ABSTRACT
Epithelial ovarian cancer (EOC) represents 5% of human gynecologic cancers in the world, is heterogeneous and highly invasive with a dismal prognosis (5 year-survival rate <35%). Diagnosis of EOC is frequently made at advanced stages and, despite aggressive treatments combining surgery and chemotherapy, fatal relapse rapidly occurs and is accompanied by a peritoneal carcinosis. In this context, novel therapeutical advances are urgently required. Adoptive transfer(s) of immune effector cells, including allogeneic human Vγ9Vδ2 T lymphocytes, represent attractive targets for efficiently and safely tracking tissue-invading tumor cells and controlling tumor dissemination in the organism. Our study describes the establishment of robust and physiological orthotopic model of human EOC in mouse, that includes surgical resection (ovariectomy) and chemotherapy, which are ineluctably accompanied by a fatal peritoneal carcinosis recurrence. Through a complementary set of in vitro and in vivo experiments, we provide here a preclinical proof of interest of the antitumor efficiency of adoptive transfers of allogeneic human Vγ9Vδ2 T lymphocytes against EOC, in association with surgical debulking and standard chemotherapies (i.e., taxanes and platinum salts). Moreover, our results indicate that chemo- and immunotherapies can be combined to improve the antitumor efficiency of immunotherapeutic lines. Altogether, these results further pave the way for next-generation antitumor immunotherapies, based on local administrations of human allogeneic human Vγ9Vδ2 T lymphocytes, in association with standard treatments.
Introduction
Ovarian cancer represents the seventh most common cancer in women worldwide. Epithelial ovarian cancer (EOC) accounts for >95% of the ovarian malignancies and is the leading cause of gynecologic cancer deaths with a 5-year survival of 35%.Citation1,Citation2 The absence of symptoms at early stages is a major problem implying that most patients are diagnosed with an advanced-stage disease. Despite surgical debulking and initial response to chemotherapy mostly based on platinum salts (e.g., Carboplatin) and antimitotic agents (e.g., Paclitaxel), majority of EOC recurs. This fatal evolution is principally accompanied by the development of chemo-resistance and peritoneal carcinosis.Citation3 Firstly, this indicates that more efficient and less toxic therapeutic approaches are urgently required. Secondly, this also evidences the main location of resistant tumor EOC cells is within the peritoneal cavity thus representing a unique opportunity to target and eliminate them by local administrations using selected immune effectors.Citation4 This unique therapeutic opportunity is further supported by the results of studies that reported the feasibility and the clinical benefits of intraperitoneal adoptive transfers of lymphocytes (e.g., tumor-infiltrative T and Natural Killer (NK) lymphocytes) (seeCitation5 for a review). Importantly, they suggested that their clinical efficacy could be enhanced by optimizing associations and positioning with standard therapeutic lines (e.g., maintenance).
Non-conventional Vγ9Vδ2 T lymphocytes are almost exclusively present in primates and represent the most common peripheral γδ T lymphocyte subset in adults (80% of γδ T lymphocytes). They express a TCR composed of Vδ2 chains which are predominantly paired to Vγ9 chains.Citation6 Importantly, the antigenic activation of Vγ9Vδ2 T lymphocytes is both species-specific and contact-dependent, but is not restricted by MHC molecules, which limits the emergence of deleterious alloreactivities.Citation7 The strong and specific antigenic activation of Vγ9Vδ2 T lymphocytes is induced by low molecular weight phosphorylated non-peptidic molecules, hereafter called phosphoantigens (PAg), that are metabolic intermediates of the endogenous mevalonate pathway (e.g., IPP, isopentenyl pyrophosphate). Interestingly, cells with elevated pinocytic activity (i.e., tumor cells), following sensitization with aminobisphosphonate (NBP) compounds (e.g., zoledronate), which are pharmacologic inhibitors blocking the mevalonate pathway downstream of the PAg synthesis, activate Vγ9Vδ2 T lymphocytes through upregulated levels of endogenous PAg. This antigenic activation process is tightly regulated by adhesion molecules and NK receptors axesCitation8 as well as controlled by target cell-expressed butyrophilin CD277 molecules.Citation9,Citation10 Several studies have now reported that allogeneic human Vγ9Vδ2 T lymphocytes detect and kill a broad range of transformed target cells, with a dysregulated mevalonate pathway, from various tissue origins in vitro, including cells from human ovarian tumors.Citation11,Citation12 We have recently shown that locally administrated allogeneic human Vγ9Vδ2 T lymphocytes patrol for several days and efficiently eliminate human glioblastoma tumor cells infiltrated within the brain in vivo.Citation13
Although passive and active human γδ T lymphocyte-immunotherapies have yielded promising safety and antitumor efficiency results,Citation14 the preclinical analysis of some of these parameters in EOC, in association with standard therapies, represents a necessary stage. This study first aimed at establishing an orthotopic xenograft model of human EOC with the injection of ovarian tumor cells, followed by a tumor resection by surgery and intraperitoneal chemotherapy, in order to recapitulate human EOC disease and standard treatments in vivo. Using this model and ex vivo analysis, we next showed the antitumor efficiency of intraperitoneal transfers of allogeneic human γδ T lymphocytes, in combination, before or after, with standard chemotherapy. Together, our observations further pave the way for next-generation antitumor immunotherapies, based on local administrations of human allogeneic human Vγ9Vδ2 T lymphocytes, in association with standard treatments.
