ABSTRACT
The advancement of immune-therapeutics in cancer treatment has proven to be promising in various malignant diseases. However, in castration resistant prostate cancer (mCRPC) major Phase III trials have been unexpectedly disappointing. To contribute to a broader understanding of the role and use of immune-therapeutics in mCRPC, we conducted a systematic review. We searched the websites ClinicalTrials.gov, PubMed and ASCO Meeting Library for clinical trials employing immune checkpoint inhibitors in mCRPC. This article not only describes the rationale of individual trials, but it also summarizes the current status of the field and sheds light on strategies for future success.
1. Introduction
Androgen deprivation therapy (ADT), sometimes combined with chemotherapy, is the mainstay of the therapy of metastatic prostate cancer.Citation1–Citation4
ADT can either be achieved by bilateral orchiectomy (surgical castration) or by employing Gonadotropin-releasing hormone (GnRH) analogues (chemical castration). Usually, prostate cancer is contained by ADT and hence termed as hormone-sensitive prostate cancer (HSPC). Although most tumors respond to ADT in the beginning, 10–20% of HSPC progress despite ADT. This stage is known as castration resistant prostate cancer (CRPC), which is also referred to as hormone-refractory or androgen-independent prostate cancer. CRPC is incurable and has a poor prognosis as it metastasizes to bones, lymph nodes, lungs, liver or brain. This advanced stage is called metastatic CRPC (mCRPC). In such cases palliative chemotherapy with Docetaxel or Cabazitaxel, novel endocrine manipulations with Abiraterone, Enzalutamide, Apalutamide and Radium 223 have proven to be beneficial, improving quality of life and survival.Citation2–Citation4
Tumor cells employ and exploit a wide range of different mechanisms to evade the responses of the immune system. One of the most well-studied strategies for immune escape is the overexpression of key “immune checkpoint” proteins on T-cells. Immune checkpoint proteins co-stimulate and co-inhibit immune response, thus maintain immune homeostasis. They also prevent T-cells from being active for too long which could result in the destruction of healthy tissue. By overexpressing the immune checkpoint proteins, tumor cells inhibit T-cells in responding against tumor cells, thus allowing the latter to proliferate uncontrollably. These insights have led to the development of several immune-therapeutics called immune checkpoint inhibitors (ICPIs). ICPIs block the checkpoint proteins responsible for diminishing the immune response against the tumor cells.Citation5
In recent years, new immunotherapies based on ICPIs have emerged as attractive strategies in the treatment of advanced cancer. With the U.S. Food and Drug Administration (FDA)approval of Sipuleucel-T, the first ever dendritic cell vaccine against tumor growth, the expectations of the relevance for immune therapy approaches in prostate cancer was high. Unfortunately, first major phase III studies of ICPIs in mCRPC turned out to be negative, leaving the field disappointed and throwing the clinical development teams back to the drawing board.
In clinical practice, ICPIs can be categorized into 2 broad classes: cytotoxic T lymphocyte antigen–4 (CTLA-4) inhibitors and the programmed death receptor–1 (PD-1)/programmed death–ligand 1 (PD-L1) inhibitors. Tumor cells either induce CTLA-4 upregulation on tumor cells, suppressing the antitumor activity of T lymphocytes, thus establishing a favorable immunosuppressive microenvironment where malignant cells can survive. Other additional immune checkpoints are CD47 on M2 macrophages, VISTA and LAG3. . However, these checkpoints are still not subject of clinical trials in prostate cancer. Sections 1.1 and 1.2 provide a detailed description of the CTLA-4 and PD-1/PD-L1 pathways.
1.1. CTLA-4
CTLA-4 is an important immune checkpoint receptor that is expressed on the membrane of activated T-cells and modulates immune response by downregulation of effector T-cells and enhancing regulatory T-cell (Treg) activity.Citation6,Citation7 CTLA-4 influences T-cell activity in the priming phase of T cell activation. In a physiological state of immune response, T-cell activation results from two different key signals: 1) the presentation of an antigen to the T-cell receptor (TCR) by the major histocompatibility complex (MHC) on antigen-presenting cells (APC); 2) binding of the primary costimulatory receptor on T-cells, namely CD28, to CD80 (B7-1) and CD86 (B7-2) on APCs. T-cell activation then further increases the proliferation of T-cells themselves and the differentiation of T-cells to T memory cells.Citation8,Citation9
CTLA-4 is a CD28 homolog and competes with CD28 by binding to CD80/86 with greater affinity, thereby inhibiting T-cell activation, maintaining protective immune-tolerance and counterbalancing hyperactive immune reactions. CTLA-4 is itself subject to a positive feedback regulation loop, since activating signals from CD28:CD80/CD86 binding are followed by upregulation of CTLA-4 on the cell surface.Citation10 In contrast to effector T-cells, Tregs constitutively express CTLA-4, conferring the Tregs ability to suppress the activation of effector T-cells.Citation11 Cancer cells are able to derange the CLTA-4/B7 pathway, most prominently by PD-L overexpression, to establish an immunosuppressive microenvironment, thereby eluding antitumor responses.Citation12 The therapeutic inhibition of the CTLA-4 pathway may reverse the immunosuppressive milieu created by tumor cells and may help in reinforcing T-cell activation and proliferation and generating memory T-cells.Citation13–Citation16
1.2. PD-1/PD-L1
The PD-1/PD-L1 pathway performs a crucial role in the regulation of T-cell activity. PD-1 is a transmembrane glycoprotein T-cell co-inhibitory receptor expressed on activated CD4+ and CD8 + T-cells, B-lymphocytes, natural killer (NK) cells, and monocytes as well as tumor-infiltrating lymphocytes (TILs). The PD-1 receptor belongs to the superfamily of the B7/CD28 family and functions as a negative checkpoint inhibitor.Citation17 The two ligands for PD-1 are PD-1 ligand 1 (PD-L1; also known as B7-H1 and CD274) and programmed death–ligand 2 (PD-L2,also known as B7-DC and CD273). The binding affinity of PD-L1 to PD-1 receptor was shown to be three times greater than the affinity between PD-1 and PD-L2.18 Physiologically, the PD-1/PD-L1 pathway acts as a control in order to minimize autoimmune phenomena and potential damage to healthy tissue as well as to generate protective immune tolerance.Citation19
