ABSTRACT
The CD28H/B7-H5 pathway is a newly identified pathway of the B7 family. In human peripheral blood, the receptor CD28H is preferentially expressed on naïve T cells and repetitive stimulation of T cells leads to the loss of CD28H expression. Here we examined the expression of the CD28H/B7-H5 pathway in human peripheral tissues, as well as in human cancers. We found that CD28H is preferentially expressed on T cells with tissue-resident phenotypes (TRM). Supporting that, stimulation via IL-15 and TGF-β, presumably major cytokines essential for TRM cell homeostasis, sustains CD28H expression on T cells. The ligand B7-H5 is constitutively expressed on normal epithelium of human oral-gastrointestinal tracts. In human cancers, CD28H is preferentially present on tumor infiltrating lymphocytes (TILs) with TRM features and identifies a TRM subset with less cytotoxicity. Taken together, our studies suggest that the CD28H/B7-H5 pathway involves the interactions between TRM cells and epithelium, and could be important for human TRM homeostasis and function.
Introduction
Memory T cells can be divided into three distinct subsets based on trafficking properties: central memory T cells (TCM), effector memory T cells (TEM), and a subset recently termed as tissue resident T cells (TRM.Citation1–Citation3 TRM cells exhibit a broad tissue distribution, comprising the majority of T cells throughout the body in lymphoid, mucosal, non-lymphoid, and peripheral tissues, especially barrier tissues.Citation4 TRM signatures include the expression of CD69, and a subset of CD8 + TRM, particularly those that reside within epithelial surfaces of the intestinal mucosa and skin, also express αEβ7 integrin (often referred to as CD103.Citation4–Citation6 Many developmental cues contribute to TRM survival and site-specific retention, and may include TGF-β and IL-15.Citation7,Citation8 Besides cytokines, molecular interactions that maintain TRM cells in their resident tissues are the main subject of investigations. Adhesion molecules that are currently known to be involved in the epithelium-TRM interaction include CD103,Citation9,Citation10 CD2/CD58,Citation11 junctional adhesion-like molecule-1 (JAML-1)/coxsackie-adenovirus receptor (CAR),Citation12 and NKG2D.Citation13
The role of TRM cells recently has become an active subject of investigation.Citation14 Physiologically, the interaction between TRM and epithelial cells is essential for maintaining epithelium integrity.Citation15,Citation16 TRM cells respond rapidly to pathogen challenge at barrier sites before the recruitment of T cells from the blood.Citation17 Several recent clinical studies empathize a crucial role for TRM cells in human cancer immunity. The expression of TRM marker CD103 in tumor infiltrating lymphocytes (TILs) is associated with better prognosis and therapy response in lung and cervical cancers.Citation18,Citation19 Single-cell profiling in human breast cancer identified a subset of TILs with TRM phenotype, which contribute to cancer immunosurveillance and are the key targets of modulation by immune checkpoint inhibition.Citation20
We previously identified a new pathway of the B7 family in humans, the CD28H/B7-H5 axis.Citation21 The receptor, CD28H (also named as TMIGD2Citation22,Citation23 or IGPR-1Citation24), is constitutively expressed on all naive T cells and natural killer (NK) cells in human peripheral blood. Repeated stimulation of T cells leads to an irreversible loss of CD28H expression, and the loss of CD28H identifies a subset of effector/memory T cells with aging phenotype. The ligand B7-H5, also named as HHLA2,Citation25 is preferentially transcribed in peripheral tissues.Citation21,Citation22 It interacts with CD28H to promote T cell response, and it was also demonstrated to deliver a suppressive signal to T cells via an unknown receptor.Citation26
Here we examined CD28H expression on T cells from human normal and cancerous tissues. We found that TRM cells in peripheral tissues preferentially express CD28H. Consistently, CD28H expression in TILs of human cancers defines a subset of TILs with TRM phenotype.
