ABSTRACT
Purpose: Local tumor ablation through irreversible electroporation (IRE) may offer a novel therapeutic option for locally advanced pancreatic cancer (LAPC). It may also serve as a means of in vivo vaccination. To obtain evidence of the induction of systemic antitumor immunity following local IRE-mediated ablation, we performed an explorative immune monitoring study. Methods: In ten patients enrolled in a clinical trial exploring the safety, feasibility, and efficacy of percutaneous image-guided IRE in LAPC, we determined the frequency and activation state of lymphocytic and myeloid subsets in pre- and post-treatment peripheral blood samples using flow cytometry. Tumor-specific systemic T cell responses to the pancreatic cancer associated antigen Wilms Tumor (WT)1 were determined after in vitro stimulation in an interferon-y enzyme-linked immunospot assay (Elispot), at baseline and at 2 weeks and 3 months after IRE. Results: Our data showed a transient decrease in systemic regulatory T cells (Treg) and a simultaneous transient increase in activated PD-1+ T cells, consistent with the temporary reduction of tumor-related immune suppression after the IRE procedure. Accordingly, we found post-IRE boosting of a pre-existing WT1 specific T cell response in two out of three patients as well as the de novo induction of these responses in another two patients. There was a trend for these WT1 T cell responses to be related to longer overall survival (p = .055). Conclusions: These findings are consistent with a systemic and tumor-specific immune stimulatory effect of IRE and support the combination of percutaneous IRE with therapeutic immune modulation.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in Western Europe and the United States.Citation1 Currently, surgical resection at an early stage is the only potentially curative treatment. Early diagnosis may increase the chance of curative resection but this is only applicable to up to 15–20% of patients;Citation2 nearly 30% of newly diagnosed pancreatic cancer patients have locally advanced pancreatic carcinoma (LAPC).Citation2 The prognosis of patients suffering from LAPC is poor with a median survival of less than a year. Often surgical resection is not an option for these patients, leaving only palliative treatment.
Local ablative therapies have been developed for the treatment of isolated tumors and metastases. They are mostly based on thermal ablation, leading to tumor necrosis. However, few local ablative therapies have been developed for the treatment of LAPC as thermal ablation was considered unsafe due to the high risk of complications caused by collateral damage to the pancreas, surrounding organs, and vascular structures, increasing the risk of pancreatitis.Citation3 Irreversible electroporation (IRE) provides a promising alternative .Citation4 IRE is a new, imaging-guided technique which causes irreversible nanopores in the cell membrane by the application of high-voltage electric pulses, leading to cell death through apoptosis .Citation5 IRE is based on the pulsatile application of electric energy delivered between several electrodes that are placed around the tumor.Citation5 Due to its primarily non-thermal mechanism of action, IRE leaves supporting extracellular matrix structures unaffected, preserving the structural integrity of inlaying and adjacent tissue structures like vessels and bile ducts.Citation5 This makes the technique ideal for the selective ablation of diffusely growing malignancies that surround such structures, as is typically the case for LAPC. Several studies have investigated the safety and efficacy of open and percutaneous IRE for LAPC, with an overall complication rate ranging from 10 to 37% and preliminary evidence of improved progression-free and overall survival.Citation4,Citation6–Citation9
We recently conducted the PANFIRE phase I/II study, investigating the safety of percutaneous IRE for LAPC in 25 patients. There were no deaths directly attributable to IRE and twelve minor (grade I/II) and eleven major (9 grade III; 2 grade IV) complications were recorded. Findings further suggested prolonged time to local recurrence and overall survival, as compared to chemotherapy or best supportive care, but require confirmation through follow-up trials.Citation9
Beside local tumor ablation, IRE may also lead to systemic tumor control through the priming or boosting of tumor specific immunity, in effect resulting in in-vivo vaccination. Pre-clinically observed abscopal effects (i.e. tumor control at distant, untreated sites) are in line with this notion.Citation10 Pancreatic carcinoma is only moderately immunogenic,Citation11 which may in part be caused by local and systemic immune suppression.Citation12 Elevated frequencies of immune regulatory cells like myeloid derived suppressor cells (MDSC) and regulatory T cells (Tregs) have been implicated in immune suppression accompanying pancreatic cancer progression.Citation13,Citation14 Nevertheless, pancreatic tumors are amenable to immunotherapy with clinical benefit demonstrated after tumor-specific vaccination.Citation15–Citation17
IRE-induced apoptosis and reduced tumor load, may lead to a simultaneous release of immunogenic tumor cell remnants as well as a reduction in tumor-associated immune suppression. Moreover, because the larger vessels remain intact, antigen presenting cells like dendritic cells (DC) should be able to infiltrate the lesion and induce an immune response against antigens contained within the apoptotic bodies.Citation5,Citation18 In the present immune monitoring study we therefore set out to obtain evidence of a systemic anti-tumor immune response resulting from local IRE-mediated ablation of LAPC.
