ABSTRACT
CD47 is known to be involved in phagocyte-mediated tumor clearance; however, its expression, clinical significance, and regulatory mechanism in hepatocellular carcinoma (HCC) remain poorly understood. In the present study, we found that upregulation of CD47 expression on tumor cells was correlated with poor overall survival and recurrence-free survival in patients with HCC. Abundance of macrophages (Mφs) infiltration was found in CD47+ tumor tissues. Mechanistic studies revealed that IL-6 derived from tumor-infiltrating Mφs could upregulate CD47 expression on hepatoma cells through activation of the STAT3 pathway. Neutralization of CD47 or disruption of the IL-6–STAT3 axis reduced the ability of tumor cells to escape phagocytosis. Moreover, CD47 blockade could enhance Mφ-mediated phagocytosis in the presence of chemotherapeutic drugs, and HCC patients with lower CD47 expression were more likely to benefit from adjuvant transcatheter arterial chemoembolization (TACE) treatment. These findings revealed that Mφ-derived IL-6 was responsible for CD47 expression on hepatoma cells, which might be served as a potential prognostic marker and a predictor for patients who might benefit from adjuvant TACE treatment.
Keywords:
Introduction
Hepatocellular carcinoma (HCC) is one of the most fatal cancers and the leading cause of cancer-related mortality worldwide.Citation1 Although surgical resection provides a potentially curative treatment, intrahepatic recurrence is common and leads to poor survival of HCC patients. Palliative treatments, such as transcatheter arterial chemoembolization (TACE), are often applied in patients with recurrent tumors. However, the improvement in survival is still limited.Citation2–Citation4 Therefore, it is necessary to elucidate the molecular basis and biomarkers of HCC that might help identify patients who may benefit from adjuvant therapies.
Evasion of programmed cell removal by the innate immune system plays a critical role in tumor development and progression.Citation5–Citation11 Recent studies have revealed that tumor cells upregulate “don’t-eat-me” signals to prevent phagocytosis, thus acquiring a survival advantage.Citation12–Citation14 CD47 is an anti-phagocytic ligand broadly expressed on platelets, erythrocytes, and lymphohematopoietic cells. It delivers an inhibitory signal through the receptor signal regulatory protein alpha (SIPRα), which is expressed on phagocytes.Citation15–Citation17 Abundant expression of CD47 has been observed on tumor cells and correlates to poor survival in patients across different tumor types, such as small-cell lung cancer, melanoma, glioblastoma, and leukemia.Citation12,Citation18–Citation20 Besides, CD47 expression could also be induced by chemotherapy and mediates therapy resistance in breast cancer.Citation21 Translation of CD47-blocking therapies has shown potential activity in some cancer patients.Citation22 However, the underlying mechanisms of CD47 expression are not fully understood.
Blockade of CD47-SIRPα signaling is intended to promote the ability of myeloid cells, in particular, macrophages (Mφs) to phagocytose tumor cells and induce antitumor responses.Citation23 Nevertheless, there is substantial evidence that Mφs in the tumor microenvironment could be programmed into an altered phenotype that promotes tumor progression.Citation17,Citation24–Citation30 For example, our previous studies showed that tumor-activated monocytes (Mos)/Mφs secrete cytokines, such as TNFα, IL-1β and IL-6, to enhance tumor cell autophagy and angiogenesis in HCC.Citation31,Citation32 Given the critical role of Mos/Mφs in regulating tumor progression, we speculated that it would, in turn, influence the phagocytic signaling on tumor cells, thus resulting in impaired phagocytic clearance of the tumor.
In the present study, we demonstrated the increased expression of CD47 on HCC cells was positively correlated with the density of Mφs and poor prognosis in HCC patients. Mechanistic studies revealed that IL-6 derived from tumor-activated Mφs was responsible for the upregulation of CD47 expression on hepatoma cells via activation of the STAT3 pathway. Moreover, we provided evidence that HCC patients with lower CD47 expression were more likely to benefit from adjuvant TACE treatment.
Results
Expression and clinical significance of CD47 in HCC
To evaluate the potential role of CD47, we first investigated its expression in HCC tissues. Immunohistochemistry (IHC) staining showed that CD47 was detected on the membranes of erythrocytes, sinusoidal endothelial cells, immune cells, and parenchyma cells (). Tumor cells showed a relatively high level of CD47 expression compared to that on hepatocytes in the non-tumor region. We divided the patients into two groups based on the median expression of CD47 (H-score = 11.5). Statistical analysis showed that the presence of CD47 on tumor cells was significantly associated with Child–Pugh score, tumor number, vascular invasion, and TNM stage of patients with HCC (Supplementary Table S2). Kaplan–Meier survival analysis showed that patients with higher CD47 expression on tumor cells had shorter overall survival (OS; median, 34 months; 5-year OS, 45.4%) and recurrence-free survival (RFS; median, 11 months; 5-year OS, 27.1%) than those in patients with low CD47 expression (median OS, 57 months; 5-year OS, 67.4%; median RFS, 17 months; 5-year RFS, 43.3%; ). Univariate and multivariate analyses revealed that CD47 expression on tumor cells was an independent prognostic factor for both OS (HR = 1.768, P = .001) and RFS (HR = 1.481, P = .006) in patients with HCC ().
Table 1. Univariate and multivariate analysis of factors associated with survival and recurrence.
Figure 1. Expression and clinical significance of CD47 in HCC.
