ABSTRACT
Bermekimab is a true human monoclonal antibody that targets interleukin-1alpa (IL-1α), an inflammation-mediating alarmin. IL-1 receptor antagonist (IL-1Ra) is a natural molecule that blocks IL-1α activity by occupying the IL-1 receptor. The effect of endogenous IL-1Ra levels on the effectiveness of bermekimab is unknown. We investigated whether pre-treatment levels of circulating IL-1Ra, assessed by an enzyme-linked immunoassay, correlated with achievement of the primary outcome endpoint (effect on lean body mass and symptoms at week 8) in a Phase III study (2:1 randomization) of bermekimab versus placebo (each with best supportive care) in advanced colorectal cancer. Patients who responded to bermekimab in terms of achieving the primary endpoint had lower levels of IL-1Ra than non-responders (N = 204 patients; median = 843 vs. 1035 pg/ml, p=0.0092); no such relationship was observed in the placebo arm (N = 100 patients; 901 vs. 984 pg/ml, p = 0.55). Multivariate analysis corroborated that, in the bermekimab group, patients with lower baseline IL-1Ra levels were more likely to achieve the primary endpoint (odds ratio (OR) 1.7 (95% confidence interval (CI), 1.1 to 2.6), p = 0.017); in contrast, in the placebo arm, pre-treatment plasma IL-1Ra levels were not associated with outcome (OR 1.2 (95% CI 0.6 to 2.5), p = 0.57). The current findings demonstrate that, in a randomized phase III trial, patients with advanced colorectal cancer and lower levels of circulating IL-1Ra are more responsive to treatment with the IL-1α-targeting antibody bermekimab and these observations define a potential biomarker for anti-IL-1α therapy.
Introduction
The interleukin-1 (IL-1) family is a group of 11 cytokines that play a key role in the regulation of inflammatory and immune responses to both infectious and non-infectious insults. IL-1α and IL-1β bind to the same receptor molecule – -the type I IL-1 receptor (IL-1RI). There is a third ligand of this receptor – IL-1 receptor antagonist (IL-1Ra). IL-1Ra functions as a classic receptor antagonist and thereby prevents downstream signaling in the presence of its agonists IL-1α and IL-1β. Moreover, the affinity of IL-1Ra for the IL-1RI is greater than that for IL-1α and IL-1β. IL-1Ra is produced by various cells including the liver. Many studies report that elevated levels of circulating IL-1Ra correlate with the severity of inflammation and infection and also correlate with levels of circulating IL-6 and C-reactive protein.Citation1,Citation2
Bermekimab (formerly known as MABp1) is a true human monoclonal antibody that specifically targets IL-1α, an important alarmin, which mediates the initiation of sterile inflammation. Bermekimab differs from previous generations of therapeutic antibodies in that it was cloned directly from a human B cell (Epstein-Barr virus-immortalized) that was isolated from a person with endogenous anti-IL-1α antibodies.Citation3 The IL-1α precursor is constitutively present in diverse cells of healthy people, including mucosal epithelial cells, vascular endothelium, and platelets, and in organs such as the lungs, liver and kidneys. With stimulation such as moderate stress, hypoxia and activation of macrophages, the IL-1α precursor is found in the membrane and there acts to trigger the IL-1/IL-1R1 on adjacent cells. After injury, the IL-1α precursor is released and transits to the cell surface membrane, where it activates cells harboring the IL-1 receptor. This process jumpstarts the sterile inflammation pathway and, thus, generates a cascade of inflammatory mediators such as additional cytokines as well as chemokines. The specific blockade of IL-1α activity should therefore attenuate the inflammatory cascade in protean diseases of the bones, skin, cardiac system, and in cancer.Citation4-Citation7 Furthermore, targeting IL-1α may have a role in conditions such as heart failure and type 2 diabetes.Citation8 The role of inflammation in these two conditions has only recently been recognized.
Through its non-signaling occupation of the IL-1 receptor, the natural endogenous biologic antagonist of the IL-1 receptor – IL-1Ra – acts to block the agonist activity of IL-1α. Since bermekimab targets IL-1α, we investigated whether the circulating levels of IL-1Ra before initiating treatment with bermekimab affected the primary outcome (effect on lean body mass and symptoms) in a Phase III randomized study of bermekimab versus placebo (each with best supportive care (BSC)) in patients with advanced colorectal cancer.