Results
CD28H is preferentially expressed on human TRM cells in peripheral tissues
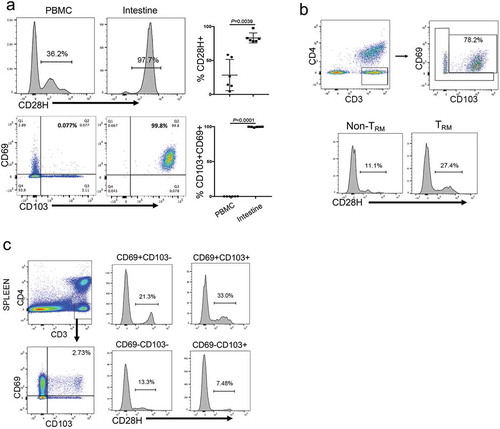
To understand CD28H expression in T cells from different human compartments, we analyzed human T cells from peripheral blood, spleen, intestine, and lung tissues by flow cytometry. Consistent with our previous finding,Citation21 a significant population of T cells in peripheral blood express CD28H, and the majority of CD28H-expressing T cells are naïve T cells (data not shown). Also, CD8 + T cells in human small intestine are all CD103 + CD69+, a major feature of TRM cells. Interestingly, in the same donor, virtually all of these cells are CD28H-positive (). In human lung, although overall only about 20% of CD8 + T cells express CD28H, there are significantly more CD28H-expressing cells within the TRM compartment (27.4% vs 11.1%) (). Thus, our results support that CD28H is preferentially expressed on T cells with resident memory phenotype (TRM). Interestingly, though few spleen CD8 + T cells exhibited TRM phenotype, the percentage of CD28H + cells (33%) in splenic TRM was still the highest among all compartments (). These results support that CD28H expressing T cells in human tissues are mainly T cells with TRM phenotype.
Figure 1. CD28H is highly expressed on human TRM cells. (a) Expressions of CD28H in CD8 + T cells from human peripheral blood and small intestine. Data shown are gated on CD3 + CD8 + cells. (b) CD28H expression in TRM cells from human lung. CD8 + T cells in human lung were further stained for CD69 and CD103 to identify TRM cells. The percentages of CD28H + T cells in the subsets of non-TRM (CD103-CD69+) and TRM (CD103 + CD69+) were indicated. (c) Expressions of CD69 and CD103 in CD8 + T cells from human spleen. The MFI of CD28H and the percentages of CD28H + in the subsets of CD103-CD69+, CD103 + CD69+, CD103 + CD69-, and CD103-CD69-.
CD28H expression on TRM cells is regulated by IL-15 and TGF-β
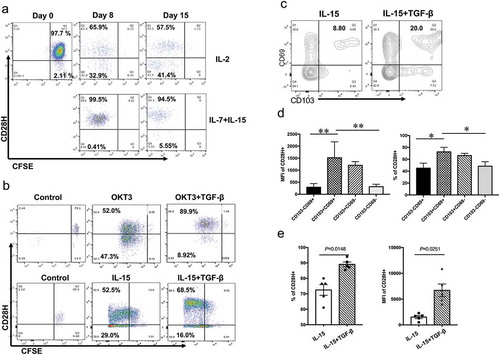
T cell activation via TCR signal leads to gradual loss of CD28H expression.Citation21 TRM cells in peripheral tissues are mainly regulated by cytokines IL-15 and TGF-β.Citation8 We hypothesized that IL-15 stimulation might retain CD28H expression on T cells. To test this, we labeled naïve human T cells with CFSE and stimulated them with cytokine IL-15 plus IL-7, or IL-2. Virtually all naïve CD8 + T cells from peripheral blood express CD28H.Citation21,Citation27 Indeed, we found that T cells stimulated with IL-2 gradually lose the expression of CD28H during division. On the contrary, IL-15 plus IL-7 stimulation retained CD28H expression as T cells went through similar rounds of cell division (). Furthermore, we found that addition of TGF-β could induce higher levels of CD28H expression in activated CD8 + T cells, regardless of stimulation by OKT3 (CD3 mAb) or IL-15 (). Cytokines IL-15 and TGF-β have been used to promote tissue residency in vitro when T cells from peripheral blood were stimulated with CD3 mAb.Citation8,Citation28,Citation29 In the same model, we analyzed CD103 and CD69 expression for TRM phenotype, and we found that the percentages of TRM cells (CD103 + CD69+) in activated CD8 + T cells significantly increased with the stimulation of TGF-β (). Moreover, the TRM cell subset in activated CD8 + T cells had higher expression of CD28H, as well as more percentages of CD28H-positive cells (). The presence of TGF-β further increased the percentages of CD28H-expressing cells (72.5% vs 89.1%, p = 0.0148), as well as the expression level of CD28H in TRM cells (MFI: 1517 vs 6699, p = 0.0251) (). Therefore, our results indicate that T cells exposed to epithelial stimulus, presumably IL-15 and TGF-β, sustain CD28H expression in human epithelium.