(a) Representative microphotographs of CD47 immunostaining in HCC tissues. The H-score = 11.0 was applied to divide patients with low and high CD47 expression. The scale bar indicates 50 μm (red arrows represent parenchymal cells, blue arrows represent endothelial/immune cells, green arrows represent erythrocytes). (b) Kaplan–Meier plots of overall survival (OS) and recurrence-free survival (RFS) rates of HCC patients stratified by CD47 expression. P value was calculated by the log-rank test. (c-d) qPCR and western blot demonstrated CD47 expression in a variety of hepatoma cell lines. (e-g) Hep3B and PLC tumor cells were labeled with CFSE and cultured with monocyte-derived-macrophages from human peripheral blood. The cells were incubated with IgG or CD47 blocking antibody; 200× magnification images of the cell are shown. Incubation with blocking anti-CD47 antibodies resulted in increased phagocytosis of CFSE-labeled Hep3B and PLC cells (arrows).
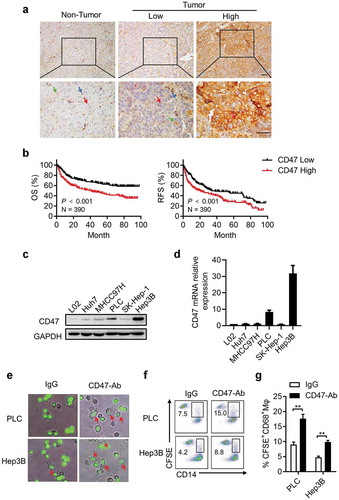
Next, we investigated the functional role of CD47 expression in five different HCC cell lines. As shown in , PLC and Hep3B cells showed high CD47 expression whereas Huh7, 97H, SK, and L02 (normal hepatocyte) cells showed low expression, as detected by both western blot and quantitative real-time PCR (qPCR). To examine the role of CD47 during phagocytosis by Mφs, Hep3B and PLC cells with high ectopic CD47 expression were labeled with CFSE and then co-cultured with Mφs derived from human peripheral blood. The results showed that tumor cells incubated with CD47-neutralizing antibodies exhibited increased phagocytosis as compared to that in the IgG control groups. Furthermore, there was an inverse correlation between CD47 expression and the phagocytic index in Hep3B and PLC cells ().
Taken together, the results suggested that CD47 expressed on hepatoma cells might promote tumor progression by resisting phagocytosis in HCC.
Mφs regulate CD47 expression on hepatoma cells
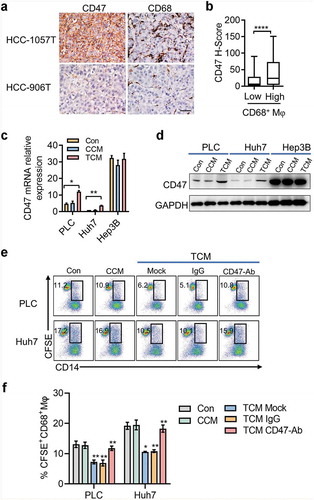
Considering the diverse impact of Mφs on cancer cells (in situ tumors), IHC was performed to examine the correlation between CD68+ Mφs and CD47-expressing tumor cells. The results showed that the density of CD68+ Mφs was positively correlated with CD47-expressing tumor cells in HCC tumor tissues (). To evaluate the effect of Mφs on CD47 expression in hepatoma cells, we incubated hepatoma cells for 24 h in conditioned medium from control human Mφs (CCM) or tumor culture supernatant (TSN)-exposed Mφs (TCM), which mimicked the tumor milieu. The results showed that TCM, but not CCM, effectively induced CD47 expression on PLC and Huh7 cells whereas the expression of CD47 on Hep3B cells was unaffected, which might be due to the higher ectopic CD47 expression on Hep3B cells (). Functional experiments showed that TCM-treated PLC and Huh7 cells had an enhanced ability to resist phagocytosis by Mφs (). These findings showed that Mφs in the tumor microenvironment could induce CD47 expression on hepatoma cells.
Figure 2. Mφs regulates CD47 expression on hepatoma cells.
(a) Representative microphotographs of CD47 expression and CD68+ Mφs accumulation in HCC tumor tissue. The scale bar indicates 50 μm. (b) Correlation between CD47 expression level and the number of Mφs. Data from 181 HCC patients shown as mean ± SEM. **** p < .0001. (c-d) Tumor cells were cultured for 48 h in conditioned medium from control Mφs (CCM) or medium from TSN-exposed Mφs (TCM). qPCR and western blot were applied to detect the CD47 expression. Data from three independent experiments shown as mean ± SEM. * p < .05. (e-f) Representative FACS analysis and summary of tumor cell phagocytosis by Mφs after treatment with CCM or TCM. Data from three independent experiments shown as mean ± SEM. ** p < .01.
IL-6 derived from tumor-activated mφs is responsible for CD47 upregulation on hepatoma cells
The above results indicated that soluble factors derived from tumor-activated Mφs (TAMs) induced CD47 expression in hepatoma cells. To determine the soluble factors accounting for the effects of TAMs on CD47 expression, we examined the levels of various cytokines in CCM and TCM and found that TAMs secreted significant amounts of IL-6, TNF-α, IL-1β, IL-12, and IL-10 (). To evaluate the contribution of these molecules to induce of CD47 expression, we initially used a specific neutralizing antibody that effectively abolished the role of IL-6, TNF-α, IL-1β, IL-12, or IL-10. qPCR and western blot showed that recombinant human (rh)IL-6 significantly induced CD47 expression on Huh7 and PLC cells in a dose-dependent manner; however, rhTNF-α, rhIL-1β, rhIL-12, and rhIL-10 had barely any impact on CD47 expression ().