When an antigen expressed by the MHC complex is presented to the T cell, inflammatory cytokines – such as IL (Interleukin)-2, IL-7 and IL-15 – are produced and induce PD-L1 expression. The binding of PD-L1 to PD-1 results in inhibition of T-cell proliferation as well as in transformation of T lymphocytes into Tregs. Hence, the inflammatory response of the immune system is limited to a locally defined area, sparing the surrounding healthy tissue and averting generalized autoimmune responses.Citation20,Citation21
However, by overexpressing PD-L1 and inducing PD-1 receptor expression on TILs and Tregs, certain tumors circumvent this specific immune system activation and generate a favorable immunosuppressive milieu, where they can thrive.Citation22 Blockade of the PD-1/PD-L1-axis by monoclonal antibodies leads to reinforcement of the antitumor activity of previously inhibited T-cells and limits the number of Tregs, thus weakening the immunosuppressive tumor microenvironment and potentially restricting tumor growth.Citation23–Citation25
Despite the similarities between CTLA-4 and PD-1 pathways regarding their inhibitory effects on T-cell activity, they differ notably with respect to their pattern of expression on various types of cells within the tumor microenvironment. In contrast to PD-1, which acts within peripheral tissues and is expressed on a variety of cells, CTLA-4 is only found on T-cells. The ligands for CTLA-4 are expressed on professional APCs residing in lymph nodes and spleen, whereas PD-L1 and PD-L2 are expressed by malignant cells and macrophages in predominantly peripheral tissue types.Citation26,Citation27
By designing monoclonal antibodies specifically inhibiting the aforementioned immune checkpoints, e.g. Ipilimumab, the tolerance of the immune system toward the cancer cells is abolished and cytotoxic T-cells can react to malignant cells.
Although the molecular pathways of the functioning of ICPIs in various tumor entities have been well studied, the current literature on mCRPC lacks a general overview of all ICPIs that are being tested and employed to date. In order to contribute to a wider understanding of their role and use in mCRPC, we compiled a comprehensive review and discuss the current debates surrounding their success and failures.
2. Methods
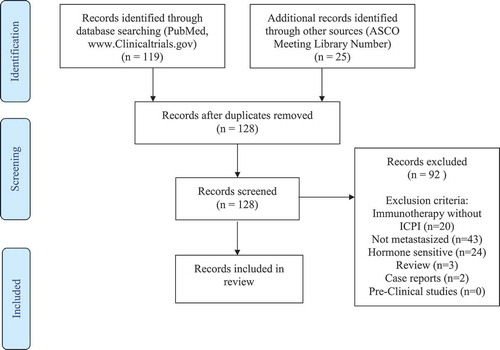
We searched the websites ClinicalTrials.gov, PubMed and ASCO Meeting Library for clinical trials testing ICPIs in mCRPC. The following keywords and combinations were used according to Medical Subject Heading database (MeSH): “prostatic” OR” prostate” AND “neoplasms” OR “cancer”, “castration resistant” AND “cancer” OR “neoplasm”, “androgen” AND “independent” OR “insensitive” OR “resistant” AND “cancer”, “hormone refractory” AND “cancer” OR “neoplasm”, combined with “immune checkpoint inhibitor”, “checkpoint inhibitor”, “immune therapy”, “Ipilimumab”, “Pembrolizumab”, “Nivolumab”, “Durvalumab”, “Tremelimumab”, “Avelumab”, “Atezolizumab”. All clinical trials studying the application of ICPI in mCRPC, which started prior to 01. December 2018 were eligible for our review. Reviews, case reports and investigations concerning HSPC and non-metastasized CRPC were excluded. Two co-authors independently searched the literature and characterized the search results. Based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines, charts the evidence acquisition process.Citation28
3. Results
The search strategy yielded 144 records. After deduplication and application of our exclusion criteria, 36 records were considered eligible for our review. The 36 clinical trials, study seven ICPIs, namely, Ipilimumab, Pembrolizumab, Nivolumab, Durvalumab, Tremelimumab, Avelumab, and Atezolizumab. provides an overview of the seven mentioned ICPIs, their brand names as well as their respective target mechanisms. Five of the ICPIs target the PD-1/PD-L1 receptor, whereas only two target the CTLA-4 receptor. Four ICPIs are members of the IgG1 subclass group. Pembrolizumab and Nivolumab belong to the IgG4 subclass. Tremelimumab is the only ICPI that belongs of the IgG2 subclass. One third of the 36 trials tested Pembrolizumab in mCRPC, followed closely by Ipilimumab, tested in nine trials. Between three and five trials were found for the remaining ICPIs. Sections 3.1–3.6, supplemented by – describe each of the ICPIs, along with the respective clinical investigations in detail. The most common endpoints were progression-free survival (PFS), overall survival (OS), overall response rate (ORR) and safety.
Table 1. Overview: clinical investigation of the seven ICPIs in mCRPC.
Table 2. Clinical investigation of ipilimumab in mCRPC.
3.1. Ipilimumab
Ipilimumab is a monoclonal IgG1 antibody that targets CTLA-4 and thus enhances the antitumor activity of T lymphocytes. Two large phase 3 trials investigated the efficacy and safety of Ipilimumab in mCRPC ().
The first trial CA184–043 included 799 patients that had progressed after Docetaxel therapy and assessed Ipilimumab after bone-directed radiotherapy (8Gy in one fraction). After randomization in a 1:1 ratio, 399 men received Ipilimumab 10mg/kg and 400 received placebo every three weeks for up to four doses with maintenance therapy every three months until disease progression or intolerable therapy-related adverse effects (TRAEs). Since the median overall survival (OS) was 11.2 months for Ipilimumab and 10.0 months in the placebo arm, the trial failed to show a statistically significant survival benefit for Ipilimumab. Apart from that, four study drug-related deaths were reported and grade 3–4 TRAE were much higher (26%) in the Ipilimumab group than in the placebo arm with only 3%.Citation29
Subset analyses on median OS of the first trial suggested that patients with poor prognostic factors, including visceral disease, anemia, elevated alkaline phosphatase and lactate dehydrogenase (LDH) or higher age had a significantly lower benefit from Ipilimumab therapy (p = .8756) than patients with favorable factors (p = .053).Citation30 This finding suggests that clinical characteristics may be important for the selection of more homogenous patient subsets that are more suitable for a therapy with Ipilimumab.