Methods
Data source: The analysis performed in this study was based on data obtained from a phase III study with bermekimab in patients with advanced colorectal cancer.Citation9
Treatment with bermekimab: Pre-treatment levels of circulating soluble IL-1Ra were measured in patients enrolled in a phase III study. Patients received an intravenous infusion of 7.5 mg/kg bermekimab or placebo given every two weeks for eight weeks.Citation9 The primary endpoint was assessed in patients who received at least one dose of bermekimab or placebo (modified intention-to-treat population), and was a composite of stable or increased lean body mass and stability or improvement in two of three symptoms (pain, fatigue, or anorexia) at week eight compared with baseline measurements.Citation9 This study was registered with ClinicalTrials.gov, number NCT02138422 and was approved by appropriate institutional review boards; all patients signed informed consent
Overall, 309 patients were randomized 2:1 to receive bermekimab plus best supportive care (BSC) (N = 207) or placebo plus BSC (N = 102). Patients had metastatic colorectal cancer refractory to standard chemotherapy (including oxaliplatin and irinotecan) and a constellation of symptoms/functional impairment (e.g. pain, fatigue, anorexia, ECOG performance 1 or 2), weight loss or elevated systemic inflammation.
Measurement of IL-1Ra: Endogenous\plasma IL-1Ra levels were measured using a commercial enzyme-linked immunoassay (ELISA) kit (human IL-1Ra Platinum ELISA from eBioscience, catalog number BMS2080). Plasma samples were frozen and stored for batch analysis. The samples were obtained on day 1 of course 1, immediately prior to the first dose of either placebo or bermekimab.
In brief, to determine IL-1Ra levels, samples were thawed and 50 µl aliquots were incubated in microtiter wells coated with anti-human IL-1Ra antibody. Wells were then washed and detection achieved by adding biotin-conjugated anti-human IL-1Ra antibody, followed by incubation with Streptavidin-HRP, and finally by addition of horseradish peroxidase (HRP) substrate solution. A colored product formed in proportion to the amount of human IL-1Ra present and absorbance was measured at 450 nm. The lower limit of assay sensitivity is 219 pg/ml.
Measurement of IL-6: Patient serum samples were subjected to an IL-6 ELISA method tested by PPD Global Central Labs (Belgium) per PPD document number GCL-LAB-0371 using Quantikine High Sensitivity Human IL-6 Immunoassay Kits (R&D Systems catalog number HS600B). This assay employs a quantitative sandwich ELISA technique. In brief, an anti-IL-6 monoclonal antibody is pre-coated onto a microtiter plate. Standards and samples are pipetted into the wells and IL-6 is bound by the immobilized antibody. Plates are washed and an enzyme-linked polyclonal antibody specific for IL-6 is added to the wells. Plates are washed to remove unbound antibody-enzyme reagent and a substrate solution is added to the wells; color develops in proportion to the amount of IL-6 bound in the initial step. The lower limit of detection for the IL-6 assay was 0.108 pg/mL.
Statistical Analysis: A multivariate logistic regression model was used to assess correlation between baseline IL-1Ra levels and primary outcome. Receiver operating characteristics (ROC) curves that graphed sensitivity versus specificity-related parameters was used to determine optimal cut off for IL-1Ra in relation to achieving the primary endpoint
Results
Patients
Plasma samples for measurement of IL-1Ra were available for 204 of 207 participants that were assigned treatment with bermekimab and 100 of 102 participants randomized to the placebo arm. All patients had advanced, metastatic colorectal cancer. The mean age of patients was 63 years (range, 31 to 84 years). Sixty one percent of patients were men. The median number of prior therapies in the metastatic setting was 3 (range, 1 to 19). There were no significant differences in age, sex distribution, baseline weight, KRAS mutation status, IL-6 levels, ECOG performance status or the number of prior lines of therapy in the bermekimab and placebo arms ().
Table 1. Pre-treatment IL-1Ra (and IL-6) plasma levels in intent-to-treat population by treatment arm.