Figure 3. IL-6 derived from tumor-activated Mφs (TAMs) is responsible for CD47 upregulation on hepatoma cells.
(a) Cytokine level of macrophages secreted after no treatment or cultured with the supernatant from Huh7 and PLC cells (TSN) for 48 hours. (b-c) PLC and Huh7 were incubated for 48 h with a concentration gradient of rhTNF-α, rhIL-1β, rhIL-6, or rhIL-10, and the CD47 expression on tumor cells was determined by qPCR and western blotting. Data from three independent experiments shown as mean ± SEM. * p < .05; ** p < .01; *** p < .001. (d-e) PLC and Huh7 cells were cultured in TCM and mAb against indicated blocking antibodies, and the CD47 expression on tumor cells was determined by qPCR and western blotting. Data from three independent experiments shown as mean ± SEM. * p < .05. (f-g) mRNA level of CD47 expression in PLC and Huh7 cells cultured in TCM and tocilizumab (5 µg/mL). Data from three independent experiments are shown as mean ± SEM. * p < .05. Western blot of PLC and Huh7 cells for CD47 expression after treatment with TCM or tocilizumab (5 µg/mL). One of three independent experiments is shown. (h-i) PLC and Huh7 cells were cultured with Mφs after treatment with IL-6 for 48 h. The percentages of phagocytosis were determined by FACS. Statistical analysis of at least three independent experiments shown as mean ± SEM. * p < .05; ** p < .01. (j-k) Representative FACS data and statistical analysis of tumor cell phagocytosis by Mφs treated with TCM or IL-6 blocking antibody or tocilizumab. Data from three independent experiments shown as mean ± SEM. ** p < .01.
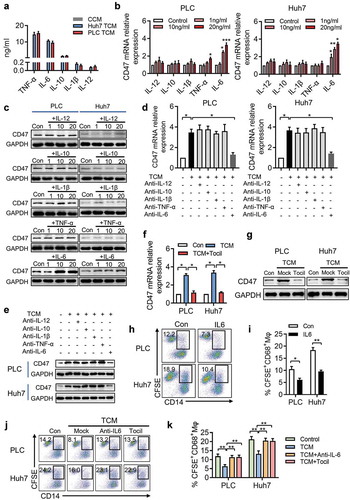
To confirm the contribution of these molecules toward the induction of CD47 expression, a specific neutralizing antibody that effectively abolished the role of IL-6, TNF-α, IL-1β, IL-12, or IL-10 was used in our conditioned medium system. As shown in , blockade of IL-6, but not TNF-α, IL-1β, IL-12, or IL-10, markedly decreased the upregulation of CD47 expression induced by TCM. These findings suggested TAM-derived IL-6, rather than TNF-α, IL-1β, IL-12, and IL-10 was responsible for the upregulation of CD47 expression on hepatoma cells. To further confirm IL-6 was necessary for TAM-enhanced CD47 expression, we treated Huh7 and PLC cells with tocilizumab, a humanized monoclonal antibody against the IL-6 receptor (IL-6R). Tocilizumab effectively abolished the upregulation of CD47 expression induced by TCM ().
We also examined whether IL-6 could enhance the ability of tumor cells to avoid Mφ-mediated phagocytosis. Consistent with the above results, Huh7 and PLC cells treated with IL-6 exhibited increased anti-phagocytosis abilities (), whereas, IL-6-neutralizing antibody and tocilizumab decreased the proportion of phagocytosis compared to that in the TCM-only treatment group (). Taken together, these results indicated that TAM-derived IL-6 could upregulate CD47 expression and enhance the ability of tumor cells to escape phagocytosis.
STAT3 signaling is essential for IL-6-induced CD47 expression in HCC
We next sought to determine the underlying mechanism of IL-6-induced CD47 expression on hepatoma cells. It has been reported that the classic pathway of IL-6 downstream signaling involved STAT3. As expected, western blot showed that IL-6-treated Huh7 and PLC cells showed increased phospho-STAT3 compared to that in the control groups, without influencing the total STAT3 expression (). We then treated Huh7 and PLC cells with NSC74859, a novel inhibitor of STAT3 signaling, and found that STAT3 signaling inhibition significantly disrupted TCM and IL-6-induced CD47 upregulation in hepatoma cells (). Consistent with this finding, inhibition of the STAT3 pathway in Huh7 and PLC cells disrupted their ability to escape Mφ-mediated phagocytosis ().
Figure 4. STAT3 signaling is essential for IL-6-induced CD47 expression in HCC.
(a) p-STAT3 and total STAT3 protein levels were assessed by western blotting. One of three representative experiments is shown. (b-c) PLC and Huh7 were cultured with IL-6 or TCM and STAT3 inhibitor NSC74859 (50 µM). CD47 expression was determined by qPCR. * p < .05; ** p < .01. (d) Western blot detected the CD47 level of PLC and Huh7 cells treated with IL-6 or TCM and STAT3 inhibitor NSC74859 (50 µM). (f-g) FACS analysis and summary of tumor cell phagocytosis by macrophages after treatment with IL-6 or TCM and NSC74859. * p < .05; ** p < .01. (h) Representative microphotographs of CD47 expression and p-STAT3+ cells in HCC tumor tissue. The scale bar indicates 50 μm. (i) The CD47 expression level was positively correlated with the density of p-STAT3+ cells. ****p < .0001.
