?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Granulysin is a protein present in the granules of human cytotoxic T lymphocytes (CTL) and natural killer (NK) cells, with cytolytic activity against microbes and tumors. Previous work demonstrated the therapeutic effect of intratumoral injection of recombinant granulysin using in vivo models of breast cancer and multiple myeloma. In the present work we have developed a granulysin gene fusion to the anti-carcinoembryonic antigen (CEA/CEACAM5) single chain Fv antibody fragment MFE23. Both granulysin and the granulysin-based immunotoxin were expressed in Pichia pastoris. The immunotoxin specifically recognized CEA, purified or expressed on the cell surface. Moreover, the bioactivity of the immunotoxin against several CEA+ cell lines was higher than that of granulysin alone. Granulysin and the immunotoxin were tested as a treatment in in vivo xenograft models in athymic mice. When injected intratumorally, both granulysin and the immunotoxin were able to inhibit tumor growth. Furthermore, systemic administration of the immunotoxin demonstrated a decrease in tumor growth in a CEA+ tumor-bearing mouse model, whereas granulysin did not exhibit a therapeutic effect. This is the first granulysin-based immunotoxin and the present work constitutes the proof of concept of its therapeutic potential.
Introduction
Granulysin is a protein with two isoforms, of 9 and 15 kDa respectively.Citation1 While the 9 kDa isoform is a cytolytic protein present in the granules of activated human cytotoxic T lymphocytes (CTL) and natural killer (NK) cells,Citation1 the 15 kDa isoform is able to induce the differentiation of monocytes to dendritic cells.Citation2 In addition, it has been reported the antimicrobial effects of 15 kDa granulysinCitation3 and the activation-induced and selective release of both isoforms.Citation4 The main role of the 9 kDa cytotoxic isoform is the killing of intracellular bacteria such as M. Tuberculosis in concert with perforin.Citation5 Recently, it has been also suggested that granulysin makes pores on the surface of bacteria and protozoan parasites, facilitating lysis exerted by granzyme B.Citation6,Citation7
Following the original observation on the ability of recombinant granulysin to kill tumor cells,Citation1 our group demonstrated that 9 kDa granulysin was able to induce apoptotic cell death in several types of tumor cells, such as the acute T cell leukemia Jurkat, several human multiple myeloma cell lines, and also on cells from B-cell chronic lymphocytic leukemia (B-CLL) patients.Citation8–Citation10 More recently, we demonstrated the efficacy of recombinant granulysin in two xenotransplantation models of human tumors in athymic mice, the mammary adenocarcinoma MDA-MB-231 and the multiple myeloma NCI-H929.Citation11,Citation12 The granulysin anti-tumoral effect in these models correlated with apoptosis induction in the tumor tissue and with a prominent NK cell infiltration into the tumor mass, indicating that granulysin-induced tumor cell death in vivo could be immunogenic. In the same study, we also demonstrated the lack of secondary effects of granulysin alone. In that study, human tumors were injected subcutaneolsy in mice, and treatments were performed by intratumoral injection. This type of treatment could be difficult to perform in internal tumors, in which a systemic treatment would be more indicated. However, systemic administration of cytotoxic agents may provoke severe off-tumor side effects. Therefore, targeted delivery of granulysin could be an alternative to increase its therapeutic index. Immunotoxins combine the cell-killing ability of a cytotoxin with the specificity of a monoclonal antibody, overcoming the limitations of each.Citation13 Single chain Fv (scFv) antibody fragments are best suited to deliver a toxic payload, given their smaller size and increased tumor penetration compared to full IgG.Citation14–Citation16
Indeed, scFv have been genetically linked to bacterial, fungal or plant toxins to obtain the so-called recombinant immunotoxins, tested in numerous clinical trials.Citation17–Citation20 Moreover, on September 2018, the U.S. Food and Drug Administration approved the first antibody-based immunotoxin, moxetumomab pasudotox, for the treatment of hairy cell leukemia.Citation21 The drug is a recombinant immunotoxin composed of a scFv anti-CD22 fused to a truncated form of Pseudomonas exotoxin A. Another fusion protein based on the same toxin and directed against EpCAM has entered Phase 3 in a bladder cancer clinical trial.Citation22
The carcinoembryonic antigen (CEA), also known as CEACAM5,Citation23 was originally described in colorectal cancer (CRC)Citation24 as one of the first ever identified tumor-associated antigens, although its expression at lower levels has been reported in normal tissues. The heterogenous CEACAM (CEA-related cell adhesion molecule) family belongs to the immunoglobulin superfamily and comprises 12 genes in humans, at least three of them expressed in epithelia: CEACAM1, CEACAM5 and CEACAM6.Citation25,Citation26 The functions attributed to CEA family members are very diverse and include modulation of tumor growth, metastasis, innate and adaptive immunity, angiogenesis, and host–microbial interaction.Citation25–Citation27 CEA is a GPI anchored molecule released by proteolytic and non-proteolytic mechanisms.Citation28 In fact, serum levels of CEA are useful in predicting prognosis and monitoring response to treatment in CRC.Citation29 Of note, the antitumoral effect of CEA-targeted therapies is not inhibited by soluble CEA, as assessed with bispecific anti-CEA x anti-CD3 antibodiesCitation30 and CAR T cells containing an anti-CEA scFv as targeting domain.Citation31 MFE23 is a scFv produced by phage display technology that binds to CEA with high affinity and has distinct tumor targeting advantages.Citation32–Citation34 Recently, a trimerbody based on the MFE23 scFv has been produced with high yield and activity in the yeast P. pastoris.Citation35 Although cross-reactivity of MFE23 with other CEACAM family molecules bearing similar N-terminal domains (such as CEACAM1 and CEACAM6) has not been addressed, their similar expression pattern may preclude recognition of non-targeted tissues.