Figure 2. IL-15 and TGF-β promote CD28H expression in TRM cells. (a) Purified naïve human T cells were labeled with CFSE and cultured with IL-2 or IL7/IL-15. The expression of CD28H and cell division (CFSE) on Day 8 and 15 were detected by flow cytometry. (b) CFSE-labeled human T cells were stimulated with CD3 mAb or IL-15, with or without TGF-β. CD28H expression on proliferating CD8 + T cells (CFSElow) was shown. (c-e) Human CD8 + T cells were stimulated with IL-15, with the presence or absence of TGF-β. (c) The expressions of CD69 and CD103 on T cells were examined. (d) The percentages and MFI of CD28H expression in each subset were determined. (e) The MFI and percentages of CD28H expression in TRM subset (CD69 + CD103+) were compared between these two culture conditions. Data were collected from five independent experiments. Data showed mean ± SD and were analyzed by F-test (MFI of CD28H) and Student’s t-test (percentage of CD28H+). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Expression of the CD28H/B7-H5 axis in human epithelium
Our early study found that the ligand B7-H5 is well-transcribed in peripheral organs.Citation21 Consistent with that, our immunohistochemistry staining indicates that B7-H5 is exclusively expressed on epithelial cells from peripheral human tissues, including intestinal tissue, the bronchial epithelia in lung (Supplemental Figure 1), and ductal epithelia in pancreas.Citation30 We further stained single cells isolated from human intestine for Ep-CAM (CD326) and CD45 to identify epithelial cells and intraepithelial lymphocytes (IELs) respectively. We found that B7-H5 is constitutively expressed on Ep-CAM + CD45− epithelial cells, while CD28H is present on virtually all CD45+ CD3 + CD8 + IELs (Supplemental ). This result suggests that TRM/epithelia interaction probably involves CD28H/B7-H5.
CD28H is enriched in CD8 + tumor-infiltrating lymphocytes (TILS) with TRM phenotype
We examined CD28H expression in TILs from surgically removed pancreatic ductal adenocarcinoma (PDAC) tissues. Though in a lower percentage compared to PBMCs, there are consistently a significant subset of CD28H-expressing CD8 + T cells in TILs from all PDAC patients we analyzed (). CD28H expression is not just limited to TILs from PDAC, as we confirmed the presence of comparable percentages of CD28H + TILs in other human cancer types, including melanoma and glioma ().
Figure 3. CD28H-positive CD8 + TILs are less differentiated antigen-experienced T cells. (a) T cells from peripheral blood and pancreatic cancer were stained for CD28H. The percentages of CD28H-expressing cells in both CD4 + and CD8 + T cell subsets were indicated. (b) The percentages of CD28H-expressing cells in CD8 + TILs from different cancer types, including PDAC, glioma, and melanoma. (c) TILs and PBMCs from different patients with PDAC were stained for CD3, CD4 and CD28H, followed with staining for CD45RO, CD45RA, or ICOS. Data shown were gated on CD3 + CD8 + T cells. (d) The expression of CD57, KLRG1 and CCR7 against CD28H in human CD8 + TILs. (e) Upon stimulation by CD3 and CD28 microbeads, TILs were stained for CD3, CD8 and CD28H, followed with intracellular staining of different cytokines as indicated. Data shown were gated on CD3 + CD8 + T cells, and further divided into two subsets based on CD28H expression. (f) TILs from different patients with PDAC were stained for CD3, CD4 and CD28H, followed with intracellular staining of perforin or Granzyme B. Data shown were gated on CD3 + CD8 + T cells, and further divided into two subsets based on CD28H expression.