Baseline IL-1Ra levels did not differ in patients randomized to bermekimab or to placebo
IL-1Ra levels (median (interquartile (IQR) range)) in plasma samples collected immediately prior to the first dosing of subjects in bermekimab or placebo treatment arms were not different: 926 (678 to 1500) versus 972 (705 to 1597) (). Thus there was no evidence of randomization bias between groups.
Pre-treatment IL-1Ra levels were significantly lower in patients who achieved the primary endpoint on bermekimab but not in the placebo group
Pre-treatment plasma IL-1Ra levels were then examined to determine if there was any association with outcomes, particularly with respect to achieving the primary endpoint of the study (). Since the IL-1Ra levels were expected to be associated with disease process, an absolutely homogenous distribution could not be assumed. This was supported by the observation of (i) overall high levels of IL-1Ra present in these subjects compared to literature reports for other healthy or even cancer populations;Citation8–Citation12 and (ii) the considerable variance among individuals. The median plasma IL-1Ra level for the intention-to-treat colorectal cancer population (including patients from both arms) was 947 pg/ml (IQR 684 to 1514 pg/ml), which is considerably above the 150–300 pg/ml reported for the healthy population.Citation10–Citation12
Table 2. IL-1Ra and treatment outcome.
For a normal distribution, it would be expected that, for several standard deviations from the mean, there should be zero distribution; and this was not the case in the population. In the population analyzed, there were outliers many standard deviations from the mean suggesting that parametric methods of analysis (student’s t-test) that assume normal distribution may be highly unreliable. Thus median values and the non-parametric Wilcoxon Rank Sum methodology were used to compare differences.
In , values are presented for the pre-treatment plasma IL-1Ra levels among patients (stratified by study arms) that achieved or failed to achieve the primary endpoint. In the bermekimab arm, pre-treatment IL-1Ra levels were significantly associated with the primary endpoint. Patients who had elevated baseline IL-1Ra were less likely to respond to bermekimab therapy in terms of achieving the primary endpoint as compared to those with lower baseline IL-1Ra levels (median = 1035 vs. 843 pg/ml, p = 0.0092). No such relationship was observed, however, in the placebo arm, median pre-treatment plasma levels for IL-1Ra were not different by patients’ outcomes with respect to the primary endpoint (984 vs. 901 pg/ml, p = 0.55).
Corroborating findings using logistic regression analysis
A univariate logistic regression model was used to further explore and qualify this association between endogenous IL-1Ra levels, treatment, and primary outcome in the Phase III study. A multivariate logistic model was also constructed by adding baseline covariates (sex and baseline weight) to the univariate model to identify other independent predictors that could be associated with the primary outcome. Results were calculated as odds ratio (OR) with the corresponding 95% confidence interval (95%CI). Values for plasma IL-1Ra levels were log transformed prior to entering into the logistic model and analysis was performed stratified by study groups.
Multivariate regression analysis corroborated the finding that, in the bermekimab group, patients with lower baseline levels of IL-1Ra were more likely to achieve clinical response(OR 1.7 (95% CI, 1.1 to 2.6), p = 0.017) (). In contrast, in the placebo arm, pre-treatment plasma IL-1Ra levels did not show any significant association with achieving the primary endpoint (OR 1.2 (95% CI 0.6 to 2.5), p = 0.57).
Determining a cut-off value for pre-treatment circulating IL-1Ra levels for maximum bermekimab responsiveness
Receiver operating characteristics (ROC) cut off of ≤ 940 pg/m versus ≥ 940 pg/ml for IL-1Ra levels is determined to be optimal for predicting outcome in bermekimab -treated patients: A ROC analysis was performed using a logistic model to determine a cut-off threshold for pre-existing IL-1ra levels in terms of the impact on responsiveness (with respect to achieving the primary endpoint) to bermekimab therapy (). Prediction accuracy using the model was evaluated along with sensitivity and specificity parameters.
Figure 1. Receiver operating characteristics curve showing optimum IL-1Ra cut-off for bermekimab treatment response. True positive rate (sensitivity) and true negative rate (specificity) are plotted on y-axis, and IL-1Ra plotted on X-axis. The optimal cut off for IL-1ra was 940 pg/ml.