Materials and methods
Human tumor cells and Vγ9Vδ2 t lymphocytes
Human EOC cells from SKOV-3 (SKOV-3-luc-D3, Perkin Elmer, Waltham, MA, USA) and SHIN-3-luc cell lines (kindly provided by M. Chérel, CRCINA Nantes France) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal calf serum (Gibco), 2 mM L-glutamine (Gibco), 10 mg/mL streptomycin (Gibco), 100 IU/mL penicillin (Gibco). Human primary cells were derived from EOC patients (CRB Santé, CHU de Rennes, France) and originated from either primary solid tumor (CKT), carcinomatosis (CKC), or ascites (CASC). These cells were cultured in adherent manner in Roswell Park Memorial Institute medium (RPMI, Gibco) supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mg/mL streptomycin, 100 IU/mL penicillin, 1 mM Sodium Pyruvate (all from Gibco) and 10 mM Hepes Buffer (Sigma Aldrich, Saint-Louis, MO, USA).
Allogeneic human Vγ9Vδ2 T lymphocytes were amplified from peripheral blood mononuclear cells (PBMC) obtained from healthy donor blood samples provided by the Etablissement Français du Sang (EFS, Nantes, France) and after Ficoll density centrifugation (Eurobio, Les Ulis, France). For specific expansions of peripheral allogeneic human Vγ9Vδ2 T lymphocytes, PBMCs were incubated with 3 µM of bromohydrin pyrophosphate (BrHPP), kindly provided by Innate Pharma (Marseille, France) in RPMI medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mg/mL streptomycin, 100 IU/mL penicillin, and 100 IU/mL human recombinant IL-2 (Proleukin, Novartis, Basel, Switzerland). After 4 days, cell cultures were supplemented with 300 IU/mL IL-2. At day 21, the purity of cultures was checked by flow cytometry. These pure (purity> 90%) human Vγ9Vδ2 T lymphocyte preparations were non-specifically expanded using mixed feeder cells, composed of 35 Gy-irradiated Epstein-Barr Virus-transformed human B lymphocytes and PBMC, and PHA-L in RPMI medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mg/mL streptomycin, 100 IU/mL penicillin, and 300 IU/mL recombinant human IL-2. After 3 weeks of culture, resting ex vivo expanded-Vγ9Vδ2 T lymphocytes were used for in vitro and in vivo assays. Tumor cells and γδ T lymphocytes were cultured at 37°C in humidified atmosphere with 5% CO2.
Flow cytometry
Human Vγ9Vδ2 T lymphocytes were stained with FITC-labelled anti-human TCR Vδ2 mAb (#IMMU389, Beckman Coulter, Brea, CA, USA) and APC-labelled anti-human CD3ε mAb (#UCHT1, Beckman Coulter), to assess the purity of the populations. SKOV-3 cells were incubated with FcR blocking reagent (Miltenyi, Bergisch Gladbach, Germany) and surface stained with either APC-labelled anti-human CD54 mAb (#HA58, BD Biosciences, Franklin Lakes, NJ, USA), PE-labelled anti-human CD44 mAb (#BJ18, Biolegend, San Diego, CA, USA) or anti-human CD166 (#3A6, BD Biosciences) followed by secondary staining with AlexaFluor 647-labeled anti-mouse IgG Αb (A21235, Life Technologies, Carlsbad, CA, USA). Acquisition was performed using an Accuri C6 PLUS flow cytometer (BD Biosciences) and the collected events were analyzed using FlowJo software (Treestar, Ashland, OR, USA).
In vitro functional assays
For CD107a surface mobilization assay, primary EOC cells or cell lines were sensitized, or not, with 50 µM of zoledronic acid (Sigma Aldrich) overnight before incubation with allogeneic human Vγ9Vδ2 T lymphocytes (effector to target ratio 1:1). The coculture was performed in RPMI medium containing 5 µM monensin (Sigma) and Alexa Fluor 647-labeled anti-human CD107a mAb (#H4A3, Biolegend). After 4 h, human Vγ9Vδ2 T lymphocytes were collected and stained with a FITC-labelled anti-human TCR Vδ2 mAb (#IMMU389, Beckman Coulter) and analyzed by flow cytometry.
For cytolytic assays, EOC tumor cells, previously treated with zoledronate overnight, were incubated with5,r(75 µCi/106 cells) for 1 h. After washes, cells were cocultured with human Vγ9Vδ2 T lymphocytes (10:1) for 4 h.51,r release activity was measured in supernatants using a MicroBeta counter (Perkin Elmer). Percentage of target cell lysis = (experimental release – spontaneous release)/(maximum release – spontaneous release) x 100. Spontaneous release and maximum release were determined by adding medium or 1% Triton X−100, respectively, to1Cr-labelled target cells in the absence of human Vγ9Vδ2 T lymphocytes.
Tumor cell-γδ t lymphocyte conjugates
Tumor SKOV-3 cells were stained with Zombie GreenTM Fixable Viability kit (Biolegend), diluted at 1:100 in phosphate buffer saline (PBS). Human Vγ9Vδ2 T lymphocytes were stained with an APC-labelled anti-human TCR Vδ2 mAb (#B6, Biolegend) for 20 min. After washes, SKOV-3 cells and Vγ9Vδ2 T lymphocytes were cocultured (1:1) in PBS in round-bottom polystyrene tubes at 37°C for 30 min. Cells were gently resuspended before analysis by flow cytometry.
Immunodeficient mice
Immunodeficient NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) female mice were obtained from Charles River Laboratories (Wilmington, MA, USA), bred in the animal facility of the Université de Nantes (UTE, SFR F. Bonamy Nantes, France) under SPF status and used at 8–12 weeks of age. This study was carried out in accordance with the recommendations of the French Regional Ethics Committee of the Pays de la Loire, France (#2168 and #10746).