In the present work, we have designed and expressed a human granulysin fusion to MFE23 as a proof of concept, and tested its antiumoral potential, both in vitro and in vivo.
Results
Design of plasmids for the mammalian production of recombinant granulysin and MFE23-granulysin immunotoxin
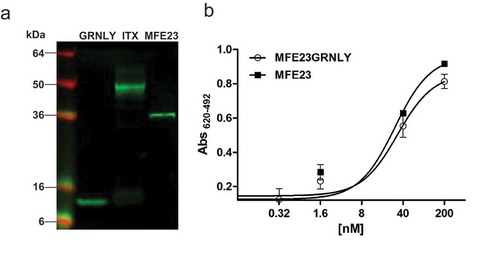
Initially, two pCR3.1-based constructs were designed for expression in mammalian cells, one comprising the 9 kDa granulysin (GRNLY) gene alone, and the other one encoding the anti-CEA scFv MF23 bound through a flexible linker of 21 aa to granulysin (). Both included a tag of six histidines to facilitate subsequent detection and purification. The two constructs were efficiently secreted to the supernatant of transfected 293T cells. The migration pattern of the immunotoxin, as assessed by Western blot analysis under reducing conditions, was consistent with its molecular weight calculated from their amino acid sequences (around 40 kDa; Suppl. Figure 1, left panel). Additionally, the MFE23-GRNLY immunotoxin in the supernatant specifically recognized human CEA immobilized on plastic, as determined by ELISA (Suppl. Figure 1, right panel).
Figure 1. Schematic representations and three-dimensional models of GRNLY and MFE23-GRNLY. The α-factor signal peptide is used to direct secretion of the recombinant proteins in P. pastoris, and the 6xHis tag is appended for immunodetection and affinity purification, respectively. Models were prepared by A. Blanco.
Production and purification of the recombinant proteins in Pichia pastoris
Subsequently, GRNLY and MFE23-GRNLY were subcloned into the pPICZalphaA vector for production in yeast cells. Both constructs were secreted to the P. pastoris supernatant, as demostrated by Western blot and ELISA (data not shown). For purification, the extracellular medium of P. pastoris cells was collected after 72 hours of methanol induction. Both GRNLY and MFE23-GRNLY were purified by immobilized metal affinity chromatography, with yields around 4 mg/L and 2 mg/L, respectively. The migration pattern of the secreted MFE23-GRNLY was consistent with the molecular weight calculated from the addition of those of GRNLY and MFE23, as assessed by reducing Western Blot (). Importantly, the yeast-produced MFE23-GRNLY was functional and recognized efficiently plastic immobilized CEA by ELISA. As shown in , the CEA binding curves of purified MFE23-GRNLY and MFE23 to CEA were equivalently dose-dependent and saturable.
Immunotoxin binding to CEA expressed on the cell surface
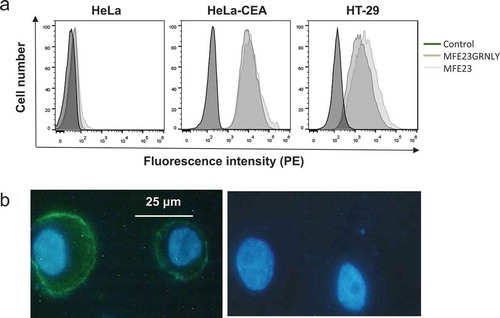
To address if the immunotoxin could bind its antigen on the cell surface, we performed flow cytometry on HeLa cells (negative control), HT-29 cells, a CEA+ colon carcinoma cell lineCitation36 and HeLa cells stably transfected with CEA (HeLa-CEA).Citation37 As shown in , incubation with the immunotoxin or with MFE23, followed by anti-His tag mouse antibody and anti-mouse IgG-PE antibody, yielded a specific labeling of both cell lines. Of note, the mean fluorescence intensity of HT-29 cells stained with the immunotoxin was slightly lower than that of MFE23, but it was equivalent in HeLa-CEA cells. We confirmed this observation using the immunotoxin in fluorescence microscopy assays on HT-29 cells in which nuclei were labelled with Hoechst 33342 (blue fluorescence). As shown in , left panel, CEA staining is observed as green fluorescence mainly on the surface of HT-29 cells.
Figure 3. MFE23-GRNLY binding to CEA expressed on the cell surface. (a) HeLa, HeLa-CEA or HT-29 cells were stained with MFE23 or MFE23GRNLY, as indicated. Dark-gray histograms correspond to the negative controls. (b) Fluorescence images of HT-29 cells stained with (left) or without MFE23GRNLY (right), followed by mouse anti-His-tag and anti-mouse IgG-FITC antibodies. Nuclei were stained with Hoechst 33342.