Results
T cell subset frequencies and activation state
Ten patients with LAPC who were enrolled in the PANFIRE study to undergo IRE were selected for this immune monitoring side study (see ). Flow cytometric analysis of PBMC was performed at baseline (T = 0, just prior to IRE), two weeks after IRE (T = 2wk), and 3 months after IRE (T = 3mnth), to assess the systemic immune modulatory effects of the IRE procedure (see for an overview of data). Pre- and post-treatment frequencies of CD4+ and CD8+ T cells remained unchanged (), as did the distribution between effector, effector-memory, central-memory, and naive T cells (assessed by CD27/CD45RA expression, data not shown). No evidence of post-treatment CD4+ or CD8+ T cell activation was observed by HLA-DR or CD25 expression (data not shown), but there was a non-significant increase in the mean proliferative fraction of CD8+ T cells (). A transient and significant decrease in total CD4+CD127−CD25+ regulatory T cells (Tregs) was observed at 2 weeks following IRE, as well as in activated Tregs (aTregs, with high FoxP3 and CD25 levels, negative for CD45RA and positive for Ki67) and in resting Tregs (rTregs with intermediate CD25 and FoxP3 levels and positive for CD45RA while lacking Ki67), Citation19,Citation20 see Supplemental . As a measure of Treg suppressive capacity CD8+Ki67+ T cell/aTreg ratios were calculated. As shown in , these ratios went up after IRE, although not significantly. For six patients, the expression of PD-1, LAG3, TIM3, and CTLA4 immune checkpoints were studied (see Supplemental ). Significant post-treatment increases in PD-1 rates in both CD4+ and CD8+ T cells were observed whereas expression of the other checkpoint remained unchanged and generally low ().
Table 1. Patients characteristics.
Table 2. Flow cytometric data of immune subsets pre- and post-IRE in patients with LAPCa.
Figure 1. The effects of IRE on immune checkpoint expression. Shown are the frequencies of the indicated checkpoints within CD4+ (a) and CD8+ (b) T cell subsets. Indicated statistical significance levels are by one-sided repeated measures ANOVA and post-hoc Dunnett’s multiple comparisons test.
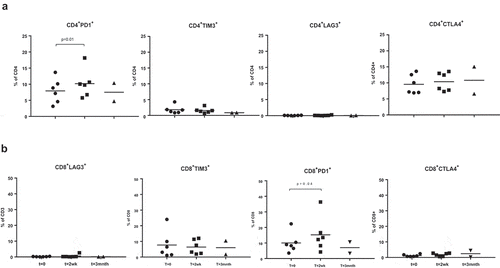
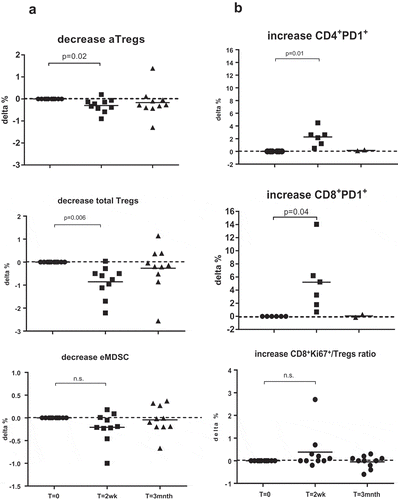
Figure 2. Consistent Treg decreases and CD4+PD1+ and CD8+PD1+ increases in all patients 2 weeks post-IRE. Shown are changes (in %) of cell frequencies relative to pre-treatment levels of the indicated suppressive subsets (a) or subsets and subset ratio associated with immune activation (b). Indicated statistical significance levels are by repeated measures ANOVA and post-hoc Dunnett’s multiple comparisons test or a two-sided Student’s T test.