The second trial, CA184–095, was conducted in chemotherapy-naïve mCRPC patients with low disease burden. Patients were randomly assigned in a 2:1 ratio to either receive Ipilimumab 10mg/kg (n = 399) or placebo (n = 199) every three weeks for up to 4 doses. Median OS was 28.7 months for Ipilimumab and 29.7 months for placebo. Similar to the first trial, grade 3–4 TRAE were significantly higher in the Ipilimumab group with 31% versus 2% in patients treated with placebo. All of the nine deaths (2%) occurred in the checkpoint inhibitor-arm.Citation31 Conclusively, Ipilimumab demonstrated no survival benefit.
Unfortunately, a phase 2 clinical trial, CA184-437, which compared the efficacy of Ipilimumab 3mg/kg versus Ipilimumab 10mg/kg in chemotherapy naïve mCRPC patients was prematurely terminated because the two aforementioned trials failed to demonstrate a survival benefit for Ipilimumab.Citation32 Since the administration of Ipilimumab as a single-agent was unsuccessful in mCRPC, current trials test the combination of Ipilimumab with other therapeutic agents, such as diverse checkpoint inhibitors, antiandrogens, cytotoxic agents and cancer vaccines.
The biological rationale for a dual anti-CTLA-4 and PD-1/PD-L1 inhibition is to affect both priming and effector phase of T-cell activity in both lymph nodes and peripheral tissues, thus overcoming the immune suppressive microenvironment of the cancer cells and making them vulnerable for immune surveillance. In preclinical mouse experiments, the superior effect of the combination immunotherapy with PD-1/PD-L1 plus CTLA-4 checkpoint inhibitors compared to a single immune checkpoint blockade was demonstrated. It was reported that this combination can largely restore T-cell rejection function in tumors and diminish the number and function of Tregs at the same time.Citation33,Citation34 Ongoing investigations with ICPIs in mCRPC based on this therapy strategy are further listed in .Citation35–Citation38
Based on pre-clinical evidence, abiraterone has immunomodulatory properties and sensitizes prostate tumor cells to T cell-mediated lysis. Thus, there is a solid rational for the combination of abiraterone with immune checkpoint inhibitors in mCRPC.Citation35
In addition, ICPIs have also been tested in combination with classic cytotoxic agents such as Docetaxel. In preclinical studies, Docetaxel was shown to augment the MHC-1 and tumor antigen expression, and to release potent cancer antigens by degrading cancer cells. The application of ICPIs can hence potentially abolish the suppression of immune surveillance and trigger immune responses against malignant cells.Citation36,Citation37 In a relevant completed phase 2 trial MDX010-07, Ipilimumab monotherapy was tested against its combination with Docetaxel in mCRPC.Citation38 The results of both of the aforementioned trials have yet to be published.
The investigational pox-viral based vaccine, PROSTVAC VF, has been employed in combination with Ipilimumab in a phase 1 trial, 050167 with 30 mCRPC patients. Its regimen consists of a recombinant vaccinia vector as a primary vaccination, followed by multiple booster vaccinations employing a recombinant fowl pox vector. It aims at enhancing immune recognition to enable the T cells to target the prostate-specific tumor-associated antigens (TAAs).Citation39 Of 24 chemotherapy-naïve participating patients, 58% had a prostate-specific antigen (PSA) decline, of which six were ≥ 50%. Median OS was 2.63 yearsCitation40 ().
3.2. Pembrolizumab
Pembrolizumab is a PD-1 inhibitor that was first tested in the prostate adenocarcinoma cohort of the phase 1b KEYNOTE-028 trial in 23 mCRPC patients (). It was given at 10mg/kg every two weeks for up to 24 months or until disease progression or intolerable toxicity. Its application resulted in partial responses in four cases with an ORR of around 17% and disease stabilization in eight patients. OS was 7.9 months and grade 3–4 TRAEs occurred in 4 participants, which included neuropathy, asthenia, fatigue and lipase increase. No deaths or discontinuations were reported.Citation41
Table 3. Clinical investigation of pembrolizumab in mCRPC.
Based on these encouraging results, the larger phase 2 KEYNOTE-199 trial was initiated and included 258 patients with Docetaxel-refractory mCRPC. They were treated with 200 mg Pembrolizumab every three weeks (Q3W) until progressive disease (PD) or intolerable toxicity. According to the Prostate Cancer Clinical Trials Working Group 3 (PCWG3)- modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria, disease control rate (DCR) lasting ≥6 months was 15% regardless of PD-L1 status. In around 13% of patients, ≥3 grade TRAEs occurred. These data are to some extent promising and warrant further trials, as a disease control rate at six months of 11% was reported, with two patients exhibiting complete responses.Citation42
The similar phase 2 trials PERSEUS1 and CC 16557, also listed in , will enroll 150 mCRPC patients, to assess the efficacy of Pembrolizumab monotherapy.Citation43,Citation44 Another phase 2 trial, CRQ 2015 applied Pembrolizumab in 28 mCRPC patients who previously progressed under Enzalutamide. The rationale behind this clinical trial was that Enzalutamide was shown by Bishop et al. to increase PD-1 and PD-L1 expression on T lymphocytes.Citation45 Recently published outcomes show that five of 28 patients (18%) had a PSA decline of higher than 50% and three of 12 patients (25%) with measurable disease at baseline achieved radiologic objective responses. Median PFS as examined by PSA increase was 3.8 months, median radiographic PFS was 10.8 months and median OS was 22.2 months. There were 8 immune related adverse events in 7 patientsCitation46 ().
