothelioma-derived versican enhances the permeability of the endothelial monolayer. We observed that the rate of albumin passing through the endothelial monolayer gaps was significantly lower, when endothelial cells were co-cultured with versican-deficient AE17 cells, compared to the control ones ().
Figure 3. Tumor-derived versican provokes vascular hyper-permeability.
Vascular permeability was determined in vivo by measuring the amount (μg) of Evans Blue binding albumin that was concentrated in the pleural cavity of mesothelioma-bearing C57Bl/6 and Balb/c mice, upon iv injection of the dye (a). Endothelial cells were co-cultured with AE17 mesothelioma cells and permeability of the endothelial monolayer was evaluated by determining the amount of FITC-albumin penetrating endothelial monolayer (in a given area) per time unit (b), *p < 0.05 compared to vector, **p < 0.01, MLEC: murine lung endothelial cells. Data arepresented as mean ± standard error of mean (sem).
Versican critically affects mesothelioma-related immune response
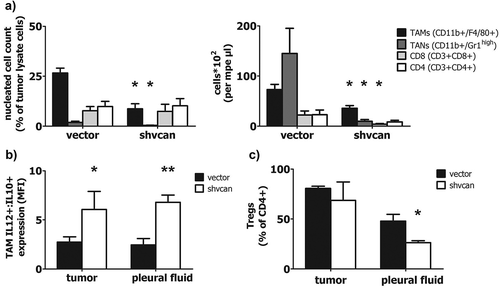
Mesothelioma tissue and pleural fluid, obtained from C57Bl/6 mice, were analyzed using flow cytometry, in order to explore the impact of versican on tumor-elicited immune response. Versican-deficient mesotheliomas contained fewer Tumor Associated Macrophages-TAMs (CD11b+ F4/80+) and Tumor Associated Neutrophils-TANs (CD11b+ GR1high), while the corresponding MPE contained fewer TAMs, TANs and CD8 + T-lymphocytes, compared to control tumor and MPE, respectively (). Furthermore, versican silencing shifted TAMs towards an anti-tumor M1 phenotype, as it is indicated by the significantly higher IL12:IL10 expression ratio, in versican-deficient tumors and corresponding MPEs, compared to control ones (). In addition, MPE CD4+ cells of mice bearing versican-expressing MPM tended to acquire a T regulatory cell (Treg) phenotype (CD4+ Foxp3+),Citation16 while versican depletion in mesothelioma cells partially inhibited this effect (). Finally, versican silencing in cancer cells had no impact on CD8+ activation, determined by CD137 expression (data not shown). Overall, versican confers anti-tumor properties in tumor immune/inflammatory milieu.
Figure 4. Tumor-derived versican sculpts a tumor-friendly immune environment.
Tumor tissue lysate and MPE obtained from C57Bl/6 mice were analyzed using flow cytometry. TAMs, TANs, CD8(+) cells, CD4(+) cells in mesothelioma tissue and MPE (a), ratio of IL12/IL10 expression by TAMs (marker of macrophage polarization) in tumor tissue and MPE (b) and percentage of Tregs among total CD4(+) cells (c), *p < 0.05, **p < 0.01 compared to vector. TAMs: Tumor Associated Macrophages, TANs: Tumor Associated Neutrophils, CD8: CD8(+) T-lymphocytes, CD4: CD4(+) T-lymphocytes, Tregs: T regulatory lymphocytes, MFI: Mean Fluorescence Intensity. Data are presented as mean ± standard error of mean (sem).
Versican promotes macrophage migration and M2 polarization and protects mesothelioma cells from macrophage-induced phagocytosis and apoptosis
Based on the observed reduction of TAMs in versican-deficient MPMs we speculated that versican elicits macrophage migration towards tumor. We therefore loaded AE17 shvcan or vector cells and Bone Marrow Derived Macrophages (BMDMs) into the two different compartments of a transwell system and we observed that significantly fewer BMDMs migrated towards AE17 shvcan cells than towards control cells (). We next asked whether tumor-derived versican modulate macrophage polarization towards M1 or M2 phenotype. Flow cytometry analysis of BMDMs, co-cultured with AE17 cells, revealed that control cells shifted macrophages towards an M2 phenotype, while versican silencing partially blocked this effect ().
Figure 5. Tumor versican critically impairs the effects of macrophages on mesothelioma cells in vitro.