Myeloid subset rates and activation state
No effects of IRE were observed on the frequency or activation state (by CD40, CD80, and PD-L1 expression, data not shown) of the peripheral blood DC/APC subsets, i.e. CD141+ cDC1, CD1c+ cDC2, and BDCA2+ pDC, nor of M-DC8/SLAN+ non-classical monocytes (). In contrast, a transient and non-significant increase in monocytes and decrease in Li−CD33+HLA-DR− early myeloid derived suppressor cells (eMDSC) was observed at 2 weeks post-IRE treatment (see ).
Treg and PD1 + T cell changes relative to start of treatment
As shown in , significant decreases in Treg rates and increases in PD1+ T cell numbers were consistent in all patients, whereas changes in eMDSC and CD8+Ki67+ T cell/aTreg ratios were more variable. Treg numbers decreased after IRE in all but one patient. This patient (08) showed early local progression (5 months post-IRE) and died at 6 months (Table II).
Tumor antigen specific T cell responses
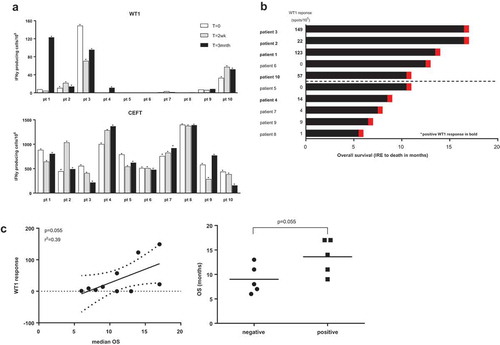
Pre- and post-treatment tumor specific T cell reactivity was assessed by IFNγ Elispot read-out, after in vitro stimulation with 15-mer overlapping peptides covering the full length of the tumor antigen WT1. As a measure of immune competence, T cell reactivity against peptides covering CD4 and CD8 T cell epitopes from CMV, EBV, Influenza (Flu), and Tetanus Toxoid (CEFT) was determined. CEFT responses were uniformly high and on the whole unaffected by IRE treatment ()). In contrast, T cell responses against WT1 were detected both pre- and post-treatment ()). Three patients harbored pre-treatment WT1-specific T cell responses; in two of these, elevated responses were observed post-treatment. In addition, in two patients WT1-specific T cell responses were de novo induced after IRE treatment.
Figure 3. Pre-existent and IRE-induced WT1 specific T cell responses: relation to overall survival (OS). (a) Pre-and post-IRE specific T cell frequencies against WT1, and CEFT (i.e. CMV, EBV, Flu and Tetanus Toxoid derived recall epitopes) as measured by IFNγ Elispot assay (expressed as number of spot-forming T cells per 100,000) after a 10-day in vitro stimulation and expansion culture. Shown are means of six parallel cultures per tested antigen. Asterisks denote positive responses – as defined in the Materials and Methods section. (b) OS, ordered by OS in months from IRE. Highest measured WT1 specific T cell rates at any time during follow-up are listed in relation to OS per patient and reveal highest frequencies in patients with above median OS (dotted line indicates median OS). Frequencies in bold face denote positive responses (n = 5) as defined in the Methods section. (c) Correlation between WT1 response and OS in patients with positive versus negative WT1 specific T cell responses. Significance levels indicated were based on 2-sided unpaired T-test.