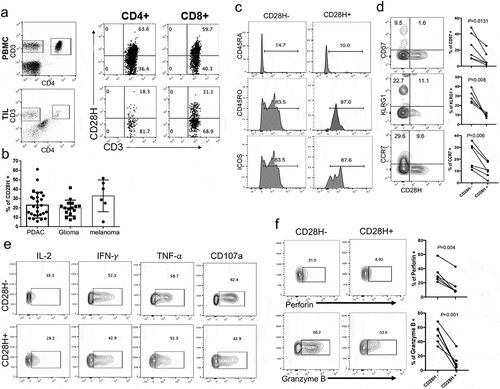
In human peripheral blood, the majority of CD28H-expressing T cells are naïve T cells.Citation21 In contrast, virtually all CD28H-positive cells in TILs are antigen-experienced or memory-phenotype T cells; they express CD45RO, are all CD45RA-negative, and the majority of them are ICOS-positive (). Many of CD28H-expressing CD8 + T cells in TILs express known immune checkpoints, including PD-1, LAG3, TIGIT and Tim3 (Supplemental Figure 3). CD28H-expressing cells in TILs do not express aging-associated molecules CD57 or KLRG1, which is similar to the compartment in peripheral blood.Citation21 Interestingly, CD28H + T cells express less CCR7 (). When we stimulated these cells in vitro for a short period, CD28H-positive cells produce significantly more IL-2, but less effector cytokine IFN-γ (). Consistently, CD28H-positive cells in TILs produced less CD107a (), perforin, and Granzyme B (). All these suggest that CD28H-expressing T cells are more of effector/memory cells with young and less-differentiated phenotype, which is consistent with a recent publication.Citation27
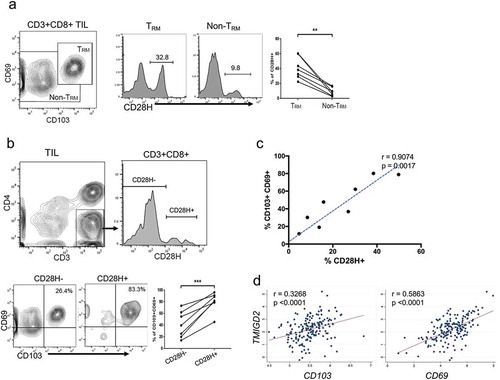
Recent research indicates that many human TILs possess features of TRM cells and are positively correlated with patient survival.Citation31–Citation33 Since TRM cells in human tissues express CD28H, we hypothesize that CD28H + T cells in human TILs could possess features of TRM cells. We examined the expressions of CD103 and CD69 on CD8 + T cells in TILs from pancreatic cancer, and we were able to confirm the presence of TILs with TRM phenotypes (, left). We compared the frequencies of CD28H expressions between the group of TRM and that of the rest subgroup. Indeed, there were significantly more CD28H expressing T cells in TILs with TRM phenotype (p < 0.01) (, right). When we divided the cell population into two subgroups according to the expression of CD28H, we found that there were significantly higher percentages of TRM cells (CD103 + CD69+) in the CD28H + group than the CD28H- counterpart (p < 0.001) (). Consistently, there is a significant, positive correlation between the percentages of TRM cells and CD28H + T cells in CD8 + TILs from pancreatic cancer (). In addition, our analysis of The Cancer Genome Atlas (TCGA) PDAC PanCancer datasetCitation34 also found a positive and significant correlation between TMIGD2 (encodes CD28H), CD103 and CD69 expression (). Thus, our results imply that the expansion of CD28H + TILs could directly affect the number of TRM cells in TILs.
Figure 4. CD28H expression identifies tumor-infiltrating lymphocytes (TILs) with TRM phenotype. (a) CD8 + TILs from pancreatic cancer were stained for CD69 and CD103 to classify into two populations: TRM and non-TRM; CD28H expression in these two populations was compared. (b) TILs were stained for CD3 and CD4 to identify CD8 + TILs, and TILs were further divided into two subsets based on CD28H expression; The percentages of TRM cells (CD103+ CD69+) in each group were indicated. (c) Plot of frequencies of CD28H-expressing T cells with those of TRM cells in pancreatic cancer. (d) Correlation analysis between the expression of CD103, CD69 and TMIGD2 (encoding CD28H) genes in pancreatic cancer patients from the TCGA cohort (n = 177 patients; r = Pearson’s correlation coefficient). Data showed mean ± SD and were analyzed by Student’s t-test. **P < 0.01.