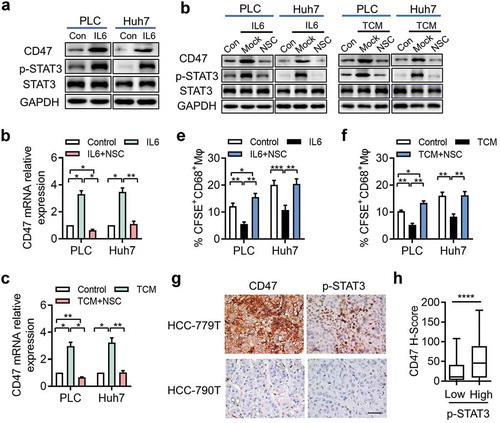
To validate the effect of the STAT3 pathway on CD47 expression in HCC, we examined the densities of p-STAT3+ cells and CD47-expressing tumor cells in situ. Correlation analysis revealed that STAT3 phosphorylation was positively associated with the level of CD47 expression on tumor cells (). These data indicated that disruption of the STAT3 pathway inhibited IL-6-mediated upregulation of CD47 expression in HCC.
CD47 expression on hepatoma cells decreases the efficacy of TACE
To further explore the clinical significance of CD47 expression in HCC treatment, we tested the impact of CD47 blockade in hepatoma cells treated with doxorubicin or 5-fluorouracil (FU), two chemotherapeutic drugs commonly used in TACE. Blockade of CD47 function by a neutralizing antibody in the presence of a high dose of doxorubicin or 5-FU that could efficiently induce apoptosis of tumor cells (data not shown) resulted in a significantly higher rate of Mφ-mediated phagocytosis of Hep3B and PLC cells (). Moreover, TCM or IL-6 treatment reduced Mφ-mediated phagocytosis in Huh7 and PLC cells treated with doxorubicin or 5-FU (). These results suggested that CD47-mediated anti-phagocytosis effects in hepatoma cells treated with chemotherapeutic drugs might decrease the efficacy of TACE.
Figure 5. CD47 expression on hepatoma cells decreases the efficacy of TACE.
(a) Representative FACS analyses of tumor cell phagocytosis by Mφs when treated with IgG or CD47 blocking antibody and doxorubicin or 5-FU. (b) Summary of (a). Data from three to six independent experiments shown as mean ± SEM. * p < .05; ** p < .01. (c) FACS analysis and summary of tumor cell phagocytosis by Mφs after treatment with IL-6 or TCM and doxorubicin or 5-FU. * p < .05; ** p < .01. (d) Kaplan–Meier plots of the overall survival rate of HCC patients who developed recurrent HCCs and received post-operative TACE treatment stratified by CD47 expression. P value was calculated by the log-rank test. (e) Kaplan–Meier plots of overall survival rate of HCC patients who developed recurrent HCCs and did or did not received post-operative TACE treatment in the CD47low or CD47high group . P value was calculated by the log-rank test.
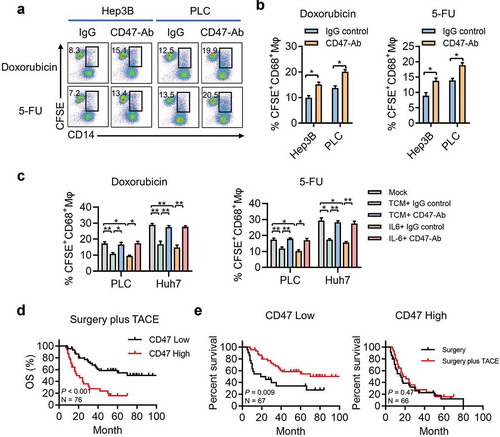
We further examined the clinical significance of CD47 expression for post-operative TACE treatment. During the follow-up, 76 patients developed recurrent HCCs and received post-operative TACE treatment. We divided the patients into two groups based on the median expression of CD47 (H-score = 15.5).The Kaplan–Meier survival analysis showed that patients with higher CD47 expression on tumor cells had a shorter OS (median, 20 months; 5-year OS, 14.8%) than did patients with low CD47 expression (median, 46 months; 5-year OS, 49.2%; ). We analyzed the survival benefits in patients with recurrent tumors who did and did not receive post-operative TACE treatment. No correlation was found in clinical characteristics between the two groups of patients (Supplementary Table 3). As compared with patients with surgery alone, post-operative TACE significantly prolonged the survival in patients with a low level of CD47 expression; whereas, in the CD47 high expression group, no significant survival benefit was found (). These data indicated that CD47 expression might be a potential predictive factor for the response to adjuvant TACE treatment in patients with HCC.
Discussion
Blockade of the innate immune checkpoint regulator CD47 has demonstrated potential efficacy in a variety of preclinical models. In the present study, we demonstrated that CD47 was significantly upregulated in HCC tumor cells and could serve as an independent prognostic factor for both OS and RFS in patients with HCC. IL-6 derived from TAMs upregulated CD47 expression through activation of STAT3 signaling, and subsequently led to an increase in the anti-phagocytotic capacity of tumor cells. Moreover, CD47 blockade could also enhance Mφ-mediated phagocytosis in the presence of chemotherapeutic drugs, and HCC patients with lower CD47 expression were more likely to benefit from adjuvant TACE treatment.