WT1 T cell reactivity in relation to overall survival
When patients were ordered by overall survival (OS), i.e. time from IRE to death (in months), it became clear that higher WT1 specific T cell frequencies (at any time during follow-up) were observed in patients with above median OS (11.1 months) ()). Indeed, a correlation was observed between median OS (from the time of IRE) and WT1 response, although this was not significant due to the small number of patients in this pilot study ()). Accordingly, higher mean OS was observed in patients with a positive post-IRE WT1 T cell response as compared to patients with a negative response (p = .055, )).
Discussion
Little is known about immune responses induced by IRE and the ability of these responses in pancreatic cancer to provide both local and systemic protection against recurrence. Our findings are encouraging in that they provide evidence for a transient lifting of systemic suppression by Tregs. This was accompanied by a transient increase of both CD4+PD-1+ and CD8+PD-1+ T cell numbers, which could point to T cell activation, as recent evidence indicates that PD-1+ T cells identify tumor specific T cells, not only in the tumor microenvironment but also in peripheral blood.Citation21 The selective upregulation of PD-1 and low or even absent expression of TIM3 and LAG3 argue against a state of exhaustion, but rather indicate activation of effector T cells that may be amenable to PD-1 blockade and thereby the release of brakes conceivably imposed on them by PD-L1 expressed in the tumor microenvironment.Citation22,Citation23 In keeping with this notion we found detectable (and durable) T cell responses to the tumor antigen WT1, which in turn were more prominent in patients with above median OS.
Minimally invasive interventional techniques for in situ tumor destruction are gaining ground clinically. Unlike with surgical resection, the treated malignancy is not removed from the body, but apoptotic or necrotic cell remnants induced by the ablative technique remain available to be taken up by phagocytic leukocytes. If apoptosis induction is accompanied by the release of damage-associated molecular patterns (like e.g. ATP and HMGB1), infiltrating DC will become activated and transport tumor fragments to draining lymph nodes where adaptive immune induction can take place. In effect, this local ablation thus serves to achieve in situ tumor vaccination.Citation24,Citation25 As a result, a durable and systemic antitumor T cell response may be induced that in turn can effectuate regression in distant, untreated metastases. In keeping with this notion, case reports of spontaneous regression of metastases following RFA of a primary tumor and enhancement of tumor-specific T-cell responses have been reported.Citation26
Thus far evidence of post-IRE induced specific antitumor immunity in man has been lacking. A major theoretic benefit of IRE is the sparing of larger vessels, which remain intact due to the largely non-thermal mechanism of action. Consequently, immune effector cells should be able to infiltrate the lesion and DC should be able to migrate to draining lymph nodes to induce a systemic immune response subsequent to IRE. Evidence for such post-IRE immune priming was provided in a study by Neal and colleagues, who studied the effects of IRE on subcutaneous renal cell tumors in an immune competent versus immune compromised mouse model.Citation27 Based on tumor burden and the progression-free interval, antitumor responses were substantially more durable in the IRE-treated immune competent mice relative to the IRE-treated immune deficient mice and sham controls. This was accompanied by robust T cell infiltration rates at the ablation border. Tumor rechallenge after IRE-mediated ablation in the immune competent mice resulted in an increased delay in tumor outgrowth or even in complete prevention of tumor growth. These findings clearly point to a protective antitumor immune response induced by IRE.Citation27 In a more recent report Bulvik et al. have compared the effects of IRE with those of RFA and found higher levels of systemic IL-6 post-IRE, which may indicate Damage-Associated Molecular Pattern (DAMP) mediated immune activation.Citation10 In a subcutaneous hepatocellular carcinoma model delayed tumor outgrowth was observed after IRE. Moreover, in the border zone surrounding the treated lesions, leukocyte infiltration into the ablation zone was demonstrated in IRE-treated, but not in RFA-treated lesions, indicating that not only larger vessels, but also micro-vessels were preserved after IRE.Citation10 Recently Shao et al. contributed to the evidence of IRE’s immune potentiating abilities by demonstrating that upon in vitro treatment of B16 murine melanoma cells, IRE induced more robust protein/antigen release and T-cell activation as compared to cryo- or heat-ablation.Citation28