In vitro cytotoxic effect of MFE23-GRNLY AND GRNLY
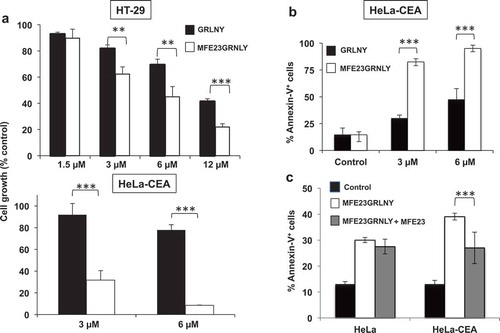
Next, we addressed if the immunotoxin could promote preferential killing of the CEA-positive cell lines HT-29 and HeLa-CEA in vitro compared with granulysin alone. Indeed, we observed that the MFE23-GRNLY immunotoxin exhibited a higher cytotoxicity than granulysin alone on HT-29 cells, reducing the IC50 from 12 to 6 µM (, upper panel). At 12 µM, the percentage of living cells treated with granulysin after 24 h was twice that of HT-29 cells treated with the immunotoxin (41% vs. 20%, respectively). We also demonstrated the enhanced cytotoxicity of the immunotoxin compared with granulysin alone on HeLa-CEA cells, and observed that this effect was even higher than that observed in HT-29 cells: the IC50 was around 6 µM for granulysin alone, while it was lower than 3 µM for MFE23GRNLY (, lower panel). In fact, a 6 µM concentration of the immunotoxin caused almost 100% of HeLa-CEA cell death in vitro. We also demonstrated that this massive cell death induction by the immunotoxin on HeLa-CEA cells was apoptotic, characterized by phosphatidylserine exposure on the plasma membrane, as assessed by annexin-V-FITC staining (). To ascertain that the increase in cytotoxicity observed with MFE23GRNLY was dependent on CEA expression on target cells, we have used MFE23 to block CEA in HeLa-CEA cells before adding MFE23GRNLY. As shown in , MFE23 preincubation did not inhibit significantly the basal cytotoxicity of MFE23GRNLY on HeLa cells. On the other hand, the cytotoxicity of MFE23GRNLY on HeLa-CEA cells was reduced by MFE23 close to the level observed in HeLa cells.
Figure 4. In vitro cytotoxicity of GRNLY and MFE23-GRNLY on HT-29 and HeLa-CEA cells. (a) HT-29 or HeLa-CEA cells were incubated with increasing concentrations of recombinant granulysin (black bars) or of the chimeric MFE23-GRNLY (white bars) during 24h. Cell growth was estimated using the MTT reduction method and results shown the percentage of the growth of control, untreated cells. (b) Apoptotic cell death induced by granulysin or by MFE23-GRNLY on HeLa-CEA cells was determined by detection of PS exposure by staining with Annexin-V-FITC and flow cytometry. (c) HeLa or HeLa-CEA cells were preincubated or not at 4ºC during 1 h with a supernatant containing MFE23, and then treated with 2 µM MFE23GRNLY during 24 h at 37ºC, as indicated. Results are the mean ± SD of at least 2 different experiments made in duplicate. **, P< .02; ***, P< .01.
Therapeutic activity of intratumoral MFE23-GRNLY and GRNLY
Once ascertained the in vitro functional activity of the MFE23-GRNLY immunotoxin, we sought to demonstrate its anti-tumoral potential in vivo. In a previous study, we had reported the therapeutic effect of the intratumoral injection of GRNLY.Citation11 To assess the effect of MFE23-GRNLY immunotoxin in this context, HT-29 cells were injected subcutaneously (s.c.) in athymic mice and when tumor volume reached 100 mm3, mice were treated every two days with intratumoral injections of PBS (control group), GRNLY or MFE23GRNLY. Overall, immunotoxin inhibited the increase in tumor size compared with the control group, with statistically significant differences from the third injection onwards. In fact, average tumor volume in the MFE23GRNLY group at the end of the experiment was less than 50% with respect to that of the control group (). Similar results were obtained if the volumes () or the weights () of the resected tumors in each group were compared. Differences between the effect of the immunotoxin and GRNLY alone were not statistically significant.
Figure 5. Granulysin or MFE23GRNLY intratumoral treatment of athymic mice bearing HT-29 cancer xenografts. (a) 2 × 106 HT-29 colon carcinoma cells were injected s.c. in athymic mice. When tumor volume had arrived to 0.1 cm3, mice were divided in three groups, control (black squares), treated with recombinant granulysin (white triangles) or treated with MFE23GRNLY (white circles). Mice in each group received intratumoral injections of PBS, GRNLY or MFE23GRNLY, respectively, every 2 days for 10 times and two days later mice were sacrificed. Data are the mean±SD of the tumor volume in each group of the study. *, P < .05. (b) Tumor volumes after resection in the three experimental groups described in A. *, P < .05. (c) Tumor weights after resection in the three experimental groups described in A. *, P < .05.
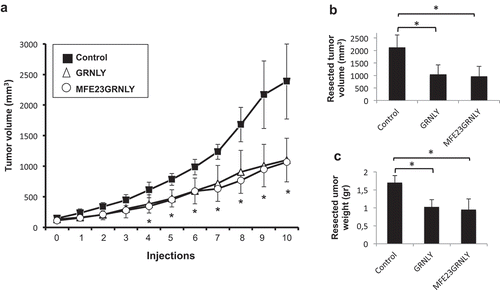
Two days after the last injections, mice were euthanised and histological studies were performed. As shown in Supplemental , detection of activated caspase-3 and DAPI nuclear staining were highly suggestive of apoptosis induction in tumors treated with GRNLY or MFE23GRNLY and recapitulated the results obtained previously in the mammary adenocarcinoma and multiple myeloma in vivo models.Citation11
Anti-tumor effect of MFE23GRNLY upon systemic administration
Intratumoral injection is frequently unfeasible in a clinical context. In fact, the rationale behind immunotoxin design was the targeted delivery of granulysin after systemic administration. Hence, we have next used HeLa-CEA cells xenotransplanted in athymic mice to test the effect of systemic treatment with GRNLY or with MFE23GRNLY.