CD47 has been identified as a marker of self-identification through binding to phagocytic cells that express its receptor SIRP-a, to escape attacking by the innate immune cells.Citation15,Citation33,Citation34 Anti-CD47 antibodies or SIRPα-Fc fusion proteins have been used to block CD47-SIRPα signaling to promote phagocytosis of tumor cells in many murine models.Citation12,Citation13,Citation35 Upregulation of CD47 has been reported in hematologic malignancies and certain types of solid tumors; however, the in situ expression of CD47 in patients with HCC is still unclear.Citation36–Citation39 In this study, we demonstrated that CD47 could be detected on the membrane of erythrocytes, sinusoidal endothelial cells, and some immune cells, while high expression of CD47 was found on hepatoma cells in HCC tumor tissues. Survival analysis showed that high expression of CD47 on tumor cells was an independent prognostic factor for HCC patients. Moreover, anti-CD47 antibody increased the phagocytosis of hepatoma cells by Mφs. These data suggests that CD47 could be a potential target in HCC treatment.
The phagocytosis process regulated by CD47-SIRPα signaling was dependent on phagocytes, particularly Mφs. We found an accumulation of Mφs in HCC tumor tissues; however, the abundance of Mφs did not facilitate tumor clearance but was positively correlated with CD47 expression on the tumor cell. Our previous studies showed that tumor-activated Mos/Mφs secrete cytokines, such as TNFα, IL-1β, and IL-6, that could influence tumor progression.Citation40 In this study, we found that IL-6 secreted from TAM promoted tumor resistance to phagocytosis by upregulating CD47 expression. Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor (IL-6R) that has been widely used to inhibit inflammation in rheumatoid arthritis.Citation41,Citation42 Our data showed that inhibition of IL-6 signaling by Tocilizumab could antagonize the effect of TAM-mediated anti-phagocytosis, making it a potential drug for HCC therapy. Overall, these data revealed the dual roles of tumor-educated Mφs in mediating phagocytosis in HCC.
Other mechanisms have been reported to regulate CD47 expression, such as the MYC oncogene up-regulated CD47 expression on the leukemia and lymphoma tumor cell surface, and MYC inactivation down-regulated CD47 expression and enhanced the anti-tumor immune response in mouse models.Citation43 Besides, P21waf1 has been reported to upregulate CD47 expression in LSA74T colorectal cells and MCF7 breast cells;Citation44 miR-708 could directly target CD47 through binding to 3′UTR and was inversely correlated with CD47 expression in acute lymphoblastic leukemia tumor cells.Citation45 Our study showed that TAM-derived IL-6 upregulated CD47 expression on hepatoma cells via the STAT3 pathway and that inhibition of IL-6 signaling by neutralizing antibody or STAT3 phosphorylation could antagonize the effect of TAM-induced CD47 expression and the anti-phagocytotic capacity of tumor cells. A recent report showed that CD47 expression could be regulated by TNF-α-induced nuclear factor kappa B (NF-κB) activation on hepatoma cells.Citation39 Our data found that TNF-α exhibited a limited effect in the inducement of CD47 expression at the RNA level in PLC cells, which might be due to the inactivation of STAT3 signaling pathway in PLC and Huh7 cells when treated with TNF-α (data not shown). Moreover, a positive correlation between the expression of p-STAT3 and CD47 was found in HCC tissues. Although the underlying mechanism by which the STAT3 pathway regulates CD47 expression needs further exploration, these data indicate that the mechanisms underlying CD47 expression vary in different tumor models and tumor microenvironments.
TACE has been commonly used as a palliative treatment for unresectable or recurrent HCC. Chemotherapeutic drugs such as doxorubicin and 5-FU are commonly used for TACE and have been approved to play an important role in the survival advantage of TACE.Citation46,Citation47 Treatment of human or murine TNBC breast cancer cells with carboplatin, doxorubicin, gemcitabine, or paclitaxel has been reported to mediate transcriptional induction of CD47 expression.Citation21 However, the apoptosis of cancer cells might also be induced by chemotherapy that leads to CD47 inactivation and ineffective antibody treatment. We first examined the anti-phagocytotic effect of CD47 on apoptotic tumor cells in vitro. The results suggested that CD47 on apoptotic hepatoma cells could also resist Mφ-mediated phagocytosis. Clinical sample analysis further confirmed that the expression of CD47 could influence the efficacy of post-operation TACE. Moreover, HCC patients with low CD47 expression showed a longer OS in surgery plus TACE treatment group in comparison with surgery alone; whereas no treatment advantage was found among patients in the high-CD47 group. Considering the limited benefit of palliative TACE and the extra burden for the patients,Citation48,Citation49 CD47 might help identify high-risk patients who might benefit from adjuvant treatments.
In conclusion, our data revealed a novel mechanism by which tumors utilize the cytokine from tumor-activated Mφs to upregulate CD47 expression, which subsequently promotes their resistance to phagocytosis and facilitates tumor progression in HCC patients. CD47 expression might thus serve as a potential prognostic marker and a predictor for patients who might benefit from adjuvant TACE treatment.
Material and methods
Patients and specimens
A total of 390 patients who received curative resection for HCC at Sun Yat-sen University Cancer Center between 2005 and 2010 were randomly enrolled as previously described.Citation50 The inclusion criteria used in patient enrollment were the absence of anticancer therapies prior to the operation and availability of follow-up data. During the follow-up period, 223 patients developed recurrent HCCs and 166 of them received further treatments, including a second surgical resection, TACE, or radiofrequency ablation. Overall survival (OS) was defined as the interval between the time of surgery to death or the last follow-up. Recurrence-free survival (RFS) was defined as the time from the date of surgery to recurrence, or the last follow-up if no recurrence was observed. The clinical pathological characteristics of the patients are summarized in Supplementary Table S1.