In a recent publication of Pandit et al.,Citation29 CD4+ Th cell and Treg rates in peripheral blood were monitored in 11 patients with pancreatic cancer, who were treated by in situ IRE. A control group of four patients undergoing pancreatectomy was also included. All CD4+ T cell subsets (including CD4+CD25+FoxP3+ Tregs) decreased 1 day after treatment in both groups, followed by a steady increase in the pancreatectomy group, whereas in the IRE group these rates remained low up to day 5. Although we did not assess IRE-induced changes in peripheral Treg rates until 2 weeks post-IRE, our observation of significant decreases in activated and total Treg frequencies at that time (but of note, not of FoxP3+ activated Th cells, data not shown), are in line with the findings by Pandit and colleagues, as is the magnitude of the observed decreases. The combined data from their and our study suggest that Treg rates drop by 24h and remain significantly decreased until at least two weeks post-IRE; after three months Treg frequencies appear to be recovering. This points to a transient but actionable therapeutic window in which tumor-related immune suppression appears to be lifted and is in line with observations for other ablative interventions like RFA.Citation30 The observed immune stimulatory effects of intraoperative IRE observed by others, are consistent with our own observations of decreased Treg levels and expansion of WT1-specific T cells in 4/10 patients following percutaneous IRE. We found pre-treatment T cell reactivity to WT1 in 3/10 patients. This is surprisingly high for a supposedly weakly immunogenic tumor type like pancreatic cancerCitation11 and is promising as such natural immunity may be boosted to enhance antitumor efficacy. Indeed, in two of these patients we found increased WT1 specific T cell frequencies after IRE. Even more promising was the observation that in two additional patients de novo WT1 specific T cell responses were induced after IRE. WT1 has been reported to be expressed in 75% of pancreatic tumors and not at all in healthy pancreatic tissues,Citation31 confirming it as a bona fide immune target antigen. Although caution is warranted not to over-interpret our findings in this small group of patients, the seeming relationship between WT1 responsiveness and longer OS is promising. In line with this, WT1 specific vaccination was shown to provide clinical benefit in a subgroup of patients with pancreatic cancer.Citation15
Whereas local ablation through IRE may readily lead to in vivo vaccination in intrinsically immunogenic tumors (with high neo-antigen load), this will likely prove more challenging in weakly immunogenic tumors. The latter may benefit from therapies combining local ablation with immune stimulation, e.g. by intratumoral delivery of Toll-like receptor ligands (TLR-L) and/or immune checkpoint inhibition.Citation24,Citation25 In pancreatic cancer a lack of infiltrating DC and prevailing immune suppression is well documented and tumor cell apoptosis is a rare event.Citation11 These unfavorable circumstances all conspire to restrict the immunogenic potential of pancreatic cancer, but could well be overcome by immunogenic tumor cell death induction by IRE combined with further immune modulation, as was recently demonstrated by Zhao et al. in an orthotopic murine pancreatic cancer model. Combination of IRE with checkpoint inhibition by anti-PD1 significantly promoted tumor infiltration by CD8+ cytotoxic T cells and significantly prolonged survival. Most strikingly, IRE combined with anti-PD1 treatment achieved a durable memory T cell response with a cure rate of 36–43%.Citation32 Indeed, our finding of specific and transient upregulation of PD-1 levels on T cells provides a clear rationale for such a future combination therapy with PD-1 blockade in man.Citation23
Patients and methods
Patients and IRE procedure
The first ten patients (out of 25) with LAPC who were enrolled in the PANFIRE study between February 2013 and June 2014 were selected for this immune monitoring side study ().Citation9 The local institutional review board approved this trial (PANFIRE, clinicaltrials.gov NCT01939665). Study design and conduct were in accordance with the Declaration of Helsinki and were undertaken in accordance with the STROBE statement for observational studies.Citation33 Written informed consent was obtained prior to treatment. Patients were enrolled with histologically proven LAPC (maximum axial tumor diameter 5 cm). Locally advanced disease (stage III) was defined as per the 7th edition of the American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer.Citation34 Previous chemotherapy was allowed, provided it had been completed six weeks prior to the procedure. The IRE procedure was performed percutaneously using computed tomography (CT)-fluoroscopy guidance, as described previously.Citation9,Citation35 Size and shape of the tumor, including a 5mm tumor-free margin, determined the number and configuration of the electrodes (NanoKnife, AngioDynamics Latham, NY). Pulses were delivered until complete ablation of the macroscopically visible tumor was achieved. Three cycles of 30 pulses (1500 V/cm, 90 usec duration) were administered sequentially for each electrode pair, to reach a total of 100 pulses per pair. Upon completion of the ablation procedure, a transcatheter arterial and portal venous phase CT scan was made to confirm technical success (i.e. the absence of any residual tumor enhancement) and to detect early complications. At the time of writing all ten patients included in this side study had died of the disease at a median overall disease specific survival of 11.1 months from IRE (range 6.3–17.1 months).