Orthotopic xenograft model of EOC
For human EOC cells orthotopic implantation, NSG mice received 0,15 µg/g buprenorphine by subcutaneous injection 15 min before being anesthetized with isoflurane 2%. Skin was disinfected using povidone-iodine 5% (e.g., Vetidine) and a 5 mm incision was made, on the left flank, parallel to the spine, between the last rib and the iliac crest. Muscle and peritoneum were also incised before exteriorizing the left ovary by carefully pulling the fat attached to this organ using atraumatic forceps. Tumor cells suspended in PBS were injected directly into the ovary, using a NanoFil syringe (WPI, Sarasota, FL). After repositioning the ovary, muscles and skin were closed with Vicryl 4–0 (Ethicon, Somerville, NJ). Local analgesia was enhanced by application of lidocaine gel 2% (Xylocaine, AstraZeneca, Cambridge, UK) on the scar. Mice stayed in a warm environment until full recovery and received 0,15 µg/g buprenorphine twice a day for 48 h after surgery. For ovariectomy and tumor resection, anesthesia and analgesia were carried out in the same manner as described above. Skin, muscles, and peritoneum were incised on the scar. Next, the left ovary and uterine horn were exposed and clamped close to the bottom of the uterine horn. A single ligature was placed around the uterine horn and blood vessels before being cut just above the clamp and the ligature.
Any visible tumor cells were also removed from the peritoneum or the adipose tissue. Peritoneum, muscles, and skin were closed using Vicryl 4–0 before applying analgesia as previously described. Surgery was performed under sterile conditions and mice were placed on heating pad to maintain body temperature.
In vivo activation assays
5.105 SKOV-3 cells were intraperitoneally injected in NSG mice and five days later, 1.10Citation7 of human Vγ9Vδ2 T lymphocytes were injected, with or without 20 µg zoledronate (Zometa, Novartis), following the same route. The next day, mice were euthanized and a peritoneal wash was performed using 5 mL PBS. Collected cells were stained with Alexa Fluor 647-labeled anti-human CD69 mAb (#FN50, Biolegend) and FITC-labelled anti-human TCR Vδ2 mAb and analyzed by flow cytometry.
Bioluminescence imaging
Following orthotopic implantation of SKOV-3luc cells, the development of ovarian tumors and peritoneal carcinosis were monitored by bioluminescence assays once a week. Mice were intraperitoneally injected with 1.5 mg of D-luciferin (Interchim, San Diego, CA), 8 min before anesthesia with isoflurane 2% and optical imaging with Biospace Imager (Biospace Lab, Nesles-la-Vallée, France). In some case, after euthanasia, imaging of whole body, cut open peritoneum and separated organs were performed.
Chemotherapy and immunotherapy
Paclitaxel (6 mg/mL, Fresenius Kabi, Bad Homburg vor der Höhe, Germany) and Carboplatin (10 mg/mL, Fresenius Kabi) were used as pharmacological chemotherapeutic drugs. Chemotherapies were intraperitoneally injected and used at the maximum tolerable dosage (MTD) concentrations for NSG mice: 15 mg/kg Paclitaxel and 12 mg/kg Carboplatin after tumor resection.Citation15 For in vitro experiments, Paclitaxel was used at 300 µg/mL and Carboplatin was used at 375 µg/mL, which corresponds to the chemotherapy concentration used in vivo. In this study, the immunotherapy tested is based on intraperitoneal injections of 2.10Citation7 human allogeneic Vγ9Vδ2 T cells and 20 µg zoledronate (Zometa).
Immunohistochemistry analysis
Resected ovaries or carcinosis tumors were collected from xenografted mice, fixed with 4% paraformaldehyde (PFA)-PBS, embedded in paraffin wax and serially sectioned. Sections were incubated with 2% bovine serum albumin (BSA) and then with polyclonal rabbit anti-human MHC class I Ab (clone EPR1394Y, Abcam, Cambridge, UK). Revelation was performed by using polymer histofine rabbit to mouse coupled to HRP (Microm Microtech France, Francheville, France) and a DAB detection system (Leica, Wetzlar, Germany). Slides were scanned using the NanoZoomer 2.0 HT (Hamamatsu Photonics K.K., Hamamatsu, Japan).
Statistical analysis
Data are expressed as mean ± SD and were analyzed using GraphPad Prism 6.0 software (GraphPad Software, Inc., San Diego, CA). Two-way ANOVA and Mann & Whitney or log rank tests (see indicated p value) were calculated to reveal significant differences.
Results
Establishment of a preclinical orthotopic human EOC model in mouse
Owing to the species-restricted reactivity of Vγ9Vδ2 T lymphocytes and the lack of natural counterparts in rodents, our study first aimed at designing a preclinical model of human EOC xenografts in vivo allowing assessment of combined conventional therapies and adoptive transfers of allogeneic human Vγ9Vδ2 T lymphocytes. By lacking a large range of immune cells, including lymphocytes and NK cells, NSG mice represented a relevant recipient mouse strain that support a better engraftment of cells from human origin as compared to other immunocompromised mice. To select the most relevant model, EOC tumor cells should be recognized by allogeneic human Vγ9Vδ2 T lymphocytes (naturally or after NBP treatment) and their orthotopic implantation should lead to a discernible solid tumor by bioluminescence. Moreover, after primary tumor resection a fatal recurrence of the disease should take place through a typical peritoneal carcinosis.