Aside from monotherapy studies, Pembrolizumab is currently being tested in several trials in combination with a variety of substances. An upcoming phase 1b/2 clinical trial – KEYNOTE-365 will enroll 210 mCRPC patients assigned to three cohorts: 200 mg Pembrolizumab (Q3W) with 400mg Olaparib twice daily (cohort A), 75mg/m2 Docetaxel (Q3W) + 5mg prednisone twice daily for a maximum of 10 cycles (cohort B), and 160mg Enzalutamide once daily (cohort C), respectively. Primary end points are safety and PSA response.Citation47 Preliminary results are listed in .
Another relevant phase 1/2 trial, UW15014, explores the combination of the DNA vaccine pTVG-HP with Pembrolizumab in mCRPC patientsCitation48 (). pTVG-HP is a plasmid DNA cancer vaccine which encodes for prostatic acid phosphatase (PAP), and hence stimulates the immune system against PAP-expressing prostate cancer cells. The preclinical background for this clinical trial were mouse experiments performed by McNeel et al, showing that the vaccination with pTVG-HP induced PD-1 expression on CD8-positive T-cells and PD-L1 expression in tumor cells.Citation49–Citation52
Guadecitabine, another novel therapeutic agent, will be assessed in combination with Pembrolizumab in the phase 1 HyPeR trial which will enroll 35 patients with NSCLC and mCRPC.Citation53 Guadecitabine is a second generation hypomethylating dinucleotide antimetabolite that is linked to guanosine via a phosphodiester bond, with potential antineoplastic activity.Citation54
Additionally, an ongoing phase 2 trial, 16–498 evaluates the addition of Pembrolizumab to Radium-223 in mCRPC patients.Citation55
An innovative concept is explored in a phase 2 trial, 2015–135, that combines Pembrolizumab with HER2Bi-armed activated T-cells. These T-cells have been exposed to the anti-CD3 murine antibody muromonab-CD3 and IL-2 for 14 days and then armed with anti-CD3 and an anti-Her2 bispecific antibody (Her2Bi). They are capable of attaching to CD3-expressing T- cells and HER2/neu-expressing tumor cells, thus selectively cross-linking T-cells and tumor cells, which may then activate cytotoxic T lymphocytes (CTLs).Citation56
Of interest is yet another trial MK-7123–034. Here, patients with solid tumors including mCRPC, are being treated with both Pembrolizumab and a new CXCR2 antagonist, Navarixin in 120 participants in a further phase 2 trail.Citation57
A recent development in the field is ARRx (AZD5312), an androgen receptor (AR) antisense oligonucleotide that targets the AR, mRNA, thus inhibiting AR-induced tumor cell growth and promoting apoptosis in AR-overexpressing cancer cells.Citation58 Its efficacy is evaluated in combination with Pembrolizumab in the phase 2 ARRO-CITO trialCitation59 ().
3.3. Nivolumab
Alike Pembrolizumab, Nivolumab is a human IgG4 anti-PD-1 monoclonal antibody currently being evaluated for the treatment of mCRPC. enlists the 3 Phase 2 trials investigating Nivolumab. In the ImmunoProst trial, Nivolumab is used as monotherapy after taxane-based chemotherapy. In the IRB18-0154 and the CheckMate 9KD trials, Nivolumab is being tested in combination with other drugs; in the former with Rucaparib, and in the latter with Rucaparib, Docetaxel or Enzalutamide.Citation60,Citation61 The reporting of results is still in progress.
Table 4. Clinical investigation of nivolumab in mCRPC.
3.4. Durvalumab and tremelimumab
Durvalumab binds to PD-1 and is currently being tested in two phase II trials with Tremelimumab. Tremelimumab is another ICPI directed against CTLA-4 and is currently being tested only in combination with Durvalumab in two trials. These trials evaluate the efficacy of 1500mg Durvalumab QW4 and 75mg Tremelimumab Q4W in mCRPC patientsCitation62–Citation65 ().
Table 5. Clinical investigation of durvalumab and tremelimumab in mCRPC.
In a large phase 1/2 clinical trial, 15-C-0145, Durvalumab is combined with Olaparib, and/or Cediranib. Olaparib is a Poly-ADP-Ribose-Polymerase (PARP) inhibitor. Cediranib is a novel orally available inhibitor of vascular endothelial growth factor (VEGFR) tyrosine kinases.Citation66 Preliminary results are listed in .
The accumulation of adenosine within the tumor microenvironment is of pivotal relevance, since immune cells expressing adenosine 2A receptor (A2AR) can exert an immunosuppressive effect that can promote tumor growth and diminish the efficacy of immune checkpoint inhibitors.Citation67,Citation68 Thus, a phase 1 trial, REFMAL 435 investigating the safety of Durvalumab in combination with the orally available adenosine receptor antagonist AZD4635 is currently ongoingCitation63 ().
3.5. Avelumab
Avelumab is an IgG1 monoclonal antibody binding to PD-L1. The phase 1/2 PAVE-1 trial studies the safety and benefit of Avelumab in combination with PT-112, a novel platinum-pyrophosphate agent. This combination is tested in different types of cancer, including mCRPC.Citation69 The large-scale phase 1 JAVELIN Solid Tumor trial enlisted over 1700 patients with tumors, including 18 mCRPC patients and explored Avelumab in these participants. The investigators concluded that overall treatment with Avelumab was safe and tolerable, with 2 patients exhibiting grade 3 asymptomatic TRAEs (amylase and lipase elevations). In seven patients, disease stabilization was achieved.Citation70 The phase 2 Parp Medley trial will enroll over 300 patients with solid tumors including mCRPC and combine the effects of Avelumab with Talazoparib, another PARP inhibitorCitation71 ().
Table 6. Clinical investigation of avelumab in mCRPC.
Table 7. Clinical investigation of atezolizumab in mCRPC.
