ABSTRACT
Oncolytic viruses (OVs) are a novel class of cancer biotherapeutics with the ability to kill cancers and trigger anti-tumor immunity. Using murine models of cancer in pre-clinical proof-of-concept studies, we found that neoadjuvant OV administration before surgery efficiently prevents relapse, controls metastases and sensitizes tumors to immune checkpoint inhibitors (ICIs).
Cancer immunotherapies allow for immune cells to efficiently target cancers. While exciting clinical successes are observed using ICIs (antibodies that prevent the inhibition of immune cells) in cancers such as melanoma, other malignancies like breast cancer are more resistant. In particular, triple-negative breast cancer (TNBC) is the type of breast cancer with the least treatment options and the worst prognosis. The recent Impassion130 trialCitation1 testing PD-L1 blockade plus nab-paclitaxel (vs nab-paclitaxel alone) for TNBC treatment showed improved progression-free and overall survival rates of 7.2 vs 5.5 and 25.0 vs 15.5 months, respectively, which led to the approval of the first cancer immunotherapy (Atezolizumab) for TNBC patients earlier this year. Many factors such as cancer immunogenicity, pre-established anti-tumor immunity, an immunosuppressive tumor niche, the gut microbiome, the expression of immune checkpoint ligands by tumors and immune cell infiltration have all been reported to impact the therapeutic efficacy of such treatment strategies.Citation2 Therefore, a therapeutic approach that would positively impact several of these factors may provide an ideal pre-treatment to condition tumors to ICIs. Given that OVs are now well recognized to induce anti-tumor immunity and trigger local inflammation (which can induce immune checkpoint ligands), and locally recruit immune cells, several groups have tested the combination of different viruses with ICIs. Some of the early studies include the combinations of: the oncolytic Newcastle disease virus with anti-CTLA4 by James Allison’s group,Citation3 an oncolytic Adenovirus with anti-PD1 by Woller et. al.Citation4 and an oncolytic Vaccinia virus with anti-PD-L1 by David Bartlett’s group.Citation5
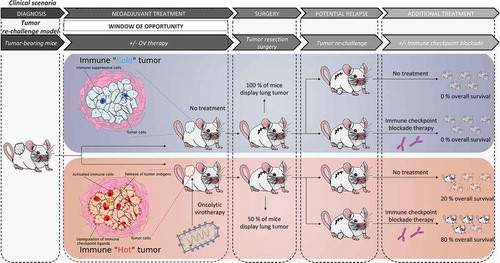
A key challenge that remains is to determine the optimal treatment regimens that could be easily incorporated into the standard treatment of breast cancer patients. In a recent study,Citation6 which is summarized in , we established a clinically relevant mouse model that recapitulates the treatment paradigm of breast cancer patients to test the combination of early OV-therapy and ICIs. Our multi-step model consists of; (1) seeding breast tumors; (2) treating with OVs; (3) resecting the tumors; (4) re-challenging the animals with tumor cells and (5) administering ICIs. In a clinical scenario, these steps would correspond to; (1) cancer onset; (2) window of opportunity for neoadjuvant therapy; (3) surgery; (4) relapse and (5) treatment. The artificially forced relapse mimics the worst case clinical scenario and allows for the testing of a second set of therapies. We then used 3 different metastatic breast cancer cell lines (4T1, EMT6, E0771), which are syngeneic to 2 different mice strains (BALB/c and C57BL/6). Our study demonstrated that oncolytic Maraba virus (MRB) treatment before surgery controlled the growth of primary tumors as well as systemic metastases. Furthermore, even without direct treatment, the animals that received pre-surgical OV-therapy were better protected against tumor re-challenge, with 20–30% of the animals being cured and the rest of the cohort showing smaller secondary tumors compared to controls. This long-term protection was observed in all 3 tumor models and when the OV was administered either intratumorally or intravenously, but was completely lost when using a UV-inactivated virus, re-challenging with a different cancer cell line or using immune-compromised mice, suggesting that the protection observed in our model is immune-mediated. Our study also showed that the fate of the animals was improved by OV treatment: not only were some mice cured, but most of the others did not succumb to their illness and were instead sacrificed for ethical reasons (their secondary tumors having grown too big over time), while control animals mostly succumbed to the metastatic spread of the disease, a condition that is much more difficult to treat.
Consistent with previous reports using different OVs, we observed the production of various chemokines and pro-inflammatory cytokines upon virus infection. We also found more immune cells infiltrating the tumors of the MRB-treated mice and detected a strong type I interferon-dependent anti-tumor immune response, demonstrating that MRB creates a pro-inflammatory niche. Along with this inflammation, we observed the induction of PD-L1, as well as the accumulation of intra-tumoral regulatory T cells following virus treatment, which suggests that alleviating immune suppression with ICIs could be particularly beneficial. We therefore tested treating the mice with both anti-PD1 and -CTLA4 post re-challenge. Excitingly, while all 3 tumor models were found to be refractory to ICIs, 60–90% of the animals that were previously treated with the OV MRB were cured by the combination, which shows that prior OV treatment sensitizes refractory breast cancers to ICIs.
In a follow-up study,Citation7 we tested other OVs in our tumor re-challenge model and found that while all of the viruses tested (Herpes simplex virus-1 (HSV), Reovirus (Reo), Vesicular stomatitis virus (VSV), Adenovirus (Ad) and MRB, all oncolytic variants) improved the fate of the animals as observed in our first study, all but HSV could control primary tumors and all but Reo could both control secondary tumors and prolong survival, which suggests, unsurprisingly, that different OVs have different anti-tumor mechanisms. Nevertheless, the observation that, for all OVs, fewer animals succumbed from metastases indicates that neoadjuvant OV-therapy before surgery is a promising strategy for breast cancer treatment.
An increasing number of TNBC patients receive neoadjuvant treatment before undergoing surgery. Neoadjuvant therapies are therefore an existing clinical paradigm for these patients. Together, our pre-clinical proof-of-concept studies suggest that OV-therapy in this window of opportunity can be efficient on its own, but may also provide alternative options for relapsing patients, who could subsequently become more responsive to ICIs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi:10.1056/NEJMoa1809615.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi:10.1158/2159-8290.CD-18-0367.
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi:10.1126/scitranslmed.3008095.
- Woller N, Gürlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, Saborowski M, Geffers R, Manns MP, Wirth TC, et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther. 2015;23:1630–1640. doi:10.1038/mt.2015.115.
- Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. doi:10.1038/ncomms14754.
- Bourgeois-Daigneault M-C, Gutfraind A, Shekhtman L, Cui Q, Kachko A, Cotler SJ, Hajarizadeh B, Sacks-Davis R, Page K, Boodram B, et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci Transl Med. 2018;10:eaao1641. doi:10.1126/scitranslmed.aao4496.
- Martin NT, Roy DG, Workenhe ST, van Den Wollenberg DJM, Hoeben RC, Mossman KL, Bell JC, Bourgeois-Daigneault M-C. Pre-surgical neoadjuvant oncolytic virotherapy confers protection against rechallenge in a murine model of breast cancer. Sci Rep. 2019;9:1865. doi:10.1038/s41598-018-38385-7.