First, the reactivity of allogeneic Vγ9Vδ2 T lymphocytes against EOC cells from ovarian cancer patients (pEOC; n = 10 patients; Supplemental Table 1) and commercial cell lines (cEOC; SKOV-3, SHIN-3), sensitized or not with zoledronate was analyzed. While Vγ9Vδ2 T lymphocytes have a poor natural reaction against tumor cells from pEOC and cEOC, zoledronate sensitization induced a strong reactivity of Vγ9Vδ2 T lymphocytes, whatever the tumor origin (CKT, CKC, CASC) ()). Next, pEOC and cEOC tumor cells were injected in the ovary of immunodeficient NSG mice ()). In the pEOC conditions, no disease symptoms (e.g., weight loss, production of ascites) were detected up to 6 months after implantation. In line with the results of biological examinations that did not evidence signs of peritoneal carcinosis, both immunochemistry and necropsy analyses revealed the absence or a sub-optimal growth of tumor cells ()).
Figure 1. Primary EOC cells as cellular basis for the establishment of physiological EOC mouse model. (a) Primary EOC cells (pEOC originated from either primary solid tumor (CKT), carcinomatosis (CKC), or ascites (CASC); light blue symbols) and cell lines (cEOC; dark blue symbols) were sensitized (filled symbols), or not (empty symbols), with zoledronate overnight before a 4h-coculture with human allogeneic Vγ9Vδ2 T lymphocytes (ET ratio 1). Expression of CD107a was analyzed on γδ T lymphocytes by flow cytometry. Results are expressed as percentage (%) of CD107a+ cells among Vδ2+ T lymphocytes (n = 4, each point correspond to different donors of Vγ9Vδ2 T lymphocytes). (b-c) Design of orthotopic engraftment of human EOC cells in the ovary of NSG mice and analysis. Six months after injection, ovaries were collected, fixed, sectioned and stained with anti-human MHC class I mAb. (c) Representative stained sections of NSG mice ovaries engrafted with three different primary EOC cells (from three different patients).
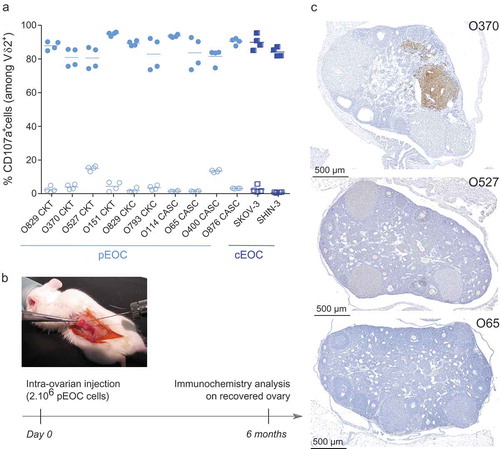
In contrast, 3 weeks after orthotopic injection of cEOC (20.10Citation3 SKOV-3 cells injected in the ovary of female mice), discernable tumor developed around the injected ovary (); picture 1). At this timepoint, tumor resection, with ovariectomy, was performed to mimic surgery of patients (); picture 2). One week after ovariectomy, residual tumor cells were detected only on surgical site (); picture 3). Importantly, an extended peritoneal carcinosis was detected 45 days after ovariectomy (); pictures 4 & 5) accompanied by invasion of lungs and all peritoneal organs, which recapitulates human disease recurrence (); pictures 6–10). Chemotherapies (Carboplatin and Paclitaxel) were intraperitoneally delivered, at recommended MTD for NSG mice (15 mg/kg Paclitaxel and 12 mg/kg Carboplatin).Citation15 Bioluminescence monitoring analysis showed a significant decrease of peritoneal carcinosis, 45 days after tumor resection ()). Compared analysis of survival rates between treated and untreated mice confirmed this observation (66 vs 50 days, respectively) ()). Of note, similar results were obtained using tumor cells from either SKOV-3 or SHIN-3 lines (data not shown). Moreover, anatomopathological analysis performed on resected ovary and peritoneal carcinosis tumors indicated the presence of high-grade serous carcinoma tumors (Supplemental Figure S1).
Figure 2. Establishment of a preclinical orthotopic human EOC mice model. (a) Representative optical and bioluminescence pictures showing the chronological development of orthotopic EOC xenografts (pictures 1 to 5) and subsequent peritoneal carcinosis (pictures 6 to 10) in NSG mice. (b) Bioluminescence analysis of mice performed at day 45 after tumor resection in surgery (surgery alone) or surgery combined to intraperitoneal injection of chemotherapy at days 10, 12, 14 and 16 after surgery (surgery + chemotherapy) (n = 10 mice; **** p < 0.0001). Results are expressed as cpm/cm2. (c) Survival curves of mice treated by surgery alone (-■-) or surgery and chemotherapy (-■-) (n = 10 mice per group; Log rank analysis, **** p < 0.0001).
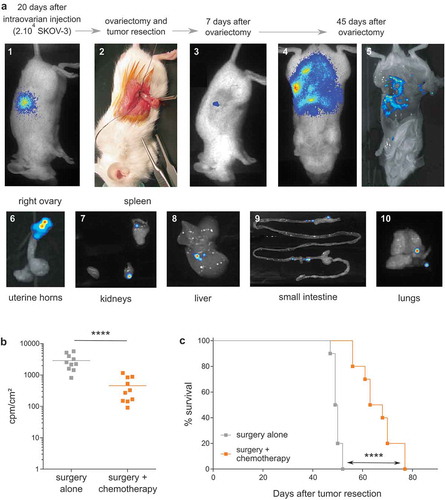
Collectively, these results show the establishment of a relevant preclinical physiological model of human EOC in mouse. This is evidence that: (i) human EOC tumor cells, originating from patients and cell lines, activate allogeneic human Vγ9Vδ2 T lymphocytes only upon NBP sensitization, (ii) EOC cells from lines (i.e., SKOV-3), but not from patients, display growth kinetics and peritoneal tumor cell dissemination criteria that are compatible with establishment of preclinical orthotopic EOC xenograft models in mouse, (iii) as it is the case for patients, the combination of resection surgery and chemotherapy lines improves survival of EOC mice.