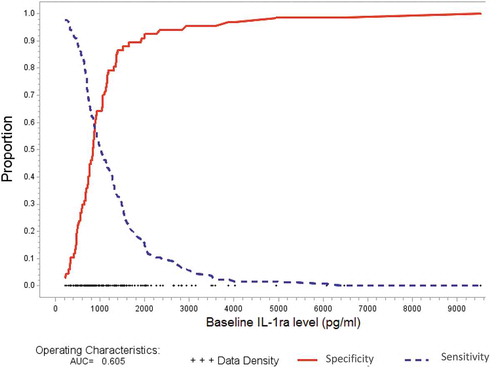
The ROC analysis, evaluating the discriminatory ability of an optimal IL-1Ra cut-off that correctly identifies the clinical response, demonstrated that a cut-off value for circulating IL-1ra levels was 940 pg/ml. This cut-off value would be expected to provide the best combination of sensitivity and specificity for identifying subjects that would achieve the primary endpoint in response to bermekimab therapy (specificity 0.55, sensitivity 0.64, AUC 0.61). The logistic regression analysis demonstrated the cut-off value to be significantly associated with primary endpoint (unadjusted OR 2.23 (95%CI 1.22 to 4.08), p = 0.009). The association remained after adjusting for sex and baseline weight (adjusted OR 2.28 (95%CI 1.24 to 4.19), p = 0.0082. AUC 0.64.).
The ROC cut-off value was also used to assess outcome for subjects randomized to the placebo group. In the placebo group, baseline IL-1Ra plasma levels in subjects did not predict outcomes with respect to the primary endpoint of the study. Not surprisingly, therefore, the IL-1Ra cut-off did not have any impact on outcomes in either univariate (OR 1.39 (95% CI 0.51 to 3.78) p = 0.52) or multivariate analysis when adjusted for sex and baseline weight (p = 0.60).
A sensitivity analysis for different IL-1Ra cut-off values was performed in bermekimab-treated population. From this analysis, the best cut-off obtained was confirmed to be that which was generated using the ROC method (i.e., 940 pg/ml) (data not shown).
Validating that bermekimab -treated but not placebo-treated patients are more likely to achieve primary outcome at ROC cut off for IL-1Ra level of 940 pg/ml: The ROC value was then validated based on the actual IL-1Ra plasma level data and outcomes for subjects using the cut-off value (<940 vs. ≥940) (). Using the ROC value and study findings, the sensitivy of IL-1RA for predicting responders (true positives or probability that responders will have IL-1Ra cut-off below 940 pg/ml) for the model was found to be 0.64. Conversely, the specificity (true negatives or probability that non-responders will have IL-1Ra values ≥940 pg/ml) for the model was 0.55. Positive predictive value (the proportion of patients with IL-1Ra <940 pg/ml that achieved the endpoint) was 0.41; negative predictive value (proportion of patients with IL-Ra levels ≥940 pg/ml that did not achieve the endpoint) was 0.76.
When using the optimized cut off from the ROC curve (<940 pg/ml versus ≥ 940 pg/ml for IL-1ra level), bermekimab-treated patients with low IL-1Ra were more likely to achieve the study endpoint (41% versus 24%; p = 0.01) while there was no difference in rate of endpoint achievement relative to the optimized cut off in patients receiving placebo (22% versus 17%; p = 0.6) (). Finally when comparing bermekimab- versus placebo-treated patients with <940 pg/ml of IL-1Ra, the former were more likely to achieve the study endpoint (relative risk (95% CI) = 1.9 (1.05 to 3.45); P = 0.03). There was no difference in likelihood of achieving the study endpoint between bermekimab- and placebo-treated patients for the group with IL-1Ra levels ≥ 940 pg/ml (relative risk (95% CI) = 1.44 (0.72–2.87); p = 0.3).
IL-6 levels
Because mechanisms inducing various cytokine-related molecules may be similar and because IL-6 is pertinent to features of many malignancies, we also examined IL-6 levels (plasma samples for measurement of IL-6 were available for 207 patients in the bermekimab-treated group and for 102 patients in the placebo group). IL-6 levels did not differ in the treatment versus placebo group at baseline (). As with IL-1Ra, achievement of the primary endpoint was associated with lower IL-6 levels in bermekimab-treated patients versus the placebo group (38% versus 16% of patients with low IL-6 in each arm achieved the endpoint; p = 0.002); in patients with higher IL-6 levels, there was no difference in the rate of achieving the primary endpoint (28% versus 22%; p = 0.36) ().