Macrophages, co-cultured with versican-deficient AE17 mesothelioma cells, exhibited reduced migration towards cancer cells (a), increased M1 polarization (b) and a higher phagocytosis of tumor cells (c) compared to macrophages co-cultured with control AE17 cells. Versican-deficient cells exhibited higher apoptosis rate compared to control ones in co-culture with macrophages (d). Number of migrated BMDMs per hpf towards AE17-vector or AE17-shvcan cells (% of macrophages migrated towards vector cells – 400X magnification) (a), IL-12/IL-10 expression ratio in macrophages cultivated alone or with AE17-vector or AE17-shvcan cells (b), number of AE17 GFP (+) fragments-containing tomato macrophages co-cultured with AE17-vector or AE17-shvcan cells per hpf (400X magnification) (c) and percentage of apoptotic AE17-vector or AE17-shvcan cells cultured alone or with macrophages (d), *p < 0.05 compared to vector, # p < 0.05 compared to shvcan, $ p < 0.05 compared to macrophages, ** p < 0.01. BMDMs: Bone Marrow Derived Macrophages, MFI: Mean Fluorescence Intensity. Data are presented as mean ± standard error of mean (sem).
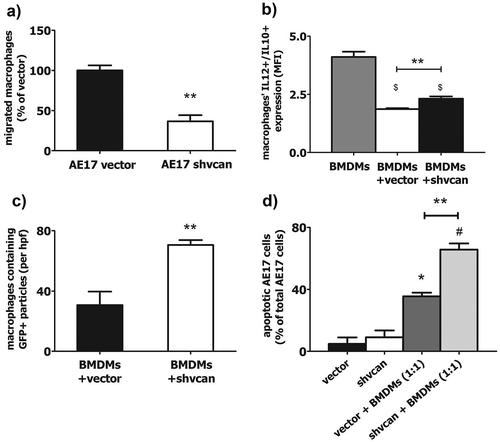
Trying to decipher versican’s tumor-limiting effects, which are restricted to the in vivo setting, and having shown that silencing of tumor-derived versican partially restores anti-tumor phenotype of TAMs, we assumed that versican-deficient mesothelioma cells might also be more sensitive to the cytotoxic activities of macrophages. For this purpose, tomato macrophages were co-cultured with AE17 vector or shvcan (GFP expressing) cells and they were viewed under an immunofluorescence microscope. Macrophages co-cultured with versican-deficient AE17 cells contained more GFP(+) fragments of mesothelioma cells than macrophages co-cultured with control AE17 cells, indicating a higher macrophage-phagocytosis capacity against versican-deficient tumor cells (, Fig. S5). In a similar set of experiments, versican-deficient tumor cells displayed increased apoptosis compared to control ones, when co-cultured with syngeneic macrophages (), albeit basal apoptotic rate did not differ between AE17 vector and shvcan cells, as it was revealed by flow cytometry. On the other hand, the apoptotic and proliferating pattern of macrophages was similar, regardless if they were cultivated in the presence of versican- expressing or versican-deficient mesothelioma cells (data not shown).
Versican is overexpressed in human mesothelioma tissue and mesothelioma-associated MPE and higher mRNA levels of the protein are linked to non-epithelioid mesothelioma
Trying to define the clinical relevance of versican in mesothelioma, we investigated its expression in mesotheliomas and control pleural tissues from patients, who underwent bullectomy (pneumothorax group). Higher expression of versican was evident in cytoplasm and ECM of MPMs, compared to benign pleura (, Fig. S6), as it was determined by immunohistochemistry. Similarly, versican levels were substantially higher in pleural fluid of patients with MPM-associated MPE, compared to the corresponding serum and the effusion and serum of patients with heart failure (). Finally, multivariate statistical analysis of “The Cancer Genome Atlas” (TCGA) data, regarding 87 patients with mesothelioma, revealed that versican expression is not an independent predictor of survival (Table S1A). Among the four factors tested (age, gender, drug therapy and histological type), in line with previous observations,Citation17 epithelioid type was found to be marginally associated with longer survival in univariate analysis (Table S1C), though this trend was not confirmed in the multivariate analysis. In addition, a strong association between low versican mRNA expression and epithelioid type was observed (Table S1B,D, Fig. S7).
Figure 6. High versican levels are observed in human mesothelioma tissue and pleural fluid.