Zoledronate sensitizes EOC tumor cells in vivo
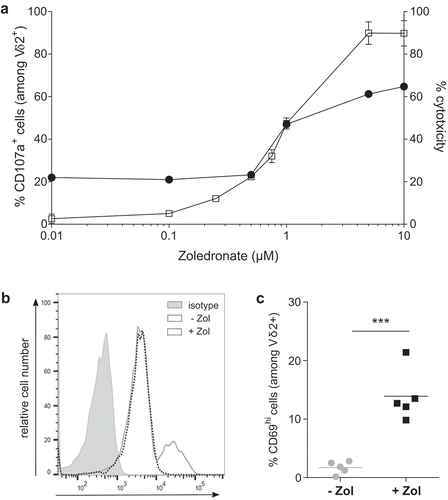
Following establishment of a physiological preclinical human EOC model, our study focused on in vivo reactivity of allogeneic Vγ9Vδ2 T lymphocytes against tumor cells, which is a key prerequisite for the efficiency of immunotherapeutic approaches. In line with our last results, allogeneic human Vγ9Vδ2 T lymphocytes not only recognized (CD107a) but also killedCitation5Citation1Cr release human SKOV-3 tumor cells, in an NBP dose-dependent manner ()). One day following intraperitoneal injection (lymphocytes ± NBP) in SKOV-3 tumor-bearing mice, expression levels of CD69, an activation marker, was analyzed and compared to allogeneic Vγ9Vδ2 T lymphocytes. As shown in (,)) independent experiments (n = 5) showed that NBP represents key pharmacological compounds to efficiently and in a dose-dependent manner sensitize EOC tumor cells to be recognized by allogeneic human Vγ9Vδ2 T lymphocytes in vivo.
Figure 3. Zoledronate is essential to efficiently sensitize human EOC tumor cells to Vγ9Vδ2 T lymphocyte recognition in vitro and in vivo. (a) Reactivity and cytotoxicity of allogeneic human Vγ9Vδ2 T lymphocytes against human EOC cells. In reactivity (CD107a) and cytotoxicity (51Crrelease) assays, SKOV-3 cells were sensitized by zoledronate overnight before a 4 h-coculture with human allogeneic Vγ9Vδ2 T lymphocytes performed at ET ratio 1 (-□-) and 10 (-●-). Results are expressed as % of CD107a+ cells among Vδ2+ T lymphocytes and % cytotoxicity (mean ± SD; n = 4), respectively. (b-c) Vγ9Vδ2 T lymphocytes were intraperitoneally injected, with or without zoledronate, in SKOV-3 tumor-bearing NSG mice. After peritoneal wash, CD69 expression was analyzed on Vδ2+ T lymphocytes. Representative histogram (b) or compiled results (c) of CD69 expression on Vγ9Vδ2 T lymphocytes (n = 5; *** p < 0.001).
The reactivity of vγ9vδ2 t lymphocytes against EOC cells can be affected by chemotherapy
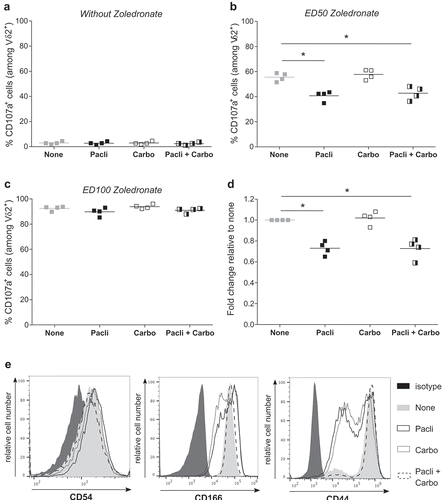
Our study next aimed at defining ideal time windows for Vγ9Vδ2 immunotherapies, related to standard platinum salts- and taxanes-based chemotherapies and their relationships. SKOV-3 tumor cells treated with chemotherapeutic agents, alone or in combination, and sensitized with zoledronate were co-cultured with Vγ9Vδ2 T lymphocytes. EOC tumor cells are only recognized upon NBP sensitization and chemotherapies did not induce any cell stress-related reactivity ()). Interestingly, NBP-induced reactivity was significantly reduced in the presence of Paclitaxel, but not Carboplatin, and only under suboptimal NBP-sensitization ()), as maximal NBP-sensitization reverse side effects of Paclitaxel on Vγ9Vδ2 T lymphocytes reactivity ()). Previous studies indicated that some antimitotic agents, such as paclitaxel, reduce the expression of adhesion molecules and consequently can affect the overall anti-tumor reactivity of effector immune cells.Citation16 Conjugate formation assays confirmed that Paclitaxel, alone or in combination with Carboplatin, decreased cellular interactions between allogeneic human Vγ9Vδ2 T cells and EOC tumor cells (); Supplemental Figure S2A). CD54 is an important cell adhesion molecule expressed by the SKOV-3 cells but its low expression was not affected by chemotherapeutic agents (), left). Interestingly, expression of CD166 and CD44, other important cell adhesion molecules (Supplemental Figure S2B),Citation17 were strongly decreased after Paclitaxel treatment, alone or in combination with Carboplatin (), middle and right). Together, these results indicate that: (i) standard Paclitaxel-based chemotherapies, alone or in combination, hamper the antitumor reactivity of allogeneic human Vγ9Vδ2 T lymphocyte effectors by reducing cellular interactions, via a decreased expression of some adhesion molecules, such as CD44 or CD166, (ii) Vγ9Vδ2 immunotherapies should not be delivered simultaneously, or immediately after, Paclitaxel-based chemotherapy treatment.