Collection and processing of peripheral blood
Heparinized blood (50 ml) was collected from the patients just before the IRE procedure (T = 0), at 2 weeks (T = 2wk) and at 3 months (T = 3mnth) after treatment. The timing was based on the priming/boosting kinetics of tumor-specific effector T cells, which we have found optimal by 2–3 weeks in previous studies and together with the 3-month time point allows for assessment of long-term T cell memory.Citation36,Citation37 Immediately after sampling, peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation (Nycomed AS, Oslo, Sweden) and cryopreserved for later analysis, as described previously .Citation36
Flow cytometry
Multiparametric flow cytometry was performed to compare frequencies and activation status of both lymphocytic and myeloid subsets in PBMC before and after treatment, as described previously.Citation19,Citation20,Citation36,Citation38 See Supplemental Text for further details. For gating procedures, see Supplemental and .
T cell in vitro restimulation and IFNγ Elispot assay
Functional T cell responses to WT1 and recall antigens were determined following a previously described in vitro (re)stimulation and expansion protocol.Citation39 Cryopreserved PBMC were thawed and incubated at a 1:1 ratio with irradiated autologous PBMC pulsed with one of the following long peptide pools at 1 µg/ml per peptide: 1) a pool of 110 15-mer overlapping peptides spanning the entire Wilms tumor-1 protein (JPT Peptide Technologies); 2) CEFT positive control pool of 27 peptides selected from defined HLA Class I and II-restricted epitopes from CMV, EBV, Influenza and Tetanus Toxoid (JPT Peptide Technologies). PBMC were cultured for 10 days in the presence of IL-2 (10 IU/ml; Novartis) and IL-15 (10 ng/ml; eBioscience). The culture medium was changed every 3 to 4 days during in vitro stimulation. Cells were harvested at day 10 and seeded in 2 × 6 replicate split wells at a density of 2x105/well in a multiscreen 96-well plate (Millipore) coated with an IFNγ catch antibody (Mabtech). Cells were either rechallenged overnight with the peptides to which they were initially stimulated or cultured with a DMSO-vehicle control. Next day the cells were removed and the plates rinsed and developed according to manufacturer’s instructions (Mabtech). The spots were counted with a fully automated Elispot reader system (AID). Specific spots (i.e. antigen-specific T cell frequencies) were calculated by subtracting the mean number of spots of the DMSO control from the mean number of spots in the experimental wells. Antigen-specific T cell frequencies were considered to reflect positive responses when I) antigen-specific frequency was more than 2 times the background, II) the mean spot counts in the experimental wells exceeded those in the control wells by at least 10, and III) the mean number of spots in the experimental wells was significantly higher than the mean number of spots in the control wells as determined by an unpaired T-test.
Statistical analyses
Statistically significant decreases in the percentage of T cells after treatment were analyzed with one-sided repeated measures ANOVA and a post-hoc Dunnett’s multiple comparisons test or a two-sided Student’s T test. The two-sided Student’s unpaired T-test was used to test differences in survival and WT1 specific T cell frequencies between patient subgroups. Statistical analyses were performed with Prism GraphPad software (version 6.02). Differences with P < .05 were considered significant.