CD28H expression identifies CD8 + TRM cells with less cytotoxicity
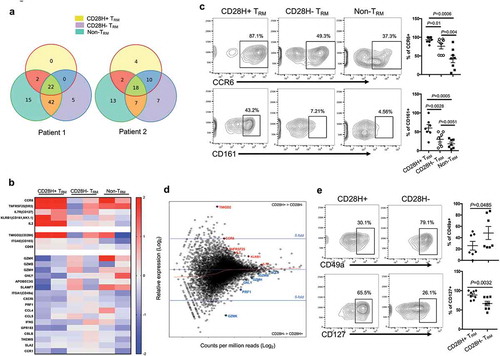
To further characterize CD28H + T cells in human cancer, we examined transcript profiles for subsets of TILs based on CD28H expression by genome-wide RNA-seq technique. TILs isolated from pancreatic cancer were further sorted into three CD8 + T cell subsets based on the expression of CD69, CD103, and CD28H: CD28H + TRM, CD28H-TRM, non-TRM. 151bp single-end RNA-Seq was performed on isolated total RNA materials, and the full-length transcripts were recovered from de novo transcriptome assembly. We found that identified TCR subtypes overlapped greatly among these three T cell groups (), suggesting they could derive from the same T cell lineages and recognize similar antigen profiles. Gene enrichment analysis indicated that CD28H + TRM cells have the highest transcript level of cytokine IL-2, which is consistent with our early analysis (). CD28H + TRM cells are higher in the expression of CCR6 and CD161, two genes important for memory T cell differentiation (). Flow cytometry analysis further confirmed that there are significantly more CCR6 and CD161-expressing cells in TRM subsets than non-TRM cells, and the CD28H + TRM subset also has the most CCR6 and CD161-expressing cells (). Cytotoxicity-related genes, including PRF1, GZMB, GZMH and GZMK, are the main difference between these two different TRM subsets, based on CD28H expression (). CD49a, a molecule recently related to TRM cells with high cytotoxicit,Citation35 is significantly less transcribed in the CD28H + TRM subset. We also noticed that CD28H + T cells express more transcripts for IL7R (CD127), which is associated with the long-term survival of T cells (). Supporting these findings, flow cytometry analysis of TILs from multiple tumor tissues found that CD28H + TRM cells express less CD49a but more surface IL7R, further demonstrating this CD28H + T subset is composed of young TRM cells with less cytotoxicity ().
Figure 5. Characterizing CD28H + TRM cells in human TILs. (a, b, d) CD8 + TILs from human pancreatic cancer were sorted into three different groups based on the expression of CD28H, CD69 and CD103; RNAseq was performed. TCR subtype overlapping (a) and relative expression of the core T cell function genes (b) in three different CD8 + T cell subsets. (c) CCR6 and CD161 expressions in three different T cell subsets from different cancer patients. (d) MAPlot of average expression of genes in CD28H + vs CD28H- TRM cells with select genes highlighted. (e) The expressions of CD49a and CD127 in the two different TRM subsets from different cancer patients.
Discussion
The CD28H/B7-H5 axis is a recently identified pathway of the B7 family. Due to the absence of a CD28H ortholog in rodents and limited access to fresh human tissues, it is still unclear how CD28H is expressed on T cells in peripheral tissues. Our study here demonstrated that the ligand B7-H5 is constitutively expressed on epithelial cells, while the receptor CD28H is expressed on adjacent TRM cells. Consistently, in human cancer, CD28H is preferentially expressed on a subset of TILs with TRM phenotype. Thus, our data suggest that the CD28H/B7-H5 interaction could be one of the important mechanisms for human CD8 + TRM cell retention or homeostasis.
Epithelial cells that line tissue vasculature constitute a major source of TGF-β and IL-15. They are therefore likely candidates to imprint infiltrating T cells with signals, which allow them to reside and survive in the mucosa microenvironment. Our study results showed that IL-15 and TGF-β are together capable of inducing a population of resident T cells, while also imposing a highly constrained (CD28H+) phenotype to facilitate their long-term survival in the face of antigenic challenge. TCR engagement, for example by in situ recognition of antigen epitopes, can substitute for these cytokine signals or can synergize with TGF-β to further promote T cell residency in the human mucosa. Our data suggest that a TCR signal or the bystander cytokine IL-15 with the exposure to TGF-β can achieve optimal induction of CD28H-positive TRM cells. Since B7-H5 is constitutively expressed on epithelium, it would be interesting to investigate whether and how the CD28H/B7-H5 interaction regulates human TRM cell homeostasis and functions.