3.6. Atezolizumab
Similar to Avelumab, Atezolizumab is an IgG1 monoclonal antibody inhibiting PD-L1.
catalogs five trials. In a phase 1 clinical trial, BO30013 this checkpoint inhibitor is combined with Radium-223.Citation72 Further, the phase 1 study Rosser-2015-4 combines Atezolizumab and Sipuleucel-T to study their safety in asymptomatic or oligosymptomatic mCRPC patients.Citation73 The phase 3 trial IMbassador250 applies Atezolizumab in conjunction with Enzalutamide in over 700 patients with mCRPC.Citation74
Similar to AZD4635, CPI-444 is an orally available adenosine receptor antagonist and is given with or without Atezolizumab for the treatment of various advanced cancers, including mCRPC, in a large phase 1 study CPI-444-001.Citation75 This study aims at enrolling over 500 patients.
Cabozantinib is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR-2 that is approved for the treatment of medullary thyroid cancer.Citation76 In the double-blind large-scale COMET-1 phase 3 study, Cabozantinib alone was compared with prednisone in mCRPC patients refractory to chemotherapy, however failed to meet its primary endpoint OS.Citation77 Currently, it is tested along with Atezolizumab in a phase 1/2 trial, XL 184-021, in the treatment of several tumor types including mCRPC.Citation78
4. Discussion
Although some clinical benefits of single agent therapy with Pembrolizumab in the phase 2 Keynote-199 trial have been demonstrated and were independent from PD-L1 status, outcomes of ICPI therapy in mCRPC so far are disenchanting when compared with other cancer entities. The two landmark trials with Ipilimumab (CA 184-043 and CA 184-095) which failed to prolong OS and partially caused severe adverse effects (AEs) independent from the disease stage status, are in sharp contrast to the success of ICPIs in melanoma or lung cancer. A subset analyses in the latter trial demonstrated at least a significantly higher clinical benefit in patients with favorable prognostic factors, thus encouraging further studies and raising interesting debates on reasons of failure and strategies to overcome them.
4.1. Debate 1: molecular biology, immunogenic aspects and external risk factors of prostate cancer
Considering how prostate cancer differs from other malignant diseases such as melanoma or small cell lung cancer, where currently big successes of immune therapy are being celebrated may shed light on the negative results from mCRPC trials. ICPIs achieved good and durable responses in melanoma, non-small cell lung and gastric cancer, to name a few. In the period prior to ICPI, these cancer types had a dismal prognosis and systemic therapeutic options were quite limitedCitation79–Citation81 when local therapy failed. In contrast, the progression of prostate cancer is contained and controlled by endocrine intervention and more recently chemotherapy, creating a more heterogeneous patient population at the time of treatment.
Another important feature of prostate cancer is its intra- and inter-tumoral genetic and epigenetic heterogeneity from the beginning. This heterogeneity is both spatial and temporal. Unlike other carcinomas, frequent chromosome alterations result in a high frequency of gene fusion events. The presence of different subclones, each with distinct genomic and phenotypic characteristics adds to the complexity and diversity.Citation82–Citation85 These differences in tumor biology may be important reasons for these modest results of ICPIs in mCRPC. Thus, a better understanding of the biology of mCRPC and the involved complex underlying mechanisms and the intricate pathway networks is urgently needed.
Although prostate cancer is thought to be an immunogenic malignancy, the immune system in most patients with prostate cancer is weaker due to the older age of the patients.Citation86 Low levels of TILs potentially based also on the relative low tumor mutational burden (TMB) of prostate cancer patients compared to other cancer entities may be additional factors. Since ICPIs are dependent on a functioning immune system, there might not be enough immunologic capacity to unleash the full potential of the ICPIs On the other hand if an adequate immune reaction in an older patient is achieved, side effects are less well tolerated thus weakening the therapeutic index.
With respect to external risk factors, there is lack of proof on which factors play a significant role in the carcinogenesis of prostate cancer.Citation87 By contrast the risk factors for the other common cancer diseases are well known: Smoking is the single most important external risk factor for the development of lung cancer, UV radiation for melanoma and Helicobacter pylori infection for gastric cancer, respectively.Citation88–Citation91 Clearly defined risk factors may be the reason for a more uniform disease.
4.2. Debate 2: selection of patient population
Unsatisfactory results may also be caused in part by suboptimal patient selection, study design and management of side effects. One way to optimize patient selection would be to allocate patients with similar clinical characteristics and features to the same subset to create a more homogenous patient collective.
Another more promising avenue would be to select patients according to biological markers. A classic example is microsatellite instability (MSI), which may not only select a particular more homogenous genetic subtype, but also one with consequences for immunogenicity. It was shown that deficient mismatch repair (dMMR) of DNA in cells causes microsatellite instability (MSI) meaning that the number of repeats of microsatellites differs from the repeat number of the corresponding normal DNA.Citation92 MSI in turn causes genetic hypermutability ultimately resulting in higher mutational load. It has been demonstrated in several experiments that the impairment of the genomic integrity renders cancer cells more susceptible to ICPI. In metastatic prostate cancer, MSI and dMMR occur in up to 12%.Citation93,Citation94 Thus, the dMMR/MSI status could serve as a powerful and reliable biomarker for a more homogenous patient classification, optimization of patient selection for clinical trials and for therapy responsiveness and prognosis.Citation95,Citation96
4.3. Debate 3: potential of combination therapies
The most commonly found combination is the combination between ICPIs targeting CTL-4 and PDL-1. A combination successful in other tumor entities holds particular promise in Prostate cancer patients for reasons discussed above. The same is true for combination with vaccines where a stimulation of tumorigenicity might unleash the potential of ICPIs. Less obvious rationale is behind the combination of ICPIs with therapeutics of proven efficacy in prostate cancer e.g. new hormonal agents, PARP inhibitors (ex. Talazoparib) and even less in the combination with as yet unproven substances.
Combination therapies have shown to be beneficial largely because of their synergistic and/or additive effects against cancer cells. For example, pre-clinical data on the combination of Enzalutamide with ICPIs have shown promising results; however, they should be carefully chosen to avoid increased toxicity. Pressing challenges are the cost of ICPI therapy, particularly for combination therapies. ICPIs are extremely cost-intensive and pose a relevant economic burden for health-care services. Further research is warranted to identify powerful predictive biomarkers, optimize patient selection and study design and to establish safe, effective and cost-efficient therapy options.