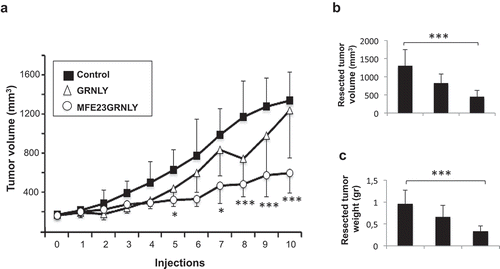
As shown in , when recombinant GRNLY was administered intraperitoneally (i.p.) to HeLa-CEA tumor-bearing mice, it did not result in a significant reduction in tumor size. On the contrary, MFE23GRNLY clearly decreased tumor size, being this reduction statistically significant from the 5th injection onwards (). Relative to the control group, mean tumor size was reduced close to 60% by the immunotoxin at the end of the experiment (). This percentage was increased to 70% when the size of the resected tumors was analyzed (), or the weights of the resected tumors determined (). Systemic GRNLY treatment also resulted in size and weight reduction of the resected tumors (38% and 35%, respectively), but the differences with the control group were not statistically significant.
Figure 6. Systemic treatment of athymic mice bearing HeLa-CEA cancer xenografts. (a) 3 × 106 HeLa-CEA cells suspended in Matrigel were injected s.c. in athymic mice. When tumor volume had arrived to 0.175 cm3, mice were divided in three groups, control (black squares), treated with recombinant granulysin (white triangles) or treated with MFE23GRNLY (white circles). Mice in each group received intraperitoneal injections of PBS, GRNLY or MFE23GRNLY, respectively, every 2 days for 10 times and two days later mice were sacrificed. Data are the mean±SD of the tumor volume in each group of the study. *, P < .05; ***, P < .01. b and c. Tumor sizes (b) and tumor weights (c) after resection in the three experimental groups of the experiment shown in A. ***, P < .01.
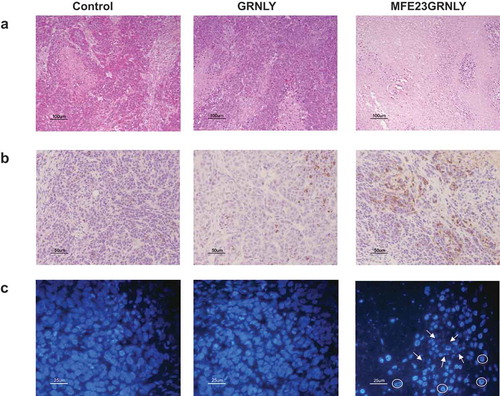
Histological studies on tissue sections obtained from resected tumors were also performed. As shown in the hematoxilin/eosin images (), untreated tumors from the control group or from the GRNLY-treated group presented the typical compact tissue structure of a fast-growing tumor. On the contrary, systemic injection of MFE23GRNLY caused a dramatic loss of cellularity in HeLa-CEA derived tumors (right panel of ). Active caspase-3 immunochemistry gave some limited labeling in GRNLY-treated tumors (middle panel of ), but this staining was much more extensive in the case of MFE23GRNLY-treated mice (right panel of ). Upon DAPI labeling, untreated tumors or tumors treated by systemic GRNLY injection displayed nuclei with non-condensed chromatin (, left and middle panels, respectively). On the contrary, MFE23GRNLY-treated tumors showed less cellularity, condensed chromatin and fragmented nuclei (indicated with arrows in the right panel of ). In addition, they also showed numerous cells with condensed chromatin concentrated in the nuclear margins (indicated with circles in the same image). This nuclear morphology is characteristic of apoptosis-inducing factor (AIF)-mediated cell death.Citation10
Figure 7. Histochemistry and immunohistochemistry in tissue sections of HeLa-CEA-derived tumors. (a) Representative images of H&E staining on sections form resected HeLa-CEA-derived tumors from the control group, from the granulysin-treated (GRNLY) or the MFE23GRNLY-treated groups of the experiment shown in Figure 7. (b) Tumor sections were incubated with an antibody against active caspase-3 and revealed by DAB staining. (c) Nuclei were stained using DAPI and photographed in a fluorescence microscope. Arrows indicate apoptotic, fragmented nuclei and circles indicate marginated chromatin nuclear phenotype.
Discussion
We demonstrated previously the efficacy of recombinant granulysin in two xenotransplantation models of human tumors in athymic mice.Citation11,Citation12 In that study, human tumors were injected subcutaneolsy in mice, and treatments were performed by intratumoral injection. In the present study, we have explored the therapeutic potential of a systemically administered immunotoxin containing the anti-CEA scFv MFE23 for the targeted delivery of human granulysin. First of all, the production of granulysin and of MFE23GRNLY in the yeast Pichia pastoris was optimized. Recombinant proteins obtained in this way are devoid of bacterial endotoxin and are amenable to clinical use,Citation38 while those produced in E. coli contain LPS (seeCitation11 for granulysin itself). In addition, we obtained a better granulysin production yield than in E. coli (8 vs 2 mg per batch).
We demonstrated in vitro by several methods that the immunotoxin retained its specific binding to CEA, either immobilized on plastic or expressed on the cell surface. Binding curves of MFE23 and MFE23GRNLY were overlapping, demonstrating that fusion to granulysin did not affect the binding capacity of the scFv. Moreover, the immunotoxin increased granulysin cytotoxicity in vitro against several CEA-expressing cell lines. In vivo experiments using human tumor xenografts in athymic mice demonstrated that the intratumoral injection of the immunotoxin recapitulated the in vivo therapeutic effect of granulysin. Most importantly, the systemic administration of MFE23GRNLY exhibited an antitumoral effect against CEA+ tumors at a relatively low dose, that was absent in mice treated with granulysin.