Figure 4. Paclitaxel alters the reactivity of Vγ9Vδ2 T lymphocytes by inducing a decrease of the expression of adhesion molecules on tumor cells. (a-b-c) SKOV-3 cell lines were treated for 1 h with the following chemotherapies: Paclitaxel alone (Pacli), Carboplatin alone (Carbo), or both (Pacli + Carbo). After zoledronate sensitization performed at different concentrations: without (a), ED50 (b) or ED100 (c), tumor cells were co-cultured for 4 h with Vγ9Vδ2 T lymphocytes (ET ratio 1) which were analyzed for expression of CD107a by flow cytometry. Results are expressed as % of CD107a+ cells among Vδ2+ T lymphocytes (n = 4; * p < 0,05). (d) SKOV-3 cells were stained with Zombie Green and Vγ9Vδ2 T lymphocytes were stained with anti-Vδ2 mAb before incubation during 30 min and flow cytometry analysis. % double positive events were recovered and results are expressed as fold change relative to the none condition (n = 4; * p < 0,05). (e) Representative histograms of extracellular staining of CD54 (left) CD166 (middle) and CD44 (right) on SKOV-3 cells after chemotherapeutic treatments.
Adoptive transfers of allogeneic human Vγ9Vδ2 T lymphocytes, combined with surgery and chemotherapy enhance the survival of EOC mice
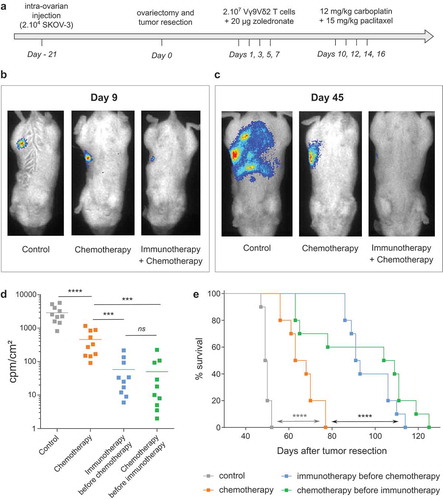
Finally, based on the results of these complementary experiments, our study aimed at assessing the impact of combined standard therapies and immunotherapies in vivo. As described above, our preclinical model relied on the development of EOC tumors following intra-ovarian injection of tumor cell. After 3 weeks, tumor resection by surgery (ovariectomy) was performed and chemotherapy was delivered by intraperitoneal injections of Carboplatin and Paclitaxel ()). Immunotherapy lines were based on intraperitoneal simultaneous injections of NBP (zoledronate), to sensitize EOC tumor cells, and allogeneic human ex vivo-amplified Vγ9Vδ2 T lymphocytes. Due to the interference of Paclitaxel on the antitumor reactivity of Vγ9Vδ2 T lymphocytes, these therapies could not be simultaneously delivered. Vγ9Vδ2 T lymphocytes and NBP were delivered before chemotherapy (days 1, 3, 5 and 7 after tumor resection) to avoid the inhibition effects of Paclitaxel ()). The sole activity of immunotherapy was previously assessed in another model of ovarian cancer (data not shown) and confirmed here thanks to bioluminescence ()). In fact, at day 9 (2 days after the last injection of immunotherapy but before the first injection of chemotherapy) bioluminescence imaging showed that mice treated with Vγ9Vδ2 T lymphocytes had reduced bioluminescence signals due to an elimination of EOC tumor cells ()). At day 45, a massive peritoneal carcinosis had developed in untreated mice, while mice treated by chemotherapy alone showed a small restricted tumor area ()). Strikingly, bioluminescence signals were weak, even absent, in mice treated by combined immunotherapy and chemotherapy which is linked to an important elimination of EOC tumor cells (,)). The enhanced efficacy of combined immunotherapy and chemotherapy was also significantly evidenced on survival curves showing that EOC mice treated by combined Vγ9Vδ2-immunotherapy and chemotherapy exhibited a higher median survival compared to mice treated by chemotherapy alone (90 days vs 66 days) ()). Reverse combination assays, in which immunotherapy was delivered several days after chemotherapy (days 19, 21, 23 and 25 after tumor resection) to avoid the inhibiting effects of Paclitaxel, were also performed. As for experiments described above, this combined treatment decreased the peritoneal carcinosis and significantly improved the survival of EOC mice (, )).
Figure 5. in vivo combination of chemotherapy and immunotherapy strongly improves EOC mice survival. (a) Design of the trial. (b-c) Representative bioluminescence pictures of one mice per group at day 9 (b) and day 45 (c) after tumor resection. (d) Compiled results of bioluminescence intensities for each group. (n = 10 mice; *** p < 0.001). (e) Survival curves of control mice (-■-), mice treated by chemotherapy (-■-), immunotherapy before chemotherapy (-■-) or chemotherapy before immunotherapy (-■-) (n = 10 mice per group; Log rank test, **** p < 0.0001).
Altogether, these in vivo experiments with a murine physiological EOC model, clearly suggested that in EOC patients, following debulking surgery, immunotherapies (adoptive transfer of allogeneic human Vγ9Vδ2 T lymphocytes) could be administrated in complement to standard chemotherapy, before or after, to enhance the elimination of infiltrative EOC tumor cells and to improve their survival.