Conclusion
ICPIs have opened exciting new avenues in tumor therapy in many cancer entities. This review provides an overview over the clinical impact of ICPIs in CRPC. It also sheds light on the reasons, why the field of prostate cancer, despite being a leader in employing clinical tumor immunology tools, has fallen behind in providing solid proofs of success. Due to space restrictions and for purposes of clarity, promising fields such as CAR (chimeric antigen receptor) T- cells therapy, and innovative ICPIs controlling macrophage activity hat to be left out.
Abbreviations
| ADT | = | Androgen deprivation therapy |
| ADCC | = | Antibody-dependent cell-mediated cytotoxicity |
| APC | = | Antigen-presenting cells |
| AR | = | Androgen receptor |
| CAR | = | Chimeric antigen receptor |
| CD | = | Cluster of differentiation |
| CLTA-4 | = | Cytotoxic T-lymphocyte-associated Protein 4 |
| CRPC | = | Castration resistant prostate cancer |
| DCR | = | Disease control rate |
| DOR | = | Duration of response |
| FDA | = | U.S. Food and Drug Administration |
| GnRH | = | Gonadotropin-releasing hormone |
| HSPC | = | Hormone-sensitive prostate cancer |
| ICPI | = | Immune checkpoint inhibitor |
| IgG | = | Immunoglobulin G |
| IL | = | Interleukin |
| irAE | = | immune-related adverse effect |
| LAG3 | = | Lymphocyte-activation gene 3 |
| MAC | = | Membrane attack complex |
| mCRPC | = | metastatic castration-resistant prostate cancer |
| MeSH | = | Medical Subject Heading database |
| MHC | = | Major histocompatibility complex |
| MHC-1 | = | Major histocompatibility complex-1 |
| N/A | = | Not applicable |
| NCT | = | National Clinical Trial |
| NK cells | = | Natural killer cells |
| NSCLC | = | Non-Small cell lung cancer |
| OS | = | Overall survival |
| ORR | = | Overall response rate |
| PARP | = | Poly-ADP-Ribose-Polymerase |
| PCWG3 | = | Prostate Cancer Clinical Trials Working Group 3 |
| PD | = | Progressive disease |
| PD-1 | = | Programmed cell death protein 1 |
| PD-L1 | = | Programmed death-ligand 1 |
| PD-L2 | = | Programmed death-ligand 2 |
| PFS | = | Progression-free survival |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-analysis |
| PSA | = | Prostate specific antigen |
| Q3W | = | every three weeks |
| Q4W | = | every four weeks |
| RECIST | = | Response evaluation criteria in solid tumors |
| SD | = | Stable disease |
| TAA | = | Tumor-associated antigens |
| TCR | = | T cell receptor |
| TIL | = | Tumor-infiltrating lymphocytes |
| TMB | = | Tumor mutation burden |
| TRAE | = | Therapy-related adverse effects |
| Treg | = | Regulatory T cell |
| VEGFR | = | Vascular endothelial growth factor receptor |
| VEGFR-2 | = | Vascular endothelial growth factor receptor 2 |
| VISTA | = | V-domain Ig suppressor of T cell activation |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Prof. Dr. Martha Eibl for helpful discussions in the course of writing this review
References
- American Cancer Society. About prostate cancer. 2016 [accessed 2018 Oct 11]. https://www.cancer.org/cancer/prostate-cancer.html.
- Prostate Cancer Foundation. Prostate cancer treatment. 2018 [accessed 2018 Oct 11]. https://www.pcf.org/about-prostate-cancer/prostate-cancer-treatment/.
- National Cancer Institute. Prostate cancer treatment. 2018 [accessed 2018 Oct 11]. https://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq.
- Cancer.Net. Prostate cancer guide. 2018 [accessed 2018 Oct 11]. https://www.cancer.net/cancer-types/prostate-cancer/types-treatment.
- Institute, N.C. Immune checkpoint inhibitor. [accessed 2019 May]. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor.
- Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:1–13. doi:10.1016/j.jaut.2013.06.006.
- Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241(1):180–205. doi:10.1111/j.1600-065X.2011.01011.x.
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi:10.1146/annurev.immunol.14.1.233.
- Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101(2):169–177. doi:10.1046/j.1365-2567.2000.00121.x.
- Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543.
- Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119(22):5155–5163. doi:10.1182/blood-2011-11-388918.
- Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(Suppl 4):2–9. doi:10.1634/theoncologist.13-S4-2.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi:10.1126/science.271.5256.1734.
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18(2):206–213. doi:10.1016/j.coi.2006.01.011.
- Movva S, Verschraegen C. The monoclonal antibody to cytotoxic T lymphocyte antigen 4, ipilimumab (MDX-010), a novel treatment strategy in cancer management. Expert Opin Biol Ther. 2009;9(2):231–241. doi:10.1517/14712590802643347.
- Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, Chin K, Canetta R, Humphrey R. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37(5):533–546. doi:10.1053/j.seminoncol.2010.09.015.
- Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34(11):556–563. doi:10.1016/j.it.2013.07.003.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi:10.1146/annurev.immunol.26.021607.090331.
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi:10.1084/jem.20090847.
- Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–6746. doi:10.4049/jimmunol.181.10.6738.
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi:10.1111/j.1600-065X.2009.00767.x.
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi:10.1073/pnas.192461099.
- Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol. 2018;11(5):475–486. doi:10.1080/17512433.2018.1464388.
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561.
- Dotti G. Blocking PD-1 in cancer immunotherapy. Blood. 2009;114(8):1457–1458. doi:10.1182/blood-2009-05-223412.
- Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239.
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi:10.3389/fonc.2018.00086.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van Den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi:10.1016/S1470-2045(14)70189-5.
- Drake C, Kwon ED, Fizazi K, Bossi A, van Den Eertwegh AJ, Logothetis C, Scher HI, Beer TM, McHenry B, Liu D, et al. Results of subset analyses on overall survival (OS) from study CA184-043: ipilimumab (Ipi) versus placebo (Pbo) in post-docetaxel metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2014 [accessed 2018 Oct 11];32:2. doi:10.1200/jco.2014.32.4_suppl.2.