TILs are rich in tumor-reactive T cells, but the majority of them are dysfunctional or exhausted.Citation36,Citation37 It is extremely valuable to identify functional T cell subtypes in TILs for adaptive cellular therapy (ACT) or future immunomodulation strategies.Citation38 Recent studies identified that some TILs bear TRM phenotype and are associated with better prognosis in cancer patients.Citation18,Citation19 In this study, we analyzed TILs from human cancers relevant to TRM phenotype and CD28H expression. We found that CD28H-expressing T cells within TILs are particularly enriched within the CD8 + TRM compartment. Consistently, CD28H-expressing TILs are antigen-experienced cells and do not express CCR7 (). The expression of CD28H is positively associated with CD103 and CD69 expression in human pancreatic cancer from TCGA dataset (). CD28H + cells in CD8 + TILs are identified as a superior T cell subset with a phenotype of young, less differentiated T cells.Citation27 CD28H + TRM cells express higher levels of IL-2, IL7R, and CCR6, and are less cytotoxic. Thus, CD28H expression could be associated with better prognosis in human cancer. Immune checkpoints, such as PD-1, LAG3, TIGIT, and Tim3, are well-expressed on CD28H + TRM cells (supplemental Figure 3), suggesting that these cells can be modulated by checkpoint inhibitors and contribute to their therapeutic efficacy in clinic. Adding the ability of TRM cells to persist long-term in tissues, targeting CD28H + T cells in cancer may exhibit durable clinical responses.
Taken together, our study found that CD28H identifies human TRM cells in both normal tissues and cancer. It remains to be seen how the CD28H pathway regulates human TRM cells in normal tissues and cancer. Furthermore, as solid human tumors express different levels of ligand B7-H5, it is interesting to further investigate whether and how CD28H + TRM cells in TILs can be affected by tumor-expressing B7-H5.
Materials and method
Human tissue collection
Human tissues of PDAC, melanoma, glioma, and normal tissues were obtained from patients who were undergoing surgery at The University of Colorado Hospital according to an Institutional Review Board (IRB)–approved protocol and patient consent. All human subject studies conducted conform to the principles of the Declaration of Helsinki.
TILs and IEL isolation
The method for fresh isolation of TILs from PDAC, melanoma, and glioma tissues has been described in detail before.Citation39 Briefly, surgically-removed fresh human tumors or normal intestine tissues were washed with PBS twice before being homogenized and digested with liberase enzyme (Roche) at 37°C for 45 min. After centrifugation, pellets were resuspended in 30% Percoll and carefully loaded on the top of a 70% Percoll solution. The Percoll gradient was centrifuged at room temperature at 1000Xg for 30 min with brake off. Mononuclear cells between the layers of the 30% and 70% Percoll were collected and washed with complete RPMI 1640 before flow cytometry analysis or in vitro culture.
Antibodies
Anti-human CD4 was purchased from eBioscience. Antibodies for human CD3, CD103, CD69, CD45RO, ICOS, CD45RA, CD57, KLRG1, CCR7, Ki67, IL-2, IFN-γ, TNF-α, Perforin, Granuzyme B, CD326, and CD45 were obtained from Biolegend. The generation of hamster anti-human CD28H mAb (clone 4–5, IgG1) and mouse anti-human B7-H5 mAb (clone 5B6-2, IgG1) has been described previously.Citation21 All other antibodies and staining dyes used in flow cytometry and immunohistochemistry were purchased from BD Bioscience.
Immunohistochemistry staining and flow cytometry analysis
Immunohistochemistry staining of B7-H5 on human frozen tissues has been described previously.Citation30 For cell surface staining and analysis by flow cytometry, cells were washed twice in fluorescence-activated cell sorting (FACS) wash buffer [D-PBS + 2% bovine serum albumin (BSA)] and stained for 30 minutes at 4℃ with antibodies conjugated with fluorochromes against different T cell surface and intracellular markers. Intracellular staining of cytokines was done according to manufacturer’s protocol (BD Biosciences). Proliferation was assessed by intracellular staining for Ki-67 with an anti–Ki-67-APC antibody (BD Biosciences). Activation of stimulated cells was followed by staining with CFSE according to manufacturer’s protocol. Dead cells were excluded with SYTOX ® Blue Dead Cell Stain Kit (Thermo Fisher Scientific). Flow cytometric analysis was conducted with an FACS LSRII flow cytometer (Becton Dickinson), and data were analyzed by FlowJo software (Tree Star Inc.)
