ABSTRACT
Expression of arginase-1 (ARG1) is an immunosuppressive feature of tumor microenvironment that leads to depletion of ʟ-arginine, a nutrient required for T-cells expansion. Ovarian carcinoma cells release extracellular vesicles carrying enzymatically active ARG1, that contributes to local and systemic immune suppression, which can be restored by ARG inhibitor.
The results of recent clinical trials revealed that the immune system can eradicate cancer. Despite unprecedented cures in multiple types of cancer, the efficacy of immunotherapy in patients with epithelial ovarian cancer turned out to be rather limited.Citation1 Many mechanisms seem to be responsible for this inefficiency. Some are based on the local production of immunosuppressive mediators or recruitment of regulatory cells that impair the activity of lymphocytes. Other, include increased production of molecular brakes, known as checkpoints, that interfere with the immune response. A recently emerging and one of the most prominent features of tumor microenvironment (TME) that impacts local immune response is amino-acid metabolism, especially that of ʟ-tryptophan and ʟ-arginine. ʟ-arginine is a substrate for four enzymes including arginases (ARG1 and 2), that convert it to ʟ-ornithine and urea. T-cell proliferation is impaired at low ʟ-arginine concentrations, thereby depletion of this amino-acid impairs the development of immune response to tumor-associated neoantigens. In the TME and in secondary lymphoid organs ARG production is mainly attributed to myeloid-derived suppressor cells (MDSCs) and immunosuppressive M2-type tumor-associated macrophages (TAMs), which is linked to inflammatory processes driving tumorigenesis.Citation2 High ARG expression has been documented in blood of patients with hematologic malignancies such as acute myeloid leukemia and in the microenvironment of solid tumors like neuroblastoma, squamous cell carcinoma, lung, and colorectal cancer.Citation3,Citation4 Recently, it has been shown that, similar to MDSCs and TAMs, some tumor cells themselves harness ʟ-arginine depletion to mitigate immunosurveillance.Citation5 Upregulated ARG has been linked to poor patients’ survival; therefore, modulation of TME properties by inhibiting ARG appears to be a promising immunotherapeutic approach that might improve antitumor immune response.Citation4,Citation5
Extracellular vesicles (EVs) are spherical-shaped, nanoscale particles carrying specific set of lipids, proteins or nucleic acids and are involved in intercellular communication. Secreted EVs might directly interact with various immune cells including B, T or NK cells, MDSCs and dendritic cells (DCs), exerting activating or inhibitory effects.Citation6 EVs derived from the malignant lesions possess the molecular signature of the parental cells and are investigated as potential diagnostic biomarkers for early cancer detection.Citation7 Exosomes, which are a well-characterized subpopulation of EVs, have been linked to tumor progression and metastasis.Citation8 Ovarian carcinoma (OvCa) is an asymptomatic at early stages, deadly cancer characterized by ascites accumulation in the abdomen and is known to secrete a substantial amount of EVs loaded with immunomodulatory cargo.Citation9
We have recently shown that ARG1 in EVs participates in the formation of the local immunosuppressive microenvironment in OvCa and alters systemic immune response.Citation10 We have detected ARG1 expression in both established OvCa cell lines as well as in tumor cells isolated from the ascites from OvCa patients. Immunohistochemistry of tissue sections of primary ovarian tumors revealed cytoplasmic staining for ARG1 with moderate to high intensity in 66.7% cases of a 84 patients cohort. Transcriptomic data analysis indicated worse survival in a cohort of patients displaying high ARG1 expression pattern in OvCa primary tumor in comparison with those with low ARG1 expression. ARG activity in serum samples