Control blood samples were obtained from 20 healthy blood donors attending the Guangzhou Blood Center, all of whom showed negative findings for antibodies against hepatitis C virus, hepatitis B virus, HIV, and syphilis. All samples were anonymously coded in accordance with local ethical guidelines as stipulated by the Declaration of Helsinki with written informed consent and a protocol approved by the Review Board of Sun Yat-sen University Cancer Center.
Tissue microarray construction
Tissues were used to construct a tissue microarray (TMA), as described previously.Citation50 In brief, tissue blocks containing the invading edges of tumoral HCC tissue were used for TMA construction. Each representative area was pre-marked in the paraffin-embedded blocks on the basis of the hematoxylin and eosin staining, excluding necrotic and hemorrhagic areas, and duplicates of 1-mm cylinders from the tumor tissues were obtained from each patient to ensure reproducibility and homogeneity.
Immunohistochemistry and image analysis
Paraffin-embedded and formalin-fixed samples were cut into 4-µm sections, which were then processed for immunohistochemistry (IHC) analysis.Citation51 Sections were dewaxed in xylene and rehydrated through a decreasing ethanol series. Subsequently, sections were placed in 0.3% H2O2 for 10 min at room temperature to diminish the activity of endogenous peroxidase. Epitope retrieval was performed by boiling the sections in 10 mM citrate buffer for 10 min. The sections were then incubated with anti-CD47 antibody (dilution 1:500, R&D, Cat# AF4670), CD68 antibody (dilution 1:200, Dako, Cat#0879), or p-STAT3 (dilution 1:200, Cell Signaling, Cat#19145) overnight at 4°C. Horseradish peroxidase-conjugated anti-sheep IgG (dilution 1:500, R&D, Cat# HF016) was used as a secondary detection reagent and developed with 3,3′-diaminobenzidine (DAB). All sections were counterstained with Mayer’s hematoxylin and mounted with a non-aqueous mounting medium.
Vectra-Inform image analysis system (Perkin-Elmer Applied Biosystems) was used to assess the expression level of CD47; for quantitation, the signals on the multicolored images were unmixed using the spectral library by recognizing their unique spectral curves. InForm image analysis software (version 2.0.1; Perkin-Elmer Applied Biosystems) was used to analyze images by identifying subcellular compartments (nucleus, cytoplasm, and membrane) and tissue compartments. The H-score was calculated by quantifying target signals in selected tissues and cellular compartments of interest in accordance with the manufacturer’s protocol.
Confocal microscopy analysis
To quantify CFSE+ macrophages in culture system, a standard fluorescence-field scanning protocal was developed. Images were acquired using a laser confocal microscope (Olympus, Essex, UK) and analyzed using FV-ASW Viewer software (Olympus).
Cell lines
The normal hepatocyte line L02 and HCC cell lines SK-Hep-1, PLC, and Hep3B were obtained from ATCC, and MHC97H and Huh7 were obtained from Shi Lab in Sun Yat-Sen University Cancer Center. Cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Cat#C11995500BT) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Cat#30067334) in a 5% CO2 atmosphere at 37°C, except PLC which were cultured in Roswell Park Memorial Institute 1640 medium (RPMI1640; Life Technologies, Cat# C11875500BT).
Immunoblotting
Immunoblotting was performed as previously described.Citation32 Protein was extracted using a protein extraction kit (Thermo Fisher, Cat#89900) according to the manufacturer’s instructions. SDS-PAGE was used to separate an equal amount of cellular proteins. The primary antibodies were as follows: anti-CD47 (Abcam, Cat#ab175388), anti-β-Actin (Cell Signaling, Cat#4970), anti-GAPDH (Cell Signaling, Cat#5174), anti-p-STAT3 (Cell Signaling, Cat#19145), and anti-STAT3 (Cell Signaling, Cat#12640). The membranes were visualized by using a commercial ECL kit (Millipore, Cat#P90720).
In vitro phagocytosis assay
Monocytes (Mos) were isolated from the blood from healthy donors using anti-CD14 magnetic beads (Miltenyi Biotechnology, Cat#130–091-097). Mφs had developed from Mos after 7 days’ culture in DMEM supplemented with AB serum. To perform the in vitro phagocytosis assay, Mφs were incubated in serum-free medium for 2 h prior to co-culture with tumor cells, which were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Cat#V12883) according to the manufacturer’s protocol. CD47 blocking antibody (R&D, Cat#AF4670) or IgG control (R&D, Cat#5–001-A) were added at a gradient concentration and incubated for 2 h at 37°C. Confocal microscopy and flow cytometry were used to detect phagocytosis. The phagocytic index was calculated as the number of phagocytosed CFSE+ cells per 100 Mφs.
Flow cytometry
For surface marker staining, the cells were prepared and suspended in PBS solution supplemented with 1% heat-inactivated fetal bovine serum. An AF700 anti-CD14 (BD Biosciences, Cat#557923) was used to identify macrophages. Data were acquired on a Cytoflex flow cytometer (Beckman Colter, USA) and analyzed with FlowJo software.