Contingent analysis of versican expression in tissue sections from patients with pneumothorax (n = 9) or MPM (n = 16), p < 0.001 and p < 0.01 compared to pneumothorax [cytoplasmic and extracellular matrix (ECM) staining, respectively] (a) and versican levels in pleural fluid and corresponding serum obtained from patients with heart failure (hf, n = 10) or MPM (n = 10) (b), *p < 0.05 compared to hf, # p < 0.05 compared to serum. IHC: immunohistochemistry. (a): Data are presented as a frequency distribution graph. (b): Data are presented as mean ± standard error of mean (sem).
![Figure 6. High versican levels are observed in human mesothelioma tissue and pleural fluid.Contingent analysis of versican expression in tissue sections from patients with pneumothorax (n = 9) or MPM (n = 16), p < 0.001 and p < 0.01 compared to pneumothorax [cytoplasmic and extracellular matrix (ECM) staining, respectively] (a) and versican levels in pleural fluid and corresponding serum obtained from patients with heart failure (hf, n = 10) or MPM (n = 10) (b), *p < 0.05 compared to hf, # p < 0.05 compared to serum. IHC: immunohistochemistry. (a): Data are presented as a frequency distribution graph. (b): Data are presented as mean ± standard error of mean (sem).](/cms/asset/f22d51ed-9031-4945-ab22-08aa2805b9ae/koni_a_1537427_f0006_b.gif)
Discussion
We herein examined the role of versican in mesothelioma progression. Using syngeneic mouse models and RNA interference techniques we found that tumor cell versican silencing: a) had no impact on tumor cell growth in vitro, but critically affected the proliferation and survival of tumor cells in vivo to curtail mesothelioma progression, b) hindered the accumulation of pro-tumor innate immune cells in the tumor milieu and enhanced the tumoricidal properties of macrophages in co-culture with mesothelioma cells and c) attenuated the vascular permeability of mesothelioma-affected pleura, the permeability of an endothelial monolayer in co-culture with mesothelioma cells and the size of mesothelioma-related MPE. In addition, we demonstrated that versican is overexpressed in human MPM tissue and mesothelioma-associated pleural effusion.
We here demonstrated for the first time that versican is an important promoter of mesothelioma progression. This finding comes alongside with previous observations that versican promotes tumor growth in breast cancer,Citation18 glioma,Citation9 lung adenocarcinoma Citation10 and melanoma Citation19 experimental models, either affecting tumor cell properties or modulating tumor micro-environment. Versican silencing had no impact on mesothelioma cell growth in vitro and its mesothelioma promoting activity was restricted to the in vivo setting, suggesting that versican is involved in mesothelioma-host interaction, rather than it regulates tumor cell growth.
Since a cell-autonomous pro-mesothelioma activity of tumor versican seemed highly unlikely we focused on its involvement in tumor-host interplay, including angiogenesis and tumor-associated immune response, both prominent hallmarks of cancer.Citation20 Versican silencing in MPM cells did not affect tumor angiogenesis. In connection to this, it has been recently shown Citation14 that host- and not tumor-derived versican, promotes tumor angiogenesis. As for the impact of versican in tumor immune milieu, we showed that versican-deficient tumors and pleural effusions contained fewer macrophages, neutrophils and T-lymphocytes. Given the central role of macrophages in tumor progression,Citation21 we next focused on versican-mediated reduction of TAM abundance in tumor microenvironment and we asked whether this was a result of decreased macrophage viability or impaired recruitment. In vitro studies revealed that, while macrophage viability was not dependent on tumor cell versican expression, tumor-derived versican promoted macrophage migration, in line with previous observations in pre-malignant and inflammatory lesions, which highlighted the chemo-attractive properties of versican.Citation19,Citation22,Citation23 Apart from this, mesothelioma-versican silencing shifted macrophages towards M1 anti-tumor phenotype and fostered the cytotoxic functions of macrophages, including tumor cell apoptosis and phagocytosis of versican-deficient mesothelioma cells by macrophages in in vitro co-culture systems. These findings expand previous observations on the effects of tumor versican on TAM phenotype Citation10 by unveiling its inhibitory activity against TAM-mediated tumor cell cytotoxicity. Overall we believe that the impaired tumor growth, observed in versican-deficient mesotheliomas, is more likely a result of increased phagocytosis and apoptotic stimuli by M1 macrophages against tumor cells. However, it should be noted, that the cleaved version of versican, versikine, exerts anti-tumor effects, mainly by activating CD8+ cytotoxic T-lymphocytes.Citation13 Since versikine is also decreased upon transfection of MPM cells with short-hairpin RNA-containing plasmids, as a result of the reduction of versican core protein, whether versikine exerts anti- or pro-tumor effects on our system cannot be clarified.