Discussion
Immunotherapies based on adoptive transfer of tumor-reactive T lymphocytes represent promising approaches to improve therapeutic care and survival of cancer patients. For tardily diagnosed EOC, patients rapidly relapse, after debulking surgery and aggressive chemotherapy, with a fatal and massive peritoneal carcinosis. These novel therapies are expected to efficiently eliminate malignant infiltrative peritoneal tumor cells with limited, if any, deleterious effect on healthy surrounding cells. Among safety and efficiency analysis, one important issue associated to the design of these approaches is the selection of efficient immune effectors and the positioning of these approaches with standard therapies (i.e., surgery, chemotherapy). Our study demonstrated that purified allogeneic human Vγ9Vδ2 T lymphocytes efficiently recognize and kill human EOC tumor cells. After establishing a physiological orthotopic xenograft model of human EOC from a commercially available line, this work indicated that peritoneal delivery of allogeneic ex vivo-amplified human Vγ9Vδ2 T lymphocytes could be combined to debulking surgery and chemotherapy and could significantly control dissemination of tumor cells in vivo.
This study first showed that EOC cells, originating from either primary tumors or cell lines, are all targeted by allogeneic human Vγ9Vδ2 T lymphocytes in vitro, upon NBP treatment. While yielding promising results, clinical trials revealed issues that should be addressed (e.g., reduced reactivity of Vγ9Vδ2 T lymphocytes against fresh tumors). Importantly, the antigenic activation process of Vγ9Vδ2 T lymphocytes is not restricted by MHC molecules, which eliminates any risk of deleterious direct alloreactive reactivities towards non-transformed cells. The constitution of clinical allogeneic human Vγ9Vδ2 T lymphocyte banks, established from PBMC of healthy donors, would represent a unique opportunity for designing adoptive transfer immunotherapies in cancer patients. Once selected, amplified and prepared, administration of peripheral effector γδ T lymphocytes could be hampered by the physiological status of the ovary and peritoneum.
To assess this, our study aimed at establishing a physiological orthotopic xenograft model of human EOC. As previously observed for sc and ip xenografts (NJ, ES, data not shown), elevated numbers of primary EOC cells that were injected in the ovary of immunodeficient NSG mice grown and survived poorly in vivo, making them unusable for kinetics and for reliability reasons. Increasing number of cells have been tested, but the quantity remains limited due to the size of grafted organ (mouse ovary). Patient Derived Xenograft (PDX) and patients tumor grafts, based on either collected ascites or tumor fragments, would represent alternative models as previous studies have shown excellent histologic and molecular fidelity to ovarian carcinoma.Citation18,Citation19 However, these attractive, but long-growing (i.e., several months) and low take rate, EOC models are not easy to handle and have been incompletely described for their ability to do disseminate as a relevant peritoneal carcinosis. The elevated infiltrative feature of EOC tumor cells remains a key characteristic and a challenge for novel therapeutic approaches. Bioluminescent EOC tumor cells from SKOV-3 line, a commercial human ovary cell line was selected to establish our preclinical model for the following reasons: (i) reduction of injected cell quantities (20.10Citation3 cells) and growth kinetics (<2 months), (ii) reliability and reproducibility, (iii) disease follow-up by non-invasive imaging, (iv) formation of high-grade serous tumors and development of a fatal peritoneal carcinosis.
Although recapitulating the peritoneal infiltration features of recurring human EOC tumors, this orthotopic in vivo model was established in immunodeficient NSG mice and did not take account of inflammatory and immunosuppressive tumor microenvironments. Several studies showed that peritoneal inflammation fosters the development of regulatory T lymphocytes and inhibits the maturation of myeloid cells and the cytotoxicity of effector cells.Citation20 While needing to be further assessed, this issue should be limited by the local delivery of elevated numbers of mature T cell effectors, whose low-sensitivity to inhibitory signals is further enhanced by counteracting the activating effect of NBP treatments on EOC tumors.Citation12
As almost all human EOC cells are not naturally recognized by Vγ9Vδ2 T lymphocytes, in vitro and in vivo assays were carried out with dose-dependent zoledronate sensitizations of EOC cells to trigger strong Vγ9Vδ2 T lymphocyte recognition, as already used for enhancing such reactivities in other oncological contexts. Zoledronate administrations, which were already indicated for other human pathologies, such as osteoporosis and multiple myeloma,Citation21,Citation22 have been proposed for Vγ9Vδ2 T cell-based immunotherapies. Recent preclinical approaches have suggested that NBP-sensitizations of tumor cells could be combined with adoptive transfers of Vγ9Vδ2 T cells in order to induce/enhance their antitumor reactivity. In this study, similar effects were targeted in simultaneous direct peritoneal combined injections in vivo. As expected from previous studies, combined injections of zoledronate and human allogeneic Vγ9Vδ2 T lymphocytes did not induce any adverse effects in treated mice.
This preclinical model of human EOC was next used to assess the clinical efficacy of immunotherapies and their positioning with standard treatments for EOC (debulking surgery followed by chemotherapy). In vitro chemotherapeutic assays showed that they did not enhance Vγ9Vδ2 T lymphocytes reactivity but rather induced inhibiting effects (e.g., Paclitaxel) on the expression of selected cell adhesion molecules (e.g., CD44, CD166) expressed by tumor cells and on their subsequent interactions with Vγ9Vδ2 T lymphocytes. Based on these observations, preclinical combinations of surgery, standard chemotherapy and immunotherapy were designed to limit such effects and immunotherapy was delivered before or some days after chemotherapy. In this preclinical setting, Vγ9Vδ2 T lymphocyte-immunotherapies, when combined with debulking resection surgery and chemotherapy, significantly reduced the spreading of human EOC tumors and improved the survival of treated mice.