of untreated OvCa patients with stage II and III tumors were significantly higher than in healthy controls correlating with poor prognosis. Increased ARG1 levels and activities were measured in the plasma of OvCa patients correlating with impaired proliferation ability of peripheral T-cells and downregulation of CD3ζ (CD247). CD3ζ belongs to the CD3 complex that associates with the T-cell receptor to generate and transmit activation signals in T-cells. These findings raised further questions on the potential mechanisms of immunomodulatory effects of ARG1, especially that T-cells mostly proliferate in local lymphoid organs rather than in the TME. A series of experiments demonstrated that OvCa cells release EVs that contain enzymatically active ARG1. Also, EVs isolated from the ascites of OvCa patients contained enzymatically active ARG1. EVs isolated from the ascites of over 40% of OvCa patients very strongly inhibited the proliferation and decreased CD3ζ levels in both CD4+ and CD8+ T-cell subsets. In many cases, the specific inhibition of ARG1 with ARG inhibitor or addition of excess ʟ-arginine to the culture medium partially reversed the effects of patients’-derived EVs. Likewise, inoculation of ARG1-containing EVs isolated from tumor cells strongly inhibited antigen-specific OT-I T-cells proliferation in mice. Further experiments revealed that ARG1-containing EVs are internalized by DCs in the local lymph nodes, where they abrogate the development of effective immune response. In contrast, a tagged recombinant ARG1 inoculated into mice was rapidly degraded and failed to reach DCs in the lymph nodes. Overexpression of ARG1 in murine ID8 OvCa cells led to accelerated tumor progression that was inhibited by treatment with ARG inhibitor.
Altogether, our studies reveal a novel mechanism of tumor-induced systemic T-cell dysfunction based on the activity of tumor-derived EVs containing ARG1 (). These vesicles transfer functionally active ARG1 as metabolic checkpoint molecules over a long distance to antigen-presenting cells and mitigate antitumor immune response, leading to an enhanced tumor growth in vivo. These results may also apply to other ARG-expressing tumor types and may have significant clinical implications for T-cell immunotherapy of cancer.
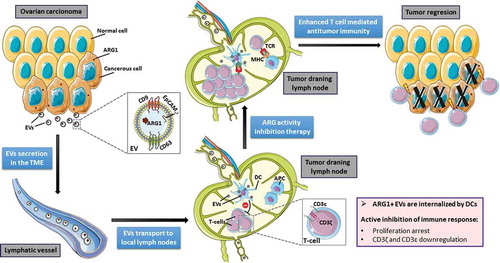
Figure 1. Suppression of T-cell mediated antitumor immune response by extracellular vesicles (EVs) containing arginase-1 (ARG1). Ovarian carcinoma (OvCa) cells produce ARG1 and release this enzyme in secreted EVs to the local tumor microenvironment (TME). EVs are being transported by lymphatic vessels to local lymph nodes. Tumor-derived ARG1-containing EVs are internalized by dendritic cells (DCs) that acquire inhibitory properties and suppress T-cell proliferation. Blocking of ARG activity restores the proliferative potential of T-cells. Activated T-cells can differentiate into effector cells exerting antitumor activity. Figure was created with images adapted from Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License.
Disclosure of potential conflicts of interest
J.G. is a shareholder and Scientific Advisory Board member in OncoArendi Therapeutics.
The remaining authors declare no potential conflicts of interests.
Abbreviations
| ARG | = | arginase |
| DCs | = | dendritic cells |
| EVs | = | extracellular vesicles |
| MDSCs | = | myeloid-derived suppressor cells |
| OvCa | = | ovarian carcinoma |
| TAMs | = | tumor-associated macrophages |
| TME | = | tumor microenvironment |
References
- Alipour S, Zoghi S, Khalili N, Hirbod-Mobarakeh A, Emens LA, Rezaei N. Specific immunotherapy in ovarian cancer: a systematic review. Immunotherapy. 2016;8:1–3. doi:10.2217/imt-2016-0034.
- Lemos H, Huang L, Prendergast GC, Mellor AL. Immune control by amino acid catabolism during tumorigenesis and therapy. Nature Rev Cancer. 2019;19:162–175. doi:10.1038/s41568-019-0106-z.
- Miret JJ, Kirschmeier P, Koyama S, Zhu M, Li YY, Naito