In vitro stimulation and T cells culture
PBMCs from healthy controls at 3 × 105 cells/well were incubated with either (i) 50 or 200 ng/ml of recombinant human IL-15, (ii) 5 or 50 ng/ml recombinant human (rh) TGFβ, or (iii) combinations of these for cytokine induction. In some experiments, immobilized anti-CD3 (eBioscience) and anti-CD28 (eBioscience) were used to stimulate human T cells. CFSE was used to label T cells to track their proliferation. All experiments were done in RPMI-1640 medium containing 10% FBS, and cells were incubated at 37 °C for 6 and 10 days. After culture, cells were stained for flow cytometry as in the prior section.
RNA-seq of TILS and gene expression analysis in pancreatic cancer
151bp single-end or paired-end RNA-Seq data were generated by University of Colorado Denver Sequencing Core facility. The data were processed using STAR software for mapping and DESeq2 for differential analysis. The Heatmap and MAplot were generated using GraphPad Prism 7.0 software (GraphPad Software) and RStudio Desktop 1.1.453 (https://www.rstudio.com/). We used the long-read RNA-Seq data to uncover the usage of TCR subtypes from different immune cell populations. The RNA-Seq data collected from Patient 1 and 2 were used because of deeper sequencing depth (an average of 100 million reads per sample). RNA-Seq de novo transcript assembly was performed using Trinity pipeline without genome guidance. Assembled full-length transcript sequences were mapped against annotated human transcript database (GENCODE.v28) using BWA aligner with mem option. After sorting and rmdup steps, TCR subtype genes were selected using in-house bash shell script. Overlap Venn diagram was generated using NeuVenn web tool (http://bioinfo5pilm46.mit.edu:318/neuvenn).
PDAC mRNA expression data from 177 patients in the TCGA PanCancer dataseCitation34 was downloaded from cBioPortal.Citation40 Correlation of log transformed mRNA expression levels of CD28H, CD69, and CD103 were evaluated.
Statistical analysis
Student’s t-test was used for statistical analysis, and p-values reflect comparison with the control sample. P-values <0.05 were considered statistically significant. The error bars in figures represent SD. For TCGA mRNA expression data, correlation of log transformed data was evaluated using the Pearson’s correlation coefficient.
Supplemental Material
Download MS Power Point (7.4 MB)Acknowledgments
We are very grateful to all patients and control volunteers who participated in this study and to all clinical staff who helped with participant recruitment. We thank the Human Immunology Flow Core at the University of Colorado Anschutz Medical Campus for flow cytometry analysis, Genomics and Microarray Core at Cancer Center for RNA-Seq service, and Emily Miller for editing the manuscript. This study is partially supported by American Cancer Society Institutional Research Grant Number 57-001-53 and Cancer League of Colorado 163479. Yuwen Zhu is supported by a Research Scholar Grant, RSG-17-106-01 LIB, from the American Cancer Society.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplementary data for the article can be accessed here.
References
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417, doi:10.1126/science.1058867.
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi:10.1038/ni.1718.
- Gebhardt T, Whitney PG, Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature, 2011;477(7363):216–219. doi:10.1038/nature10339.
- Thome JJ, Yudanin N, Ohmura Y, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell, 2014;159(4):814–828. doi:10.1016/j.cell.2014.10.026.
- Okhrimenko A, Grun JR, Westendorf K, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A, 2014;111(25):9229–9234. doi:10.1073/pnas.1318731111.
- Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory. T Cells. Sci Transl Med, 2015;7(279):279ra39. doi:10.1126/scitranslmed.3010302.
- Casey KA, Fraser KA, Schenkel JM, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol, 2012;188(10):4866–4875. doi:10.4049/jimmunol.1200402.
- Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol, 2013;14(12):1294–1301. doi:10.1038/ni.2744.
- Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192(5):761–768. doi:10.1084/jem.192.5.761
- Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9(7):836–850.