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi:10.1200/JCO.2016.69.1584.
- ClinicalTrials.gov. Safety and efficacy study of ipilimumab 3 mg/kg versus ipilimumab 10 mg/kg in subjects with metastatic castration resistant prostate cancer who are chemotherapy naive. 2016 [accessed 2018 Oct 11]. https://clinicaltrials.gov/ct2/show/NCT02279862.
- Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):39. doi:10.1186/s40425-018-0349-3.
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591–3603. doi:10.1158/0008-5472.CAN-12-4100.
- Ardiani A, Gameiro SR, Kwilas AR, Donahue RN, Hodge JW. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget. 2014;5(19):9335–9348. doi:10.18632/oncotarget.2429.
- Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–3544. doi:10.1158/1078-0432.CCR-07-4025.
- Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang K-Y, Ferrone S, Gameiro SR. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133(3):624–636. doi:10.1002/ijc.28070.
- CinicalTrials.gov. Comparison study of MDX-010 (CTLA-4) alone and combined with docetaxel in the treatment of patients with hormone refractory prostate cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT00050596?term=MDX010-07&rank=1.
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi:10.1016/S1470-2045(12)70006-2.
- Coyne GO, Jochems C, Heery C, Singh H, Surolia I, Riley R, Madan R, Dahut W, Steinberg S, Gulley J, et al. Abstract 5012: A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. 2014 [accessed 2018 Oct 11]. http://cancerres.aacrjournals.org/content/74/19_Supplement/5012.
- Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, Wallmark JM, Keam B, Delord J-P, Aggarwal R, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29(8):1807–1813. doi:10.1093/annonc/mdy232.
- De Bono J, Goh JC, Ojamaa K, Piulats Rodriguez JM, Drake CG, Hoimes CJ, Wu H, Poehlein CH, Antonarakis ES. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2018 [accessed 2018 Oct 11];36:5007. doi:10.1200/JCO.2018.36.15_suppl.5007.
- CinicalTrials.gov. Trial of pembrolizumab in metastatic castration resistant prostate cancer (PERSEUS1). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03506997?term=PERSEUS1&rank=1.
- CinicalTrials.gov. Pembrolizumab in metastatic castration resistant prostate cancer (mCRPC) with or without DNA damage repair defects. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03248570?term=CC+16557&rank=1.
- Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234–242. doi:10.18632/oncotarget.v6i1.
- Graff J, Alumkal JJ, Thompson RF, Moran A, Thomas GV, Wood MA, Drake CG, Slottke R, Beer TM. Pembrolizumab (Pembro) plus enzalutamide (Enz) in metastatic castration resistant prostate cancer (mCRPC): extended follow up. J Clin Oncol. 2018[accessed 2018 Oct 11];36:5047. doi:10.1200/JCO.2018.36.15_suppl.5047.
- CinicalTrials.gov. Study of pembrolizumab (MK-3475) combination therapies in metastatic castration-resistant prostate cancer (MK-3475-365/KEYNOTE-365). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02861573?term=KEYNOTE-365&rank=1.
- CinicalTrials.gov. Vaccine therapy and pembrolizumab in treating patients with hormone-resistant, metastatic prostate cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02499835?term=NCT02499835&rank=1.
- McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018;9(39):25586–25596. doi:10.18632/oncotarget.25387.
- Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–955. doi:10.1158/2326-6066.CIR-14-0206.
- Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8(+) T cells. Cancer Immunol Res. 2017;5(8):630–641. doi:10.1158/2326-6066.CIR-16-0374.
- Rekoske BT, Olson BM, McNeel DG. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016;5(6):e1165377. doi:10.1080/2162402X.2016.1165377.
- CinicalTrials.gov. Combination study of guadecitabine and pembrolizumab. (HyPeR). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02998567?term=NCT02998567&rank=1
- National Cancer Institute. guadecitabine. 2018 [accessed 2018 Oct 11]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/guadecitabine.
- CinicalTrials.gov. Study evaluating the addition of pembrolizumab to radium-223 in mCRPC. https://clinicaltrials.gov/ct2/show/NCT03093428?term=16-498&rank=1.
- National Cancer Institute. HER2Bi-armed activated T cells. 2018 [accessed 2018 Oct 11]. [accessed 2019 Jun 28]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/her2bi-armed-activated-t-cells.
- Chapman RW, Minnicozzi M, Celly CS, Phillips JE, Kung TT, Hipkin RW, Fan X, Rindgen D, Deno G, Bond R, et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol Exp Ther. 2007;322(2):486–493. doi:10.1124/jpet.106.119040.
- National Cancer Institute. androgen receptor antisense oligonucleotide AZD5312. 2018 [accessed 2018 Oct 11]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/androgen-receptor-antisense-oligonucleotide-azd5312.
- CinicalTrials.gov. ARRx in combination with enzalutamide in metastatic castration resistant prostate cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03300505?term=ARRO-CITO&rank=1.
- CinicalTrials.gov. Rucaparib and nivolumab in patients with prostate or endometrial cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03572478?term=IRB18-0154&rank=1.
- CinicalTrials.gov. An investigational immunotherapy study of nivolumab in combination with rucaparib, docetaxel, or enzalutamide in metastatic castration-resistant prostate cancer (CheckMate 9KD). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03338790?term=CheckMate+9KD&rank=1.
- CinicalTrials.gov. Phase I/II study of the anti-programmed death ligand-1 antibody MEDI4736 in combination with olaparib and/or cediranib for advanced solid tumors and advanced or recurrent ovarian, triple negative breast, lung, prostate and colorectal cancers. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02484404?term=NCT02484404&rank=1.
- CinicalTrials.gov. A Phase 1 clinical study of AZD4635 in patients with advanced solid malignancies. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02740985?term=REFMAL+435&rank=1.
- CinicalTrials.gov. Tremelimumab + durvalumab chemotherapy naive CRPC. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03204812?term=NCT03204812&rank=1.
- CinicalTrials.gov. Durvalumab with or without tremelimumab in metastatic castration resistant prostate cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT02788773?term=NCT02788773&rank=1.
- Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–4400. doi:10.1158/0008-5472.CAN-04-4409.
- Ma S-R, Deng -W-W, Liu J-F, Mao L, Yu G-T, Bu -L-L, Kulkarni AB, Zhang W-F, Sun Z-J. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol Cancer. 2017;16(1):99. doi:10.1186/s12943-017-0665-0.
- Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–272. doi:10.1016/j.csbj.2015.03.008.
- CinicalTrials.gov. A dose escalation and confirmation study of PT-112 in advanced solid tumors in combination with avelumab (PAVE-1). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03409458?term=PAVE-1&rank=1.
- Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL, Lepone LM, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587–598. doi:10.1016/S1470-2045(17)30239-5.
- CinicalTrials.gov. Javelin Parp Medley: Avelumab plus talazoparib in locally advanced or metastatic solid tumors. https://clinicaltrials.gov/ct2/show/NCT03330405?term=Parp+Medley&rank=1.
- CinicalTrials.gov. Safety and Tolerability of Atezolizumab (ATZ) in Combination With Radium-223 Dichloride (R-223-D) in Metastatic Castrate-Resistant Prostate Cancer (CRPC) Progressed Following Treatment With an Androgen Pathway Inhibitor. https://clinicaltrials.gov/ct2/show/NCT02814669?term=BO30013&rank=1.
- CinicalTrials.gov. Clinical study of atezolizumab (Anti-PD-L1) and Sipuleucel-T in patients who have asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03024216?term=Rosser-2015-4&rank=1.
- CinicalTrials.gov. A study of atezolizumab (Anti-PD-L1 Antibody) in combination with enzalutamide in participants with metastatic castration-resistant prostrate cancer (mCRPC) after failure of an androgen synthesis inhibitor and failure of, ineligibility for, or refusal of a taxane regimen (IMbassador250). [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03016312?term=IMbassador250&rank=1.
- National Cancer Institute. adenosine-A2A receptor antagonist CPI-444. 2018. [accessed 2019 Jun 28]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/adenosine-a2a-receptor-antagonist-cpi-444.
- National Cancer Institute. cabozantinib-s-malate. 2018 [accessed 2018 Oct 11]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/cabozantinib-s-malate.
- Smith M, De Bono J, Sternberg C, Le Moulec S, Oudard S, De Giorgi U, Krainer M, Bergman A, Hoelzer W, De Wit R, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–3013. doi:10.1200/JCO.2015.65.5597.
- CinicalTrials.gov. Study of cabozantinib in combination with atezolizumab to subjects with locally advanced or metastatic solid tumors. [accessed 2019 Jun 28]. https://clinicaltrials.gov/ct2/show/NCT03170960?term=XL184-021&rank=1.
- Pakkala S, Owonikoko TK. Immune checkpoint inhibitors in small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S460–S467. doi:10.21037/jtd.2017.12.51.
- Lugowska I, Teterycz P, Rutkowski P. Immunotherapy of melanoma. Contemp Oncol (Pozn). 2018;22(1A):61–67. doi:10.5114/wo.2018.73889.
- Taieb J, Moehler M, Boku N, Ajani JA, Yañez Ruiz E, Ryu M-H, Guenther S, Chand V, Bang Y-J. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: current status and future perspectives. Cancer Treat Rev. 2018;66:104–113. doi:10.1016/j.ctrv.2018.04.004.
- Tolkach Y, Kristiansen G. The heterogeneity of prostate cancer: a practical approach. Pathobiology. 2018;85(1–2):108–116. doi:10.1159/000477852.
- Yadav SS, Stockert JA, Hackert V, Yadav KK, Tewari AK. Intratumor heterogeneity in prostate cancer. Urol Oncol. 2018;36(8):349–360. doi:10.1016/j.urolonc.2018.05.008.
- Shoag J, Barbieri CE. Clinical variability and molecular heterogeneity in prostate cancer. Asian J Androl. 2016;18(4):543–548. doi:10.4103/1008-682X.178852.
- Boyd LK, Mao X, Lu YJ. The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol. 2012;9(11):652–664. doi:10.1038/nrurol.2012.185.
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–965. doi:10.1172/JCI64096.
- Society AC Prostate cancer risk factors. 2016 [accessed 2019 21 January]. https://www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/risk-factors.html.
- Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer. 2001;1(1):82–86. doi:10.1038/35094091.
- Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49(9):978–986. doi:10.1111/j.1365-4632.2010.04474.x.
- Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Semin Cutan Med Surg. 2011;30(4):222–228. doi:10.1016/j.sder.2011.08.003.
- Correa P, Piazuelo MB. Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7:59–64.
- National Cancer Institute. microsatellite instability. 2018 [accessed 2018 Oct 11]. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/microsatellite-instability.
- Hempelmann JA, Lockwood CM, Konnick EQ, Schweizer MT, Antonarakis ES, Lotan TL, Montgomery B, Nelson PS, Klemfuss N, Salipante SJ, et al. Microsatellite instability in prostate cancer by PCR or next-generation sequencing. J Immunother Cancer. 2018;6(1):29. doi:10.1186/s40425-018-0341-y.
- Schweizer MT, Cheng HH, Tretiakova MS, Vakar-Lopez F, Klemfuss N, Konnick EQ, Mostaghel EA, Nelson PS, Yu EY, Montgomery B, et al. Mismatch repair deficiency may be common in ductal adenocarcinoma of the prostate. Oncotarget. 2016;7(50):82504–82510. doi:10.18632/oncotarget.12697.
- Palmieri G, Colombino M, Cossu A, Marchetti A, Botti G, Ascierto PA. Genetic instability and increased mutational load: which diagnostic tool best direct patients with cancer to immunotherapy? J Transl Med. 2017;15(1):17. doi:10.1186/s12967-017-1119-6.
- Ji JH, Song H-N, Kim RB, Oh SY, Lim HY, Park JO, Park SH, Kim MJ, Lee SI, Ryou SH, et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol. 2015;45(3):256–260. doi:10.1093/jjco/hyu210.