MPEs appear at the vast majority of MPM patients and present a major source of morbidity.Citation24 Tumor-versican was also found to promote pleural vascular leakage and pleural fluid accumulation in mesothelioma bearing mice. Moreover, versican-expressing mesothelioma cells were more effective in stimulating the permeability of an endothelial monolayer than versican-deficient ones. To our knowledge, this is the first observation demonstrating that tumor versican possesses direct pro-permeability properties. However, the in vivo effect of versican silencing was more pronounced than the in vitro one, suggesting that, at least in the AE17-C57Bl/6 model, other mediators, more likely secreted by inflammatory cells Citation15,Citation25 recruited to the tumor site by tumor-versican, largely contribute to the in vivo endothelial hyper-permeability and the formation of MPE.
From a clinical viewpoint, the in vivo impact of versican silencing on mesothelioma progression, together with the finding that versican is commonly over-expressed by human mesotheliomas, suggests that this molecule might be therapeutically exploited for the treatment of MPM. Notably, a versican inhibitor would not only target tumor-derived versican and thus modulating tumor-elicited immune response and vascular hyper-permeability, but also host-derived versican, probably leading to impaired tumor angiogenesis,Citation14 achieving a more pronounced inhibition of tumor growth, than that observed here. The finding that tumor-versican silencing exerts its anti-mesothelioma effects by enhancing the antitumor activity of macrophages puts a potential anti-versican therapy among an emerging class of cancer immunotherapies that manipulate innate immunity. More interestingly, depicting the role of versican as important promoter of innate immune suppressive micro-environment, a typical feature of MPM,Citation26 suggests that this molecule may contribute in blunting the effects of novel immunotherapies aiming to stimulate the adaptive immunity against cancer and are currently tested for this malignancy.Citation27 Therefore, anti-versican regimens reversing the immunosuppressive properties of innate immunity could act synergistically with lymphocyte-activating agents (i.e. anti-PD-1/PDL-1) to elicit a more potent therapeutic effect in MPM. Whether this concept is valid requires further investigation in both pre-clinical and clinical level.
In conclusion, we here demonstrated that versican is overexpressed in human MPM and associated pleural effusions. More importantly, abolishment of tumor versican expression limited experimental mesothelioma progression, mainly by modulating tumor homing and function of TAMs. In addition, tumor versican enhances pleural vascular permeability and promotes the accumulation of MPE. These findings suggest that versican may be therapeutically exploited to devise novel treatments for MPM.
Materials and methods
Cells and animals
Murine malignant mesothelioma AE17 Citation28 and AB1 Citation29 cells were kindly provided by Dr YCG Lee (Perth, Australia). Murine Bone Marrow Derived Macrophages (BMDMs) were isolated from the bone marrow of C57Bl/6 or Tomato C57Bl/6 mice, as described previously.Citation30 Murine lung endothelial cells (MLECs) were isolated as described elsewhere.Citation31 All cell lines were routinely tested for mycoplasma spp. Versican-deficient mesothelioma stable clones were created using plasmid vectors (online data supplement) and maintained as described elsewhere.Citation32 C57Bl/6 and Balb/c mice were purchased from BSRC Al. Fleming or Hellenic Pasteur Institute, while Tomato C57Bl/6 mice were kindly offered to us by Dr. GT Stathopoulos (Patras, Greece) and were housed at the Animal Model Research Unit of “Evangelismos” Hospital (EL25BIO015, Athens, Greece), receiving food and water ad libitum. The total number of animals used (per mouse strain) was 20 and it was determined by power analysis (α = 0.8, 20% reduction of tumor weight, p = 0.05), according to data from literature. Another 10 C57Bl/6 and 5 Tomato C57Bl/6 were used for the isolation of MLECs and BMDMs.