The 1st and 2nd generation of immunotoxins were produced by chemical conjugation of bacterial or plant toxins to full length IgG. Taking advantage of recombinant DNA technology, 3rd generation immunotoxins were obtained by genetically fusion of an antibody fragment to the cytotoxic domain of a toxin. The recently approved moxetumomab pasudotox belongs to this category. Such immunotoxins are more homogeneous and stable, and have better tumor penetration due to their smaller size, but are still hampered by immunogenicity.Citation39 The immune reaction against the toxin moiety in patients with a normal immune system may reduce their clinical success, and different approaches for toxin deimmunization have been proposed.Citation40 As an alternative, 4th generation immunotoxins, known as cytolytic fusion proteins or humanized immunotoxins, replace foreign toxins with human pro-apototic proteins.Citation41 Granzyme B-based immunotoxins have been generated with therapeutic effect in preclinical models in vivo.Citation42 In this line, the granulysin-based immunotoxin described here should be devoid of immunogenicity due to the cytotoxic payload, since granulysin is also a human protein. Toxicity associated with immunotoxins can be either nonspecific or targeted.
We have shown previously that granulysin, the toxic moiety of the MFE23GRNLY immunotoxin, lacks toxicity on human PBMC.Citation8 We have also shown that granulysin has not undesirable side effects in mice, neither on the dermis/epidermis when injected s.c. nor on different organs when injected systemically, ruling out nonspecific toxicity.Citation11 The second type of toxicity of immunotoxins can be caused by the expression in normal tissues of the antigen recognized by the antibody (on target/off tumor side effects). The potential of MFE23 for discrimination between tumor and normal tissues has been demonstrated using radioiodinated antibody.Citation43 123I-labeled MFE23 showed to be safe when given intravenously to patients and to localize selectively to gastrointestinal cancers.Citation32 MFE23 has also been used in an antibody-directed enzyme prodrug clinical trial and has been found safe and well tolerated in patients, although there is some expression of CEA in normal colon. Ex vivo quantitative measurements showed that local concentrations of up to 2 µg/g can be found in some areas of tumor, while in normal mucosa levels are normally below 30 ng/g.Citation44 Furthermore, CEA in normal colon is on the apical cell surface and is a priori poorly accessible to antibodies administered systemically.
To our knowledge, this is the first granulysin-based immunotoxin and our results constitute the proof of concept of its therapeutic potential. In summary, the combination of selective cytotoxicity, high activity, and lack of immunogenicity defines this new class of immunotoxins as good candidates for development as anti-tumoral therapeutic agents.
Materials and methods
Cell culture
Jurkat cells, obtained from the ATCC, were cultured in RPMI 1640 medium supplemented with 5% FBS (Pan Biotech, Aidenbach, Germany) at 37ºC and 5% CO2 using standard procedures. The CEA-positive HT-29 colon adenocarcinoma cell line and HeLa cervix carcinoma stably transfected to express CEA (HeLa-CEA cells) were cultured in DMEM medium (Pan Biotech GmbH) supplemented with 10% FBS (Sigma). In all cases, culture media were supplemented with penicillin/streptomycin (Pan Biotech) and GlutaMAX (Invitrogen, Barcelona). All cell lines were routinely tested for mycoplasma contamination by PCR.
Bacterial strains, plasmids, and culture conditions
Escherichia coli was grown at 37°C in Luria-Bertani medium (LB; Oxoid, Basingstoke, UK). Pichia pastoris was grown at 30°C in YPD broth (Formedium) for routine maintainance and in BMGY medium (1% yeast extract, 2% peptone, 1.34% YNB, 1% glycerol, 400 μg/L biotin, and 0.1 M potassium phosphate, pH 6.0) for expansion and big scale production, followed by cultured at 18ºC in BMMY medium (1% yeast extract, 2% peptone, 1.34% YNB, 1% methanol, 400 μg/L biotin, and 0.1 M potassium phosphate, pH 6.0) for induction of the recombinant protein.
Construction of mammalian expression vectors and transient expression in 293T cells
Synthetic genes encoding 6xHis-tagged 9 kDa granulysin were synthesized by Geneart GmbH (Thermo Fisher Scientific, Regensburg, Germany) and subcloned as ClaI/XbaI or NruI/XbaI into pCR3.1-MFE23-NC1Citation45 resulting in pCR3.1-GRNLY and pCR3.1- MFE23-GRNLY, respectively. 293T cells were transiently transfected with the appropriate vector using calcium phosphate, and supernatants were analyzed for protein expression by ELISA and western blotting.
Construction of expression vectors and transformation of pichia pastoris
The ClaI/XbaI-digested fragments of pCR3.1-GRNLY and pCR3.1-MFE23-GRNLY were ligated into the ClaI/XbaI-digested backbone of plasmid pPICZαA to obtain pPICZαA-GRNLY and pPICZαA-MFE23-GRNLY. Both plasmids were amplificated in E.coli and isolated by NucleoSpin® Plasmid EasyPure (Macherey-Nagel). Plasmids were linearized with SacI (Takara) and purified by Ilustra™ GFX™ PCR DNA and Gel Band Purification kit (GE Healthcare). Generation of electrocompetent yeast cells, transformation of P. pastoris and colonies selection was carried out according to the method described in.Citation46
Expression and purification of recombinant granulysin and MFE23-granulysin immunotoxin in Pichia pastoris
The P. pastoris cells strain SMD1168 was cultured in BMGY medium at 30°C in a shaking incubator (250 rpm) overnight for growth and in BMMY medium at 18°C in a shaking incubator (250 rpm) overnight for induction. The culture was fed with 1% methanol every 24 h for 2 or 3 days, then, supernatants were concentrated using Pellicon® XL Ultracel 0,005 m2 cassettes (MerckMillipore) and proteins were purified by Ni2+ affinity chromatography (Ni-NTA agarosa, Qiagen). Eventually, eluate was concentrated and its buffer was changed using Amicon® filters (MerckMillipore) to PBS.
