ABSTRACT
Caspases are known for their ability to precipitate apoptosis. Our findings indicate that accelerating the terminal inactivation of cells dying in response to radiation therapy limit their immunogenicity as a consequence of reduced type I interferon secretion. Thus, caspase inhibitors stand out as promising combinatorial partners to improve the immunogenicity of radiation therapy in the clinic.
Apoptotic cell death involves the sequential activation of two or more cysteine-dependent aspartate-directed proteases commonly known as caspases.Citation1 Apoptosis mediates key physiological functions during embryonic and post-embryonic development as well as in the maintenance of adult tissue homeostasis, but is also involved in the demise of normal and malignant cells exposed to stress.Citation2 Consistent with this notion, progressing neoplasms tend to acquire genetic or epigenetic alterations that ultimately inhibit apoptosis-associated caspase activation.Citation3 Moreover, considerable efforts have been devoted over the past three decades to the development of caspase inhibitors for the treatment of cardiac, neurological and hepatic conditions associated with the unwarranted loss of post-mitotic cells.Citation4 However, such efforts have been largely disappointing, for the most part reflecting (1) the intimate interconnection between different cell death pathways activated by stress (implying that the inhibition of a single mechanism is generally insufficient to mediate cytoprotective effects as compensatory pathways are activated), and (2) the fact that – at least in mammals – apoptotic caspases control the kinetic of cell death, but not its occurrence (i.e., they accelerate the terminal inactivation and structural breakdown of cells succumbing to stress, but are not required for cell death to ultimately ensue).Citation4 As a single exception, the broad-spectrum caspase inhibitor emricasan has recently been granted fast track designation by the FDA for the treatment of non-alcoholic steatohepatitis.Citation5 Alongside, it has become clear that apoptotic caspases have a major influence on the signals emitted by dying cells, and hence on how cell death is perceived by the host immune system.Citation6 In particular, the key apoptotic executioner caspase 3 (CASP3) has been attributed major immunosuppressive effects, reflecting its capacity (1) to favor phosphatidylserine (PS) exposure on the surface of dying cells; (2) to promote the secretion of prostaglandin E2 (PGE2) and lysophosphatidylcholine (LPC); and (3) to inhibit type I interferon (IFN) secretion driven by mitochondrial DNA (mtDNA) released in the cytosol upon mitochondrial outer membrane permeabilization (MOMP).Citation7
Since the secretion of type I IFN by irradiated cancer cells is paramount for the initiation of a tumor-specific anticancer response with systemic outreach and associated with the establishment of immunological memory,Citation8 we set to investigate the impact of apoptotic caspases on the efficacy of radiation therapy (RT) in a preclinical model of hormone receptor (HR)+ breast cancer. We found that the deletion of Casp3 delays the emergence of biochemical markers of apoptosis including MOMP, PS exposure and plasma membrane breakdown amongst mouse mammary carcinoma TSA cells subjected to irradiation in vitro. However, the clonogenic potential of Casp3−/- TSA cells and TSA cells exposed to the pan-caspase inhibitor Z-Val-Ala-Asp fluoromethyl ketone (Z-VAD-fmk) was compromised by low-dose irradiation comparably to that of their control counterparts.Citation9 These data confirm that apoptotic caspases control the kinetic of apoptotic cell death driven by radiation therapy, but have little impact on the ultimate fate of irradiated cells.