In vivo studies
Experiments were approved by the Veterinary Administration Bureau, Prefecture of Athens, Greece under compliance to the national law and the EU Directives (protocol number: K/7890/2010). In order to create experimental MPM, we injected versican-deficient (shvcan) or versican-expressing (vector) AE17 or AB1 (5X105) cells into the pleural cavity of sex-, weight- and age- matched syngeneic C57Bl/6 and Balb/c mice, respectively.Citation33 Mice were euthanized 14 days upon tumor cell inoculation. Following sacrifice, pleural fluid was retrieved and quantified, tumor mass was collected and weighed and tumor vascular permeability was assessed using the in vivo Evans Blue assay.Citation34
Immunohistochemistry, immunofluorescence, immunoblotting and flow cytometry
Cancer cell proliferation, apoptosis and tumor angiogenesis was assessed in mesothelioma tissue as described previously Citation35 and online. Tumor tissue and cell culture lysates were analyzed by western immunoblotting in order to assess versican expression (online data supplement).Citation32 Nucleated cells isolated from mesotheliomas and pleural effusions from C57Bl/6 mice were analyzed by flow cytometry (online data supplement).
In vitro experiments
Mesothelioma cells’ viability, proliferation and apoptosis were assessed as described online. Endothelial cell monolayer permeability was evaluated using a transwell system [0.4 μm membrane pore size (mps)], (Corning) as described online. Macrophage migration studies were carried out using a different transwell system (8 μm mps) as previously described Citation32 (and online) and a similar co-culture system (0.4 μm mps) was used to investigate the in vitro effect of tumor-derived versican in macrophage phenotype and macrophage-induced tumor cell cytotoxicity (online data supplement). All in vitro experiments were performed twice and each value represents a triplicate.
Human samples
For experiments using human samples see online data supplement. Studies on human tissues were approved by the Ethics Committee of “Evangelismos” Hospital, Athens, Greece (protocol number: 322/12–4-2012). Studies using human pleural fluid were approved by the Ethics Committee of “Evangelismos” Hospital, Athens, Greece (protocol number: 379/12–7-2006).
Statistics
Quantitative variables are presented as mean ± standard error of mean (SEM) and were analyzed with Student’s t-test or one-way ANOVA, while qualitative ones are presented as a frequency distribution graph (2X2 table), and were analyzed using Chi-square test with Yates’ correction. N-Way tabulation was performed on data derived from “The Cancer Genome Atlas” (TCGA) public-access data base. Versican mRNA was considered as an independent variable, while overall survival was the dependent one. Age, gender, histological type (epithelioid mesothelioma vs non-epithelioid) and drug therapy (standard treatment with cisplatin/pemetrexed vs standard treatment plus other agents) were tested as confounding factors. P values < 0.05 were considered significant. Statistical analysis was done using the Statistical Package for the Social Sciences v.13.0.0 (IBM) and IHS EViews (Irvine). Multivariate analysis results were further validated by R software.
Availability of materials & data
Materials and data are available upon contact with the corresponding author.
Authors contribution
A.G. Pappas did most of the experimental work and wrote the first draft. S. Magkouta, I. Pateras, C. Moschos, M.E. Vazakidou, K. Psarra and V. Gourgoulis contributed in different parts of the experimental work and reviewed the manuscript. I. Skianis conducted the statistical analysis and reviewed the manuscript. I. Kalomenidis conceived the idea of the study, designed and supervised the experiments and critically contributed in writing and reviewing the manuscript.
Supplemental Material
Download (9.2 MB)Acknowledgments
We thank Dr E. Aravidou and Z. Kollia for professional veterinarian and animal care assistance, respectively.
Disclosure statement
Authors declare no conflict of interest.
Supplemental online material
Supplemental data for this article can be access on the publisher’s website.
Authors contribution
Supplemental data for this article can be access on the publisher’s website.
Additional information
Funding
References
- Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–283. doi:10.1111/j.1440-1843.2005.00714.x.
- Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in great britain from 2002 to 2050. Br J Cancer. 2005;92:587–593. doi:10.1038/sj.bjc.6602307.
- Baas P, Fennell D, Kerr KM, Van Schil PE, Haas RL, Peters S. ESMO guidelines committee. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis. Treatment Follow-Up Ann Oncol. 2015;26Suppl 5:v31–9. doi:10.1093/annonc/mdv199.
- Margolis RU, Margolis RK. Aggrecan–versican–neurocan family proteoglycans. Methods Enzymol. 1994;245:105–126.
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi:10.1006/dbio.1998.9001.
- Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fässler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–7742. doi:10.1523/JNEUROSCI.4158-08.2009.
- Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Isobe H, Nakashima N, Takeuchi J. Immunohistochemical localization of extracellular matrix components in human breast tumours with special reference to PG-M/versican. Histochem J. 1997;29:21–30.
- Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/PG-G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18:754–756. doi:10.1096/fj.03-0545fje.
- Hu F, A Dzaye OD, Hahn A, Yu Y, Scavetta RJ, Dittmar G, Kaczmarek AK, Dunning KR, Ricciardelli C, Rinnenthal JL et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro Oncol. 2015;17:200–210. doi:10.1093/neuonc/nou324.
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi:10.1038/nature07623.
- Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi:10.1016/j.matbio.2014.01.005.
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, et al. Versican V1 proteolysis in human aorta in vivo occurs at the glu441–ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi:10.1074/jbc.M009737200.
- Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, Leith C, Maroulakou I, Callander N, Miyamoto S et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128:680–685. doi:10.1182/blood-2016-03-705780.
- Asano K, Nelson CM, Nandadasa S, Aramaki-Hattori N, Lindner DJ, Alban T, Inagaki J, Ohtsuki T, Oohashi T, Apte SS. Stromal versican regulates tumor growth by promoting angiogenesis. Sci Rep. 2017;7:17225.
- Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186:487–492. doi:10.1164/rccm.201203-0465PP.
- Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16:220–233. doi:10.1038/nri.2016.26.
- Van Schil PE, Opitz I, Weder W, De Laet C, Domen A, Lauwers P, Hendriks JM, Van Meerbeeck JP. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J. 2014;44:754–764. doi:10.1183/09031936.00207213.
- Du WW, Fang L, Yang X, Sheng W, Yang BL, Seth A, Zhang Y, Yang BB, Yee AJ. The role of versican in modulating breast cancer cell self-renewal. Mol Cancer Res. 2013;11:443–455. doi:10.1158/1541-7786.MCR-12-0461.
- Kunisada M, Yogianti F, Sakumi K, Ono R, Nakabeppu Y, Nishigori C. Increased expression of versican in the inflammatory response to UVB- and reactive oxygen species-induced skin tumorigenesis. Am J Pathol. 2011;179:3056–3065. doi:10.1016/j.ajpath.2011.08.042.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013.
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi:10.1016/j.immuni.2014.06.010.
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi:10.1016/j.cell.2006.02.015.
- Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi:10.1016/j.matbio.2014.01.015.
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi:10.1016/S0140-6736(05)67025-0.
- Giannou AD, Marazioti A, Spella M, Kanellakis NI, Apostolopoulou H, Psallidas I, Prijovich ZM, Vreka M, Zazara DE, Lilis I et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125:2317–2334. doi:10.1172/JCI79840.
- Yap TA, Aerts JG, Popat S, Fennell DA. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;17:475–488. doi:10.1038/nrc.2017.42.
- Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, van Brummelen E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi:10.1016/S1470-2045(17)30169-9.
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063.
- Bielefeldt-Ohmann H, Marzo AL, Himbeck RP, Jarnicki AG, Robinson BW, Fitzpatrick DR. Interleukin-6 involvement in mesothelioma pathobiology: inhibition by interferon alpha immunotherapy. Cancer Immunol Immunother. 1995;40:241–250.
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83:14. doi:10.1002/0471142735.im1401s83. PMID: 19016445
- Van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085–1091.
- Magkouta S, Pappas A, Moschos C, Vazakidou ME, Psarra K, Kalomenidis I. Icmt inhibition exerts anti-angiogenic and anti-hyperpermeability activities impeding malignant pleural effusion. Oncotarget. 2016;7:20249–20259. doi:10.18632/oncotarget.7912.
- Vazakidou ME, Magkouta S, Moschos C, Psallidas I, Pappas A, Psarra K, Kalomenidis I. Temsirolimus targets multiple hallmarks of cancer to impede mesothelioma growth in vivo. Respirology. 2015;20:1263–1271. doi:10.1111/resp.12604.
- Stathopoulos GT, Psallidas I, Moustaki A, Moschos C, Kollintza A, Karabela S, Porfyridis I, Vassiliou S, Karatza M, Zhou Z et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst. 2008;100:1464–1476. doi:10.1093/jnci/djn325.
- Psallidas I, Stathopoulos GT, Maniatis NA, Magkouta S, Moschos C, Karabela SP, Kollintza A, Simoes DC, Kardara M, Vassiliou S et al. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene. 2013;32:528–535. doi:10.1038/onc.2012.57.