Elisa
The ability of MFE23-granulysin immunotoxin to bind human CEA was studied by ELISA as previously described.Citation37 Briefly, Maxisorp plates (Nunc A/S, Roskilde, Denmark) were coated with 0.25 μg/well CEA (Sigma-Aldrich, St. Louis, MO, USA) and after washing and blocking with 5% BSA in PBS, 100 μl of the indicated amount of purified protein was added for 1 hour at room temperature. After three washes, 1 μg/well mouse Penta·His Antibody (Qiagen GmbH, Hilden, Germany) was added for 1 hour at room temperature. After three washes, 100 μl of HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) was added for 1 hour at room temperature, after which the plate was washed and developed with OPD (Sigma).
Flow cytometry analysis of CEA binding
To analyze binding to the CEA antigen on the surface of living cells, 105 cells per well were placed in a 96-well round-bottom plate. First, cells were incubated with MF23 or with MFE23GRNLY (10 μg/ml) in PBS 5% FBS for 30 min at 4°C followed by mouse anti-histidine tag antibody (1:200; Genscript, Netherlands) and goat anti-mouse antibody bound to PE or FITC (1:200; Caltag, Barcelona). After each incubation, cells were washed with 5% FBS in PBS. Binding was determined on Jurkat cells (negative for CEA expression), and on CEA-positive HT-29 and HeLa-CEA cells using a FACScalibur flow cytometer (BD Biosciences).
Immunofluorescence staining
HT-29 cells (105 cells per well) were placed on a cover slip with poly-L-lysine in a 24-well plate and incubated at 4ºC with the immunotoxin and the corresponding antibodies as indicated above. Finally, cells were fixed with 4% paraformaldehyde and Hoechst 33342 staining was performed in order to visualize nuclei. Finally, cells were analyzed by fluorescence microscopy and photographed. Negative controls were carried out in the absence of MFE23GRNLY.
In vitro cytotoxicity assays
50 µl aliquots of 1x106/ml cell suspensions in complete medium were seeded per well in 96 well plates and GRNLY or MFE23GRNLY were added at the indicated concentrations. In control wells, the same volume of PBS was added. Cells were then incubated for 24 h at 37ºC and inhibition of cell growth and apoptotic cell death was analyzed. Cell growth was analyzed using the MTT reduction method adapted to microplates.Citation47 Briefly, cells were incubated at 37°C with 10 μl of MTT solution for 2–3 hours until formazan precipitate was visible. Then, MTT solvent was added and absorbance at 560 nm was read and compared with that of control wells. Cell death was analyzed by determination of phosphatidyl-serine exposure by flow cytometry after incubation with Annexin-V-FITC (BD Biosciences, Madrid) in annexin-binding buffer (140 mMNaCl, 2.5 mMCaCl2,10 mM Hepes/NaOH, pH 7.4) for 15 minutes.
In vivo experiments
Immune-deficient athymic mice, Swiss nu/nu strain, six-week-old males (Charles River), were used in this study. Mice experiments were performed according to the European recommendations on animal ethics and the University of Zaragoza Animal Experimentation Ethical Commission previously approved the housing and experimental protocols. Mice were kept under specific standard pathogen-free conditions (average ambient temperature 24°C, 12/12 h light/dark cycle) with water and food provided ad libitum throughout the study.
Tumor growth was analyzed by measuring the tumor daily with a precision caliper. To calculate the tumor volume, the width (A) and length (L) of the tumor were measured, and the following formula was applied:
At the end of the experiment (2 days after the last injection) mice were euthanized and the tumors were surgically excised, fixed in 10% buffered formalin and embedded in paraffin.
For intratumoral injection experiments, 2 × 106 HT-29 tumor cells were injected subcutaneously in nude mice (n = 5 mice per group). When the tumors reached a mean volume of 0.1 cm3, mice were treated with 2 nmol MFE23GRNLY or GRNLY in PBS every 2 days for 10 times. Mice in the control group received injections of PBS with the same time schedule.
In systemic treatments, 3 × 106 HeLa-CEA tumor cells suspended in Matrigel were injected subcutaneously in nude mice (n = 5 mice per group). When tumors reached a volume of 0.175 cm3, mice received intraperitoneal injections of 10 nmol GRNLY or MFE23GRNLY in PBS every 2 days for 10 times. Mice in the control group received injections of PBS with the same time schedule.
Histological and immunohistochemistry studies
Tissue sections 5 μm thick were deparaffinated, rehydrated and stained by immersing in GILL II Hematoxylin, followed by eosin staining. For the study of apoptotic nuclei, tissue sections were stained with DAPI Fluoromont-G (EMS, Madrid) for 10 min and detected in a fluorescence microscope (E600/E400, Nikon) equipped with a digital photography machine (DXM1200F, Nikon).
The expression of activated caspase-3 was investigated by immunohistochemistry using a rabbit polyclonal anti-human caspase-3 antibody (Cell Signaling, Barcelona), which recognizes the active, cleaved caspase-3 form. For antigen retrieval, the sections were boiled in 10 mM citrate buffer, pH 6.0 for 30 minutes. After blocking with 5% horse serum diluted in PBS for 1 hr at room temperature, sections were incubated at 4°C in humid chambers with the anti-caspase-3 antibody at 1/150 dilution for 1 hr followed by ready to use secondary anti-rabbit antibody (Vector Laboratories, Peterborough, UK) for 30 min. As a chromogenic substrate, DAB (Agilent, Madrid) was used, followed by hematoxylin counterstaining. Appropiate negative control stainings were also performed.