Moreover, CASP3-incompetent TSA cells secreted increased amounts of type I IFN as compared to their control counterparts upon exposure to a single RT dose of 8 Gy (which is known to mediate optimal immunostimulatory effects in this cellular model),Citation8 largely reflecting the accumulation of a cell subpopulation manifesting elevated levels of double stranded DNA (dsDNA) in the cytosol and reduced mitochondrial transmembrane potential.Citation9 We interpreted these findings to suggest that CASP3 limits the accumulation of cells with permeabilized mitochondria, and hence prone to secrete type I IFN downstream of the mtDNA-driven activation of cyclic GMP-AMP synthase (CGAS), as it accelerates the functional inactivation and structural breakdown of dying cells. Notably, Casp3−/- TSA cells growing in immunocompetent syngeneic mice were more (rather than less) sensitive to focal RT as compared their CASP3-proficient counterparts. Moreover, Casp3−/- TSA cells treated with focal RT plus an immune checkpoint blocker in vivo were superior to their CASP3-competent counterparts at generating a systemic immune response culminating with the control of a distant, non-irradiated lesion (the so-called abscopal response).Citation9
Interestingly, various genetic signatures of apoptotic deficiency, including low levels of CASP3 or APAF1 (but not CASP9), as well as elevated levels of the main antiapoptotic Bcl-2 family members (i.e., BCL2, BCL2L1 and MCL1) correlated with good (rather than poor) disease-specific survival in patients with breast cancer from the METABRIC transcriptomic dataset, although this was unrelated to type I IFN signaling (which appeared to have a negative impact on survival).Citation9 These results comfort data from other groups suggesting that indolent, chronic (as opposed to robust, acute) inflammation in the mammary tissue fosters disease progression.Citation10 Further supporting this notion, we identified solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 (SLC7A2), which has anti-inflammatory effects,Citation11 as a novel gene with independent positive prognostic value in this cohort of women with breast cancer.Citation9 This latter finding awaits independent validation in alternative patient cohorts.
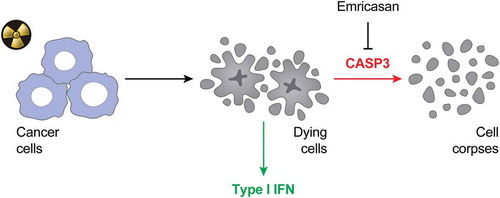
In summary, our results suggest that accelerating the functional inactivation and structural breakdown of cells succumbing to irradiation considerably limits their immunogenicity as a consequence of poor type I IFN secretion (). Thus, emricasan stands out as a promising combinatorial partner for RT. Clinical trials investigating the ability of emricasan to enhance the therapeutic activity of RT in patients with breast cancer are urgently awaited.
Figure 1. Negative impact of apoptotic caspases on the immunogenicity of radiation therapy.
The immunogenicity of cancer cells succumbing to irradiation is largely dependent on type I IFN secretion (IFN). In this context, apoptotic caspases like caspase 3 (CASP3) mediate a detrimental effect as they drive the terminal inactivation and structural breakdown of dying cells. Thus, caspase inhibition with emricasan stands out as a promising approach to boost the efficacy of radiation therapy in the clinic.
Disclosures
LG provides remunerated consulting to OmniSEQ (Buffalo, NY, USA), Astra Zeneca (Gaithersburg, MD, USA), Inzen (New York, NY, USA) and the Luke Heller TECPR2 Foundation (Boston, MA, USA), and he is member of the Scientific Advisory Committee of OmniSEQ (Buffalo, NY, USA).
Acknowledgments
The Galluzzi Lab is supported by a Breakthrough Level 2 grant from the US Department of Defense (DoD), Breast Cancer Research Program (BRCP) [#BC180476P1], by a startup grant from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, US), and by donations from Phosplatin (New York, US), the Luke Heller TECPR2 Foundation (Boston, US) and Sotio a.s. (Prague, Czech Republic).
References
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi:10.1038/s41418-017-0012-4.
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi:10.1038/nrm2952.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013.
- Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi:10.1038/cdd.2014.137.
- Mehta G, Rousell S, Burgess G, Morris M, Wright G, McPherson S, Frenette C, Cave M, Hagerty DT, Spada A, et al. A placebo-controlled, multicenter, double-blind, Phase 2 randomized trial of the Pan-Caspase inhibitor emricasan in patients with acutely decompensated cirrhosis. J Clin Exp Hepatol. 2018;8:224–234. doi:10.1016/j.jceh.2017.11.006.
- Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19:731–745. doi:10.1038/s41580-018-0068-0.
- Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–231. doi:10.1016/j.immuni.2016.01.020.
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi:10.1038/ncomms15618.
- Rodriguez-Ruiz ME, Buque A, Hensler M, Chen JH, Bloy N, Petroni G, Sato A, Yamazaki T, Fucikova J, Galluzzi L. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology. 2019.
- Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–18495. doi:10.1073/pnas.0809242105.
- Coburn LA, Singh K, Asim M, Barry DP, Allaman MM, Al-Greene NT, Hardbower DM, Polosukhina D, Williams CS, Delgado AG, et al. Loss of solute carrier family 7 member 2 exacerbates inflammation-associated colon tumorigenesis. Oncogene. 2019;38:1067–1079.