- Shao JY, Yu Y, Dustin ML. A model for CD2/CD58-mediated adhesion strengthening. Ann Biomed Eng. 2005;33(4):483–493. doi:10.1007/s10439-005-2504-5
- Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer, W H., Wilson, I A., Havran, W L. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science, 2010;329(5996):1205–1210. doi:10.1126/science.1192698.
- Prajapati K, Perez C, Rojas LBP, Burke B, Guevara-Patino JA. Functions of NKG2D in CD8(+) T cells: an opportunity for immunotherapy. Cell Mol Immunol. 2018. doi:10.1038/cmi.2017.161. 15 470–479
- Amsen D, van Gisbergen K, Hombrink P. and van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nature Immunology. 2018;19(6):538–546, doi:10.1038/s41590-018-0114-2.
- Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9(3):153–161, doi:10.1038/nri2496.
- Ariotti S, Haanen JB, Schumacher TN. Behavior and function of tissue-resident memory T cells. Adv Immunol. 2012;114:203–216. doi:10.1016/B978-0-12-396548-6.00008-1.
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897, doi:10.1016/j.immuni.2014.12.007
- Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol (Baltimore, Md: 1950)., 2015;194(7):3475–3486. doi:10.4049/jimmunol.1402711
- Komdeur FL, Prins TM, van de Wall S, Plat A, Wisman GBA, Hollema H, Daemen, T., Church, D N., de Bruyn, M., Nijman, H W. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology, 2017;6(9):e1338230. doi:10.1080/2162402X.2017.1338230.
- Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24(7):986–993. doi: 10.1038/s41591-018-0078-7.
- Zhu Y, Yao S, Iliopoulou BP, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi:10.1038/ncomms3043.
- Janakiram M, Chinai JM, Fineberg S, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res, 2015;21(10):2359–2366. doi:10.1158/1078-0432.CCR-14-1495.
- Janakiram M, Chinai JM, Zhao A, Sparano JA, Zang X. HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology. 2015;4(8):e1026534, doi:10.1080/2162402X.2015.1008371.
- Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell. 2012;23(9):1646–1656, doi:10.1091/mbc.E11-11-0934.
- Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3). Genomics. 1999;59(3):255–263, doi:10.1006/geno.1999.5877.
- Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A, 2013;110(24):9879–9884. doi:10.1073/pnas.1303524110.
- Crespo J, Vatan L, Maj T, Liu R, Kryczek I, Zou W. Phenotype and tissue distribution of CD28H(+) immune cell subsets. Oncoimmunology. 2017;6(12):e1362529, doi:10.1080/2162402X.2017.1362529.
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115(4):1091–1105. doi:10.1083/jcb.115.4.1091
- Koyama SY, Podolsky DK. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989;83(5):1768–1773, doi:10.1172/JCI114080.
- Byers JT, Paniccia A, Kaplan J, Koenig M, Kahn N, Wilson L, et al. Expression of the novel costimulatory molecule B7-H5 in pancreatic cancer. Ann Surg Oncol, 2015;22(Suppl 3):S1574–9. doi:10.1245/s10434-014-4293-2.
- Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8(8):13762–13769, doi:10.18632/oncotarget.14632.
- Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight, 2016;1(21):e88955. doi:10.1172/jci.insight.88955.
- Murray T, Fuertes Marraco SA, Baumgaertner P, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol. 2016;7:573. doi:10.3389/fimmu.2016.00573.
- Cancer Genome Atlas Research Network. Electronic address aadhe, and cancer genome atlas research n. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2): 185–203 e13. doi:10.1016/j.ccell.2017.07.007.
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a Expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity, 2017;46(2):287–300. doi:10.1016/j.immuni.2017.01.009.
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med, 1988;319(25):1676–1680. doi:10.1056/NEJM198812223192527.
- Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest, 2011;121(6):2350–2360. doi:10.1172/JCI46102.
- Zhu Y, Chen L. CD137 as a biomarker for tumor-reactive T cells: finding gold in the desert. Clin Cancer Res. 2014;20(1):3–5, doi:10.1158/1078-0432.CCR-13-2573
- Zhu Y, Yao S, Augustine MM, et al. Neuron-specific SALM5 limits inflammation in the CNS via its interaction with HVEM. Sci Adv, 2016;2(4):e1500637. doi:10.1126/sciadv.1600375.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov, 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095.