Advances in Knowledge
Percutaneous irreversible electroporation (IRE) induces a transient decrease in regulatory T cell frequencies in the peripheral blood of patients with Locally Advanced Pancreatic Cancer (LAPC) (P= .006).
IRE induces transient increases in frequencies of PD-1 expressing circulating CD4+ and CD8+ T cells in patients with LAPC (P= .01 and P= .04, respectively).
WT1-reactive and IFNγ releasing T cell rates in peripheral blood were boosted subsequent to IRE in 4 out 10 patients with LAPC.
Implications for Patient Care
IRE may create a temporary window for the successful application of immunotherapy in LAPC, in effect serving as a means of in vivo vaccination.
The selective upregulation of PD-1 on circulating T cells following percutaneous IRE provides a rationale for the subsequent administration of PD-1 checkpoint inhibitors.
Disclosure of interest
Martijn Meijerink is a paid consultant for AngioDynamics. The other authors have no competing interests to declare.
Ethics approval and consent to participate
The VU University Medical Center’s medical ethics committee (METc, OHRP# IRB00002991) approved the study registered under number 2013/155. All patients provided written informed consent prior to participation.
Availability of data and materials
Experimental data will be made available on request.
Supplemental Material
Download MS Word (16.3 KB)Supplemental Material
Download MS Word (275.4 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):1–8. doi:10.3322/caac.21208.
- Niederhuber JE, Brennan MF, Menck HR. The national cancer data base report on pancreatic cancer. Cancer. 1995;76(9):1671–1677. doi:10.1002/1097-0142(19951101)76:9<1671::aid-cncr2820760926>3.0.co;2-r.
- Pezzilli R, Serra C, Ricci C, Casadei R, Monari F, D’Ambra M, Minni F. Radiofrequency ablation for advanced ductal pancreatic carcinoma: is this approach beneficial for our patients? A systematic review. Pancreas. 2011;40(1):163–165. doi:10.1097/MPA.0b013e3181eab751.
- Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AAJM, Vieveen JM, Bouwman A, Meijer S, van Kuijk C, van Den Tol PMP, Meijerink MR. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interventional Radiol. 2014;25(7):997–1011. doi:10.1016/j.jvir.2014.01.028.
- Rubinsky B, Onik G, Mikus P. irreversible electroporation: a new ablation modality — clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi:10.1177/153303460700600106.
- Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M, Fontana M, D’Onofrio M, Martone E, Bassi C. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg. 2015;32(2):90–97. doi:10.1159/000375323.
- Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262(3):486–494; discussion 92-4. doi:10.1097/SLA.0000000000001441.
- Mansson C, Bergenfeldt M, Brahmstaedt R, Karlson BM, Nygren P, Nilsson A. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res. 2014;34:289–293.
- Scheffer HJ, Vroomen LG, de Jong MC, Melenhorst MC, Zonderhuis BM, Daams F, Vogel JA, Besselink MGH, van Kuijk C, Witvliet J, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology. 2017;282(2):585–597. doi:10.1148/radiol.2016152835.
- Bulvik BE, Rozenblum N, Gourevich S, Ahmed M, Andriyanov AV, Galun E, Goldberg SN. Irreversible electroporation versus radiofrequency ablation: a comparison of local and systemic effects in a small-animal model. Radiology. 2016;280(2):413–424. doi:10.1148/radiol.2015151166.
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–631. doi:10.1158/2326-6066.CIR-14-0027.
- Schnurr M, Duewell P, Bauer C, Rothenfusser S, Lauber K, Endres S, Kobold S. Strategies to relieve immunosuppression in pancreatic cancer. Immunotherapy. 2015;7(4):363–376. doi:10.2217/imt.15.9.
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi:10.1007/s00262-011-1028-0.
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi:10.1158/1078-0432.CCR-06-0369.
- Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20(16):4228–4239. doi:10.1158/1078-0432.CCR-14-0314.
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–1333. doi:10.1200/JCO.2014.57.4244.
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother (hagerstown, Md: 1997). 2013;36(7):382–389. doi:10.1097/CJI.0b013e31829fb7a2.