Statistical analysis
Computer-based statistical analysis was performed using GraphPad Prism 4.0 program (GrandPath Software Inc). Results are shown as mean±SD. Statistical significance was evaluated by using Student t test for non-paired variants. Differences were considered significant if P< .05.
Disclosure of potential conflicts of interest
The use of granulysin immunotoxins as an anti-tumoral treatment is protected by the patent application PCT/ES2018/P201830768 presented to the Spanish Bureau of Patents and Brands (OEPM) on 07/26/2018.
Supplemental Material
Download MS Word (793.2 KB)Acknowledgments
The authors thank Dr. María Royo and Amparo Gallur, Microscopy and HistoPathology Core Unit, Institute for Health Sciences of Aragon (Zaragoza, Spain), for their advice and technical support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol. 1997;158:1–11.
- Clayberger C, Finn MW, Wang T, Saini R, Wilson C, Barr VA, Sabatino M, Castiello L, Stroncek D, Krensky AM. 15 kDa granulysin causes differentiation of monocytes to dendritic cells but lacks cytotoxic activity. J Immunol. 2012;188:6119–6126. doi:10.4049/jimmunol.1200570.
- Wei H, Lin L, Wang C, Lee Y, Chen Y, Antimicrobial LY. Properties of an immunomodulator – 15 kDa human granulysin. PLoS One. 2016;11:e0156321. doi:10.1371/journal.pone.0156321.
- Lettau M, Dietz M, Dohmen K, Leippe M, Kabelitz D, Janssen O. Granulysin species segregate to different lysosome-related effector vesicles (LREV) and get mobilized by either classical or non-classical degranulation. Mol Immunol. 2019;107:44–53. doi:10.1016/j.molimm.2018.12.031.
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi:10.1126/science.282.5386.121.
- Dotiwala F, Mulik S, Polidoro R, Ansara J, Burleigh B, Walch M, Gazzinelli RT, Lieberman J. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nature Med. 2016;22:210–216. doi:10.1038/nm.4023.
- Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi:10.1016/j.cell.2014.03.040.
- Aporta A, Catalán E, Galán-Malo P, Ramírez-Labrada A, Pérez M, Azaceta G, Palomera L, Naval J, Marzo I, Pardo J, et al. Granulysin induces apoptotic cell death and cleavage of the autophagy regulator Atg5 in human hematological tumors. Biochem Pharmacol. 2014;87:410–423. doi:10.1016/j.bcp.2013.10.001.
- Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol. 1998;161:1758–1764.
- Pardo J, Pérez-Galán P, Gamen S, Marzo I, Monleón I, Kaspar AA, Susín SA, Kroemer G, Krensky AM, Naval J, Anel A. A role of the mitochondrial apoptosis-inducing factor (AIF) in granulysin-induced apoptosis. J Immunol. 2001;167:1222–1229. doi:10.4049/jimmunol.167.3.1222.
- Al-Wasaby S, de Miguel D, Aporta A, Naval J, Conde B, Martínez-Lostao L, Anel A. In vivo potential of recombinant granulysin against human tumors. OncoImmunol. 2015;4:e1036213. doi:10.1080/2162402X.2015.1036213.
- Martinez-Lostao L, de Miguel D, Al-Wasaby S, Gallego-Lleyda A, Anel A. Death ligands and granulysin: mechanisms of tumor cell death induction and therapeutic opportunities. Immunotherapy. 2015;7:883–892. doi:10.2217/imt.15.56.
- Pastan I, Hassan R, Fitzgerald D, Kreitman R. Immunotoxin therapy of cancer. Naure Rev Cancer. 2006;6:559–565. doi:10.1038/nrc1891.
- Chowdhury P, Viner J, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA. 1998;95:669–674. doi:10.1073/pnas.95.2.669.
- Deckert P. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr Drug Targets. 2009;10:158–175. doi:10.2174/138945009787354502.
- Sanz L, Kristensen P, Blanco B, Facteau S, Russell S, Winter G, Alvarez-Vallina L. Single-chain antibody-based gene therapy: inhibition of tumor growth by in situ production of phage-derived human antibody fragments blocking functionally active sites of cell-associated matrices. Gene Ther. 2002;9:1049–1053. doi:10.1038/sj.gt.3301725.
- Kreitman R, Stetler-Stevenso M, Jaffe E, Conlon K, Steinberg S, Wilson W, Waldmann TA, Pastan I. Complete remissions of adult T-cell leukemia with anti-CD25 recombinant immunotoxin LMB-2 and chemotherapy to block immunogenicity. Clin Cancer Res. 2016;22:310–318. doi:10.1158/1078-0432.CCR-15-1412.
- Kreitman R, Tallman M, Robak T, Coutre S, Wilson W, Stetler-Stevenson M, Fitzgerald DJ, Lechleider R, Pastan I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi:10.1200/JCO.2011.38.1756.
- Liu W, Onda M, Lee B, Kreitman R, Hassan R, Xiang L, Pastan I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA. 2012;109:11782–11787.
- Tomé-Amat J, Olombrada M, Ruiz-de-la-Herrán J, Pérez-Gómez E, Andradas C, Sánchez C, Martínez L, Martínez-del-Pozo A, Gavilanes JG, Lacadena J. Efficient in vivo antitumor effect of an immunotoxin based on ribotoxin α-sarcin in nude mice bearing human colorectal cancer xenografts. SpringerPlus. 2015;4:168. doi:10.1186/s40064-015-0943-5.