Table 3. Correlation of IL-6 levels and achievement of primary endpoint.
Discussion
We investigated pre-treatment levels of circulating IL-1Ra in patients with advanced colorectal cancer entering a double-blind, placebo-controlled Phase III study of bermekimab (9). Since IL-1Ra is an endogenous antagonist of IL-1α activity, examining baseline levels of the antagonist could provide insight into response to an exogenous IL-1α inhibitor – bermekimab.
Here we report a significant correlation between circulating IL-1Ra levels and primary outcomes of patients with colorectal cancer treated with bermekimab. Baseline IL-1Ra levels were significantly lower in patients who responded to bermekimab therapy than in those who did not (median, 843 vs. 1035 pg/ml, p = 0.0092, adjusted logistic model p = 0.017) (with response denoting, per protocol, stable or higher lean body mass and stability or improvement in two of three symptoms (anorexia, fatigue or pain) at week eight compared with pre-treatment assessmentsCitation9). A correlation between IL-1Ra levels and outcomes was not, however, seen in the placebo arm (p = 0.60, multivariate logistic model): pre-treatment levels of circulating IL-1Ra were not different between those achieving or not achieving the primary outcome in the placebo arm.
The finding that pre-treatment IL-1Ra levels correlated with primary outcomes in the bermekimab treatment group, but not in the placebo group, provides evidence that: (i) the mechanism of action for bermekimab in vivo involves targeting IL-1α; and (ii) bermekimab-specific targeting of IL-1α is indeed related to the effect on primary outcomes achieved in the Phase III clinical study.Citation9
IL-1 is a central mediator of innate immunity and inflammation.Citation10 Not surprisingly, therefore, IL-1Ra and other IL-1 family members play a role in disease progression in solid and hematological malignancies.Citation5,Citation12–Citation14 In colorectal cancer, for instance, levels of circulating IL-1Ra are elevated and associated with clinicopathological features of disease, including tumor burden and metastasis.Citation6,Citation7 The data are consistent with our study, which showed median IL-1Ra levels of 947 pg/ml; this level is significantly higher than the 150–300 pg/ml found in healthy people.Citation10-Citation12 Of interest, mutated RAS genes mediate autocrine IL-1β production in some leukemias by stimulating signal transduction pathways that activate the IL-1β promoter.Citation15 Furthermore, anti-inflammatory therapy with canakinumab, which targets IL-1β, significantly reduced incident lung cancer and lung cancer mortality.Citation16
Results from animal models and additional clinical findings support the notion that elevated IL-1Ra levels would antagonize disease progression in cancer.Citation4,Citation17 In advanced colorectal cancer, circulating IL-1Ra levels could act as a natural inhibitor of tumor-mediated IL-1α activity.Citation17-Citation19 The physiological response to the tumor, including upregulation of circulating IL-1Ra, is likely an important mechanism of antagonizing disease progression. However, over time, endogenous IL-1α antagonism could be expected to exert selection pressure on the tumor with respect to the dependence on IL-1 signaling. This selection pressure could result in the outgrowth of a tumor phenotype that is less susceptible to IL-1α antagonism. Alternatively, endogenous IL-1Ra might dampen the response of non-tumor cells in the vicinity of the malignancy. When tumors enlarge and the vascularization fails to sufficiently supply them, the tumor cells become stressed (due to hypoxia) and release IL-1alpha. With continued stress due to hypoxia, the tumor cells undergo necrotic death. The released IL-1alpha can now trigger the IL-1R1 on neighboring cells. This is the “alarmin” property.Citation20 Necrotic cells thus evoke inflammation, which brings in neutrophils and inflammatory cells to the area. These effects would be attenuated in the presence of endogenous IL-1Ra.