- Jose A, Sobrevals L, Ivorra A, Fillat C. Irreversible electroporation shows efficacy against pancreatic carcinoma without systemic toxicity in mouse models. Cancer Lett. 2012;317(1):16–23. doi:10.1016/j.canlet.2011.11.004.
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi:10.1016/j.immuni.2009.03.019.
- Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, Godkin A, Gouttefangeas C, de Gruijl TD, Koenen HJPM, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64(10):1271–1286. doi:10.1007/s00262-015-1729-x.
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22(4):433–438. doi:10.1038/nm.4051.
- Clouthier DL, Ohashi PS. Costimulation, a surprising connection for immunotherapy. Science (New York, NY). 2017;355(6332):1373–1374. doi:10.1126/science.aan1467.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349.
- Bastianpillai C, Petrides N, Shah T, Guillaumier S, Ahmed HU, Arya M. Harnessing the immunomodulatory effect of thermal and non-thermal ablative therapies for cancer treatment. Tumour Biol. 2015;36(12):9137–9146. doi:10.1007/s13277-015-4126-3.
- O’Brien MA, Power DG, Clover AJ, Bird B, Soden DM, Forde PF. Local tumour ablative therapies: opportunities for maximising immune engagement and activation. Biochim Biophys Acta. 2014;1846(2):510–523. doi:10.1016/j.bbcan.2014.09.005.
- Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66. doi:10.1148/radiol.2511072175.
- Neal RE 2nd, Rossmeisl JH Jr., Robertson JL, Arena CB, Davis EM, Singh RN, Stallings J, Davalos RV, Rubinsky B. Improved local and systemic anti-tumor efficacy for irreversible electroporation in immunocompetent versus immunodeficient mice. PLoS One. 2013;8(5):e64559. doi:10.1371/journal.pone.0064559.
- Shao Q, O’Flanagan S, Lam T, Roy P, Pelaez F, Burbach BJ, Azarin SM, Shimizu Y, Bischof JC. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int J Hyperthermia. 2019;36(1):130–138. doi:10.1080/02656736.2018.1539253.
- Pandit H, Hong YK, Li Y, Rostas J, Pulliam Z, Li SP, Martin RCG. Evaluating the regulatory immunomodulation effect of Irreversible Electroporation (IRE) in pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26(3):800–806. doi:10.1245/s10434-018-07144-3.
- Fietta AM, Morosini M, Passadore I, Cascina A, Draghi P, Dore R, Rossi S, Pozzi E, Meloni F. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum Immunol. 2009;70(7):477–486. doi:10.1016/j.humimm.2009.03.012.
- Oji Y, Nakamori S, Fujikawa M, Nakatsuka S-I, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004;95(7):583–587. doi:10.1111/j.1349-7006.2004.tb02490.x.
- Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, Melancon MP, Gupta S, Shen B, Peng W, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10(1):899. doi:10.1038/s41467-019-08782-1.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (clinical Research Ed). 2007;335(7624):806–808. doi:10.1136/bmj.39335.541782.AD.
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. doi:10.1245/s10434-009-0408-6.
- van Tilborg AA, Scheffer HJ, Nielsen K, van Waesberghe JH, Comans EF, van Kuijk C, van Den Tol PM, Meijerink MR. Transcatheter CT arterial portography and CT hepatic arteriography for liver tumor visualization during percutaneous ablation. J Vasc Interventional Radiol. 2014;25(7):1101–11.e4. doi:10.1016/j.jvir.2014.02.008.
- Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, Jooss K, Sacks N, Hege K, Lowy I, et al. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013;62(2):245–256. doi:10.1007/s00262-012-1330-5.
- Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, Haanen JBAG, van Den Eertwegh AJM, Scheper RJ, de Gruijl TD. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14(14):4532–4542. doi:10.1158/1078-0432.CCR-07-4711.
- Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, Hege K, Lowy I, Scheper RJ, Gerritsen WR, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. 2014;2:31. doi:10.1186/s40425-014-0031-3.
- Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–20415. doi:10.1073/pnas.0810114105.