Preparation of cultured supernatants
TSNs were prepared by plating 5 × 106 tumor cells in 10 mL of complete DMEM medium in 100-mm dishes for 24 h, and the medium was thereafter changed to complete medium supplemented with 10% FBS. After 2 days’ incubation, the supernatants were harvested, centrifuged, and stored in aliquots at −80°C. To generate conditioned medium, Mφs (2 × 106) were seeded in 6-well plates overnight, then treated with medium or 15% TSN for 2 h, and subsequently washed the complete media and then cultured in DMEM supplemented with 10% FBS for 16 h, which was defined as conditioned medium from the control Mφs (CCM) or conditioned medium from TCM. Thereafter, the supernatant was centrifuged and stored in aliquots at −80°C.
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent ((Life Technologies, AM9738). Equal amounts of total RNA from each sample were subjected to oligo (dT)-primed cDNA synthesis using 5 × All-In-One RT MasterMix (Abm, Cat#G492). qPCR was performed using the flowing specific forward and reverse primers: for CD47: forward, 5ʹ-AGCATGGAATGACGACAGTG-3ʹ and reverse, 5ʹ- GATGTGGCCCCTGGTAGC-3ʹ; GAPDH: forward, 5ʹ-GAGTCAACGGATTTGGTCGT-3ʹ and reverse, 5ʹ-GACAAGCTTCCCGTTCTCAG-3ʹ. Reactions were run on according to a standard protocol using the SYBR Green Teal-Time MasterMix (TOYOBO, Cat#QPS-201) on a Roche Light Cycler 480 System (Roche Diagnostics). The relative expression of CD47 was normalized to that of GAPDH.
ELISA assay
The concentrations of TNF-α, IL-6, IL-1β, IL-10, and IL-12 (eBioscience, Cat#88–7346; Cat#88–7066; Cat#88–7106; Cat#88–7261; Cat#88–8086) in the Mφs’ culture media stimulated by TSN were determined using a commercial set according to the manufacturer’s instructions.
Statistical analysis
The statistical significance of intergroup differences was analyzed by using a two-tailed Student t test, and all values are expressed as mean ± SE. Pearson correlation analysis and linear regression analysis were used to assess the correlations among groups. The OS and RFS curves were generated by the Kaplan–Meier method and the log-rank was applied to compare the groups. Prognostic factors were determined by univariate and multivariate analysis with the Cox proportional hazards model. All data were analyzed using IBM SPSS (version 21.0; IBM Corp., Armonk, NY, USA) statistical software. All data were analyzed by using two-tailed tests unless otherwise specified, and P < .05 was considered statistically significant.
Abbreviations
HCC Hepatocellular carcinoma
Mφs Macrophages
TACE Transcatheter arterial chemoembolization
SIPRα Signal regulatory protein alpha
OS Overall survival
RFS Recurrence-free survival
TMA Tissue microarray
IHC Immunohistochemistry
DAB 3,3′-diaminobenzidine
TSN Tumor culture supernatant
TCM Conditioned medium from tumor culture supernatant exposed Mφs
CCM Conditioned medium from control human Mφs
5-FU 5-fluorouracil
HBsAg Hepatitis B surface antigen
AFP α-fetoprotein; ALT: Alanine aminotransferase
TNM Tumor-node-metastasis
TNF-α Tumor necrosis factor alpha
IL-6 Interleukin 6
IL-10 Interleukin 10
IL-1β Interleukin 1 beta
IL-12 Interleukin 12
Disclosure of potential conflicts of interest
All authors declare that there are no conflicts of interest.
Supplemental Material
Download Zip (64.4 KB)Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262.
- Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–535. doi:10.1038/nrclinonc.2014.122.
- Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855–862. doi:10.1016/j.jhep.2014.11.014.
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi:10.1016/S0140-6736(02)08649-X.
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi:10.1038/ni.2703.
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi:10.1038/nature22396.
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi:10.1038/nrc3581.
- Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, Wang J, Song J, Zheng M, Sun H, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. 2017;6:e1264562. doi:10.1080/2162402X.2016.1264562.
- Stakheyeva M, Riabov V, Mitrofanova I, Litviakov N, Choynzonov E, Cherdyntseva N, Kzhyshkowska J. Role of the immune component of tumor microenvironment in the efficiency of cancer treatment: perspectives for the personalized therapy. Curr Pharm Des. 2017;23:4807–4826. doi:10.2174/1381612823666170714161703.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi:10.1038/nrclinonc.2017.101.
- Roselli M, Formica V, Cereda V, Jochems C, Richards J, Grenga I, Orlandi A, Ferroni P, Guadagni F, Schlom J. The association of clinical outcome and peripheral T-cell subsets in metastatic colorectal cancer patients receiving first-line FOLFIRI plus bevacizumab therapy. Oncoimmunology. 2016;5:e1188243. doi:10.1080/2162402X.2016.1188243.
- Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer J-P, Liu J, Lim JS, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126:2610–2620. doi:10.1172/JCI81603.
- Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu Y-X, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi:10.1038/nm.3931.
- Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10:12. doi:10.1186/s13045-016-0381-z.
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi:10.1016/j.cell.2009.05.046.
- Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi:10.1038/nri1859.
- Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol. 2011;8:285–288. doi:10.1038/cmi.2010.83.
- Ingram JR, Blomberg OS, Sockolosky JT, Ali L, Schmidt FI, Pishesha N, Espinosa C, Dougan SK, Garcia KC, Ploegh HL, et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci USA. 2017;114:10184–10189. doi:10.1073/pnas.1710776114.
- Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, et al. Disrupting the CD47-SIRP alpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017;9(381). pii:eaaf2968. doi: 10.1126/scitranslmed.aaf2968.
- Ritchie DS, Smyth MJ. A new therapeutic target for leukemia comes to the surface. Cell. 2009;138:226–228. doi:10.1016/j.cell.2009.07.005.
- Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci USA. 2018;115:E1239–E48. doi:10.1073/pnas.1718197115.
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-hodgkin’s lymphoma. N Engl J Med. 2018;379:1711–1721. doi:10.1056/NEJMoa1807315.
- Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, Bennett ML, Olson A, Azad TD, Sinha R, et al. Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci USA. 2019;116:997–1006. doi:10.1073/pnas.1721434116.
- Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi:10.1053/j.gastro.2014.08.039.
- Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin X-Y, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi:10.1016/j.jhep.2010.08.041.
- Kuang DM, Xiao X, Zhao Q, Chen MM, Li XF, Liu RX, Wei Y, Ouyang F-Z, Chen D-P, Wu Y, et al. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J Clin Invest. 2014;124:4657–4667. doi:10.1172/JCI74381.
- Liang S, Ma HY, Zhong Z, Dhar D, Liu X, Xu J, Koyama Y, Nishio T, Karin D, Karin G, et al. NADPH oxidase 1 in liver macrophages promotes inflammation and tumor development in mice. Gastroenterology. 2019;156:1156–72 e6. doi:10.1053/j.gastro.2018.11.019.
- Liu T, Larionova I, Litviakov N, Riabov V, Zavyalova M, Tsyganov M, Hoadley KA, Print C, Knowlton N, Black MA, et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology. 2018;7:e1436922. doi:10.1080/2162402X.2018.1490854.
- Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015;12:1–4. doi:10.1038/cmi.2014.83.
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi:10.1007/s00281-013-0367-7.
- Meng YM, Liang J, Wu C, Xu J, Zeng DN, Yu XJ, Hoadley KA, Print C, Knowlton N, Black MA, et al. Monocytes/Macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology. 2018;7:e1408745. doi:10.1080/2162402X.2018.1490854.
- Chen DP, Ning WR, Li XF, Wei Y, Lao XM, Wang JC, Wu Y, Zheng L. Peritumoral monocytes induce cancer cell autophagy to facilitate the progression of human hepatocellular carcinoma. Autophagy. 2018;14:1335–1346. doi:10.1080/15548627.2018.1474994.
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr., van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi:10.1016/j.cell.2009.05.045.
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi:10.1073/pnas.1121623109.
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi:10.1038/nature18935.
- Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–309. doi:10.1016/j.canlet.2015.02.036.
- Lo J, Lau EY, So FT, Lu P, Chan VS, Cheung VC, Ching RHH, Cheng BYL, Ma MKF, Ng IOL, et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. 2016;36:737–745. doi:10.1111/liv.12963.
- Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng IOL. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179–191. doi:10.1002/hep.27070.
- Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TKW. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi:10.1002/hep.27859.
- Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi:10.1084/jem.20082173.
- Bongartz T. Tocilizumab for rheumatoid and juvenile idiopathic arthritis. The Lancet. 2008;371:961–963. doi:10.1016/S0140-6736(08)60428-6.
- Felson DT. Tocilizumab versus adalimumab for rheumatoid arthritis. The Lancet. 2013;382:394–395. doi:10.1016/S0140-6736(13)61669-4.
- Casey SC, Tong L, Li YL, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi:10.1126/science.aac9935.
- Guillon J, Petit C, Moreau M, Toutain B, Henry C, Roche H, Bonichon-Lamichhane N, Salmon JP, Lemonnier J, Campone M, et al. Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment. Cell Death Dis. 2019;10:199. doi:10.1038/s41419-019-1406-7.
- Huang W, Wang WT, Fang K, Chen ZH, Sun YM, Han C, Sun L-Y, Luo X-Q, Chen Y-Q. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol Cancer. 2018;17:12. doi:10.1186/s12943-018-0768-2.
- Bruix J, Sherman M. Practice guidelines committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Shi M, Lu LG, Fang WQ, Guo RP, Chen MS, Li Y, Luo J, Xu L, Zou R-H, Lin X-J, et al. Roles played by chemolipiodolization and embolization in chemoembolization for hepatocellular carcinoma: single-blind, randomized trial. J Natl Cancer Inst. 2013;105:59–68. doi:10.1093/jnci/djs464.
- Labgaa I, Demartines N, Melloul E. Surgical resection vs. transarterial chemoembolization for intermediate stage hepatocellular carcinoma (BCLC-B): an unsolved question. Hepatology. 2019;69(2):923. doi:org/10.1002/hep.30338.
- Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011;26:1354–1360. doi:10.1111/j.1440-1746.2011.06812.x.
- Liu CQ, Xu J, Zhou ZG, Jin LL, Yu XJ, Xiao G, Lin J, Zhuang S-M, Zhang Y-J, Zheng L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br J Cancer. 2018;119:80–88. doi:10.1038/s41416-018-0144-4.
- Sun HY, Xu J, Huang M, Huang Q, Sun R, Xiao WH, Sun C. CD200R, a co-inhibitory receptor on immune cells, predicts the prognosis of human hepatocellular carcinoma. Immunol Lett. 2016;178:105–113. doi:10.1016/j.imlet.2016.08.009.