The bermekimab monoclonal antibody is derived from a natural human immune response. Primary sequence analysis of bermekimab shows that it underwent affinity maturation in vivo, and consequently has high binding affinity and specificity for IL-1α.3 The central mechanism of action for bermekimab in vivo is expected to be its neutralization of the biological activity of IL-1α, including blocking the inflammatory induction of VEGFs and matrix metalloproteinases in order to inhibit neoangiogenesis and stromal remodeling in the tumor microenvironment.Citation21 But IL-1α is commonly expressed on tumor cells where it is associated with invasiveness and dedifferentiation.Citation18,Citation19 Consequently, on tumor cells, IL-1α could act as a target, whereby bermekimab could mediate direct inhibition of tumors. As an IgG1 subclass, bermekimab activity could include antibody-directed, cell-mediated cytotoxicity, although there is no current evidence that this mechanism plays a significant role in the therapeutic activity of the antibody.
Low IL-6 levels (dichotomized at 10 pg/ml, consistent with a previous investigation in colorectal cancerCitation22) also tracked with response in the bermekimab-treated, but not in the placebo-treated group; 38% versus 16% achieved the primary endpoint in these groups, respectively (p = 0.002) (). When patients were stratified based on baseline IL-6 < 10 pg/ml (low IL-6) combined with IL-1Ra < 940 pg/ml (low IL-1Ra), the rate of achieving the primary endpoint was 46% versus 17% for bermekimab vs placebo arms, respectively (p = 0.007). It is unclear why low IL-6 levels would track with responsiveness but, since both low IL-1Ra and low IL-6 correlated with achievement of the primary endpoint in bermekimab-treated patients, it is possible that IL-1Ra and IL-6 have a common pertinent mechanism underlying their production. Indeed previous studies have suggested that both IL-1Ra and IL-6 increase in response to inflammation and that IL-6 can enhance plasma levels of IL-1Ra.Citation23,Citation24
The current findings confirm a significant association between pre-treatment circulating IL-1Ra levels and bermekimab responsiveness. Subjects who were relatively “naive” in terms of IL-1Ra antagonism were more likely to respond to therapy. The relatively lower level of plasma IL-1Ra could mean that there are less IL-1R1 occupied by IL-1Ra and this will make IL-1α (or IL-1β) more likely to be active. The lower plasma levels also may mean that the patients are not producing the level they should in response to their cancer and this is a reflection of why they need more IL-1Ra or more anti-IL-1α for disease control. Therefore, those who had more elevated pre-existing antagonist activity had disease progression that was relatively resistant to pharmacological IL-1α inhibition when presented with bermekimab. The notion that cell-mediated immune-surveillance might result in selection pressure on tumors, particularly as evidenced by loss of HLA expression in advanced disease, has been suggested (and contested) for several decades.Citation25 More recently, emergence of defects in interferon-receptor signaling and in antigen presentation due to selection pressure has been described as a mechanism of secondary resistance to checkpoint blockade.Citation26 Reports of IL-1Ra association with various malignancies adds another point of reference to the immunological and more specifically inflammasome in the natural history of cancer. The findings presented here provide important additional evidence that regulators of innate immunity may constitute a mechanism of selection pressure on tumors. This selection pressure could lead to yet undefined phenotypic changes that are part of the natural history of the disease and which possibly impact the effects of treatments that modulate the inflammatory response to the disease – notably in this case that of bermekimab.
Disclosure of Potential Conflicts of Interest
Razelle Kurzrock receives research funding from Genentech, Merck, Serono, Pfizer, Sequenom, Foundation Medicine, Konica Minolta, Grifols, Omniseq, and Guardant, as well as consultant fees from LOXO, NeoMed, XBiotech, and Actuate Therapeutics. She receives speaker fees from Roche, and has an equity interest in IDbyDNA and Curematch Inc. Tamas Hickish has received research funding, paid to his institution, from XBiotech as well as Roche and Abbvie. Lucjan Wyrwicz has received consulting fees from Halozyme, Eisai, Amgen and Roche and has received speaker fees from Amgen, Roche and Sanofi. Mark Saunders has received honoraria payments from Roche, Servier, Merck and Amgen. John Simard, Prasant Mohanty, Michael Stecher and Qian Wu were employees of XBiotech at the time of study conduct and John Simard, Michael Stecher and Qian Wu hold stock and or stock options of XBiotech. John Simard holds patents related to anti-interleukin 1α therapy. All other authors declare no competing interests.
Additional information
Funding
References
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi:10.1182/blood-2010-07-273417. Epub 2011 Feb 8.