In conclusion, our study highlights the feasibility and the antitumor effects of intraperitoneal immunotherapies, based on allogeneic human Vγ9Vδ2 T lymphocytes, using a novel and robust orthotopic human EOC mouse model. This work also clearly positioned immunotherapies with standard EOC treatments by showing that delivered Vγ9Vδ2 T lymphocytes can act, not as first-line antitumor effectors, but rather to track and eliminate residual invasive EOC cells, likely accounting for a fatal recurring peritoneal carcinosis. Therefore, these results evidenced that adoptive allogeneic immunotherapies could represent promising and effective approaches for the treatment of human EOC which remains one of the deadliest cancers.
Abbreviations
Disclosure of potential conflict of interest
The authors disclose no potential conflicts of interest.
Supplemental Material
Download PDF (10.3 MB)Acknowledgments
We thank the staff of University Hospital animal facility of Nantes for animal husbandry and care, the cellular and tissular imaging core facility of Nantes university (MicroPICell) for imaging, and the Cytometry Facility (Cytocell) from Nantes for their expert technical assistance. We also thank Pr V. Catros and technical staff of the CRB Rennes for providing primary EOC cells and Pr J-M Classe for providing chemotherapeutic agents at clinical grade format.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332.
- Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. May 29 2018. doi: 10.3322/caac.21456
- Colombo P-E, Fabbro M, Theillet C, Bibeau F, Rouanet P, Ray-Coquard I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit Rev Oncol Hematol. 2014;89(2):207–216. doi:10.1016/j.critrevonc.2013.08.017.
- Ventriglia J, Paciolla I, Pisano C, Cecere SC, Di Napoli M, Tambaro R, Califano D, Losito S, Scognamiglio G, Setola SV, et al. Immunotherapy in ovarian, endometrial and cervical cancer: state of the art and future perspectives. Cancer Treat Rev. 2017;59:109–116. doi:10.1016/j.ctrv.2017.07.008.
- Mittica G, Capellero S, Genta S, Cagnazzo C, Aglietta M, Sangiolo D, Valabrega G. Adoptive immunotherapy against ovarian cancer. J Ovarian Res. 2016;9(1):30. doi:10.1186/s13048-016-0236-9.
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi:10.1146/annurev.immunol.18.1.975.
- Chien Y, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. doi:10.1146/annurev-immunol-032713-120216.
- Thedrez A, Sabourin C, Gertner J, Devilder M-C, Allain-Maillet S, Fournié -J-J, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. doi:10.1111/j.1600-065X.2006.00468.x.
- Harly C, Guillaume Y, Nedellec S, Peigné C-M, Mönkkönen H, Mönkkönen J, Li J, Kuball J, Adams EJ, Netzer S, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120(11):2269–2279. doi:10.1182/blood-2012-05-430470.
- Harly C, Peigné C-M, Scotet E. Molecules and mechanisms implicated in the peculiar antigenic activation process of human Vγ9Vδ2 T cells. Front Immunol. 2014;5:657. doi:10.3389/fimmu.2014.00657.
- Lai D, Wang F, Chen Y, Wang C, Liu S, Lu B, Ge X, Guo L. Human ovarian cancer stem-like cells can be efficiently killed by γδ T lymphocytes. Cancer Immunol Immunother. CII. 2012;61(7):979–989. doi:10.1007/s00262-011-1166-4.
- Lavoué V, Cabillic F, Toutirais O, Thedrez A, Dessarthe B, de La Pintière CT, Daniel P, Foucher F, Bauville E, Henno S, et al. Sensitization of ovarian carcinoma cells with zoledronate restores the cytotoxic capacity of Vγ9Vδ2 T cells impaired by the prostaglandin E2 immunosuppressive factor: implications for immunotherapy. Int J Cancer. 2012;131(4):E449–462. doi:10.1002/ijc.27353.
- Jarry U, Chauvin C, Joalland N, Léger A, Minault S, Robard M, Bonneville M, Oliver L, Vallette FM, Vié H, et al. Stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology. 2016;5(6):e1168554. doi:10.1080/2162402X.2016.1168554.
- Fournié -J-J, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10(1):35–41. doi:10.1038/cmi.2012.39.
- Helland O, Popa M, Vintermyr OK, Molven A, Gjertsen BT, Bjørge L, McCormack E. First in-mouse development and application of a surgically relevant xenograft model of ovarian carcinoma. PLoS One. 2014;9(3):e89527. doi:10.1371/journal.pone.0089527.
- Loubani O, Hoskin DW. Paclitaxel inhibits natural killer cell binding to target cells by down-regulating adhesion molecule expression. Anticancer Res. 2005;25:735–741.
- Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the activation of human gamma delta T cells by tumor cells sensitized with nonpeptide antigens. J Immunol Baltim. Md 1950. 2006;177(2):877–884. doi:10.4049/jimmunol.177.2.877.
- Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, Perkins SE, AlHilli M, Butler KA, McKinstry S, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(5):1288–1297. doi:10.1158/1078-0432.CCR-13-2611.
- Liu JF, Palakurthi S, Zeng Q, Zhou S, Ivanova E, Huang W, Zervantonakis IK, Selfors LM, Shen Y, Pritchard CC, et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for preclinical evaluation of novel therapeutics. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(5):1263–1273. doi:10.1158/1078-0432.CCR-16-1237.
- Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle Georget Tex. 2010;9(2):260–268. doi:10.4161/cc.9.2.10430.
- Dhillon S, Lyseng-Williamson KA. Zoledronic acid : a review of its use in the management of bone metastases of malignancy. Drugs. 2008;68(4):507–534. doi:10.2165/00003495-200868040-00010.
- Green J, Lipton A. Anticancer properties of zoledronic acid. Cancer Invest. 2010;28(9):944–957. doi:10.3109/07357907.2010.512598.