- Kreitman R, Dearden C, Zinzani P, Delgado J, Karlin L, Robak T, Gladstone DE, le Coutre P, Dietrich S, Gotic M, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi:10.1038/s41375-018-0210-1.
- Kowalski M, Guindon J, Brazas L, Moore C, Entwistle J, Cizeau J, Jewett MA, MacDonald GC. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188:1712–1718. doi:10.1016/j.juro.2012.07.020.
- Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A, Kuroki M, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi:10.1006/excr.1999.4610.
- Gold P, Freedman S. Demonstration of tumor specific antigen in human colon carcinoma by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi:10.1084/jem.121.3.439.
- Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010;8:12. doi:10.1186/1741-7007-8-12.
- Kuespert K, Pils S, Hauck C. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571.
- Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche C, Tegtmeyer N, Kalali B, Bach NC, Sieber SA, et al. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nature Microbiol. 2016;17:16189.
- Kinugasa T, Kuroki M, Yamanaka T, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y. Non-proteolytic release of carcinoembryonic antigen from normal human colonic epithelial cells cultured in collagen gel. Int J Cancer. 1994;58:102–107. doi:10.1002/ijc.2910580117.
- Duffy M, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Clinical utility of biochemical markers in colorectal cancer: European group on tumour markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–727.
- Lutterbuese R, Raum T, Kischel R, Lutterbuese P, Schlereth B, Schaller E, Mangold S, Rau D, Meier P, Kiener PA, et al. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother. 2009;32:341–352. doi:10.1097/CJI.0b013e31819b7c70.
- Gilham D, O’Neil A, Hughes C, Guest R, Kirillova N, Lehane M, Hawkins RE. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002;25:139–151.
- Begent R, Verhaar M, Chester K, Casey J, Green A, Napier M, Hope-Stone LD, Cushen N, Keep PA, Johnson CJ, et al. Clinical evidence of efficient tumor targeting based on single-chain Fv antibody selected from a combinatorial library. Nature Med. 1996;2:979–984.
- Boehm M, Corper A, Wan T, Sohi M, Sutton B, Thornton JD, Keep PA, Chester KA, Begent RH, Perkins SJ. Crystal structure of the anti-(carcinoembryonic antigen) single-chain Fv antibody MFE-23 and a model for antigen binding based on intermolecular contacts. Biochem J. 2000;346:519–528.
- Verhaar M, Chester K, Keep P, Robson L, Pedley R, Boden J, Hawkins RE, Begent RH. A single chain Fv derived from a filamentous phage library has distinct tumor targeting advantages over one derived from a hybridoma. Int J Cancer. 1995;61:497–501. doi:10.1002/ijc.2910610412.
- Blanco-Toribio A, Lacadena J, Nuñez-Prado N, Álvarez-Cienfuegos A, Villate M, Compte M, Sanz L, Blanco FJ, Álvarez-Vallina L. Efficient production of single-chain fragment variable-based N-terminal trimerbodies in Pichia pastoris. Microb Cell Fact. 2014;13:116. doi:10.1186/1475-2859-13-1.
- Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin Cancer Res. 2016;22:3286–3297. doi:10.1158/1078-0432.CCR-15-1696.
- Cuesta A, Sánchez-Martín D, Sanz L, Bonet J, Compte M, Kremer L, Blanco FJ, Oliva B, Alvarez-Vallina L. In vivo tumor targeting and imaging with engineered trivalent antibody fragments containing collagen-derived sequences. PLoS One. 2009;4:e5381. doi:10.1371/journal.pone.0005381.
- Daly R, Hearn M. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit. 2005;18:119–138. doi:10.1002/jmr.687.
- Mazor R, Onda M, Pastan I. Immunogenicity of therapeutic recombinant immunotoxins. Immunol Rev. 2016;270:152–164. doi:10.1111/imr.12390.
- Mazor R, King E, Pastan I. Strategies to reduce the immunogenicity of recombinant immunotoxins. Am J Pathol. 2018;188:1736–1743. doi:10.1016/j.ajpath.2018.04.016.
- Mathew M, Verma R. Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Sci. 2009;100:1359–1365. doi:10.1111/j.1349-7006.2009.01192.x.
- Hlongwane P, Mungra N, Madheswaran S, Akinrinmade O, Chetty S, Barth S. Human granzyme B based targeted cytolytic fusion proteins. Biomedicines. 2018;6:E72. doi:10.3390/biomedicines6020072.
- Chester K, Pedley B, Tolner B, Violet J, Mayer A, Sharma S, Boxer G, Green A, Nagl S, Begent RH. Engineering antibodies for clinical applications in cancer. Tumor Biol. 2004;25:91–98. doi:10.1159/000077727.
- Boxer G, Stuart-Smith S, Flynn A, Green A, Begent R. Radioimmunoluminography: a tool for relating tissue antigen concentration to clinical outcome. Br J Cancer. 1999;80:922–926. doi:10.1038/sj.bjc.6690443.
- Cuesta A, Sánchez-Martín D, Blanco-Toribio A, Villate M, Enciso-Álvarez K, Alvarez-Cienfuegos A, Sainz-Pastor N, Sanz L, Blanco FJ, Alvarez-Vallina L. Improved stability of multivalent antibodies containing the human collagen XV trimerization domain. MAbs. 2012;4:226–232. doi:10.4161/mabs.4.2.19140.
- Weidner M, Taupp M, Hallam S. Expression of recombinant proteins in the methylotrophic yeast Pichia pastoris. J Vis Exp. 2010;36:e1862.
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine D, Abbot BJ, Mayo JG, Shoemaker RH, Boyd MR. Sensibility of drug screening with panels of human tumor cell lines using microculture tetrazolium assay. Cancer Res. 1988;48:589–601.