- Dinarello CA, Arend W, Sims J, Smith D, Blumberg H, O’Neill L, Goldbach-Mansky R, Pizarro T, Hoffman H, Bufler P, et al. IL-family nomenclature. Nat Immunol. 2010;11:973. doi:10.1038/ni1110-973.
- Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–666. doi:10.1016/S1470-2045(14)70155-X. Epub 2014 Apr 17.
- Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–329. doi:10.1007/s10555-010-9229-0.
- Kurzrock R, Wetzler M, Estrov Z, Talpaz M. Interleukin-1 and its inhibitors: a biologic and therapeutic model for the role of growth regulatory factors in leukemias. Cytokines Mol Ther. 1995;1:177–184.
- Ito H, Miki C. Profile of circulating levels of interleukin-1 receptor antagonist and interleukin-6 in colorectal cancer patients. Scand J Gastroenterol. 1999;34:1139–1143. doi:10.1080/003655299750024959.
- Iwagaki H, Hizuta A, Tanaka N. Interleukin-1 receptor antagonists and other markers in colorectal cancer patients. Scand J Gastroenterol. 1997;32:577–581. doi:10.3109/00365529709025103.
- Timper K, Seelig E, Tsakiris DA, Donath MY. Safety, pharmacokinetics, and preliminary efficacy of a specific anti-IL-1alpha therapeutic antibody (MABp1) in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2015;29:955–960. doi:10.1016/j.jdiacomp.2015.05.019. Epub 2015 Jun 3.
- Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi:10.1016/S1470-2045(17)30006-2. Epub 2017 Jan 14.
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi:10.1016/j.immuni.2013.11.010.
- Horst R, Jaeger M, Smeekens SP, Oosting M, Swetz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AFM, Lemmers H, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167:111–24. e13. doi:10.1016/j.cell.2016.10.018.
- Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi:10.1002/cncr.20672.
- Kurzrock R. Cytokine deregulation in cancer. Biomed Pharmacother. 2001;55:543–547.
- Wetzler M, Kurzrock R, Estrov Z, Kantarjian H, Gisslinger H, Underbrink MP, Talpaz M. Altered levels of interleukin-1 beta and interleukin-1 receptor antagonist in chronic myelogenous leukemia: clinical and prognostic correlates. Blood. 1994;84:3142–3147.
- Beaupre DM, Talpaz M, Marini FC 3rd, Cristiano RJ, Roth JA, Estrov Z, Albitar M, Freedman MH, Kurzrock R. Autocrine interleukin-1beta production in leukemia: evidence for the involvement of mutated RAS. Cancer Res. 1999;59:2971–2980.
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, CANTOS Trial Group. Effect of interleukin-1ß inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomized, double-blind, placebo-controlled trial. Lancet. 2017 Oct 21;390(10105):1833–1842. doi:10.1016/S0140-6736(17)32247-X. Epub 2017 Aug 27.
- Dinarello CA. An expanding role for interleukin-1 blockade from gout to cancer. Mol Med. 2014;20(Suppl 1):S43–S58. doi:10.2119/molmed.2014.00232.
- Ricote M, García-Tuñón I, Bethencourt FR, Fraile B, Paniagua R, Royuela M. Interleukin-1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer. 2004;100:1388–1396. doi:10.1002/cncr.20142.
- Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, Czerwenka K, Schreiber M, Seifert M, Kubista E. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res. 2003;15(9):4877–4883.
- Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin-1alpha precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013;4:391. doi:10.3389/fimmu.2013.00391.
- Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi:10.1096/fj.02-0134fje. Epub 2002 Jul 18.
- Yeh KY, Li YY, Hsieh LL, Lu CH, Chou WC, Liaw CC, Tang RP, Liao SK. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol. 2010;40:580–587. doi:10.1093/jjco/hyq010. Epub 2010 Mar 1.
- Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99(16):2079–2084.
- Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL1Ra, IL-10 and cortisol in humans. Am J Physciol Endocrinol Metab. 2003;285:E433–E437. doi:10.1152/ajpendo.00074.2003.
- Moller P, Hammerling GJ. The role of surface HLA-A,B,C molecules in tumour immunity. Cancer Surv. 1992;13:101–127.
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi:10.1056/NEJMoa1604958. Epub 2016 Jul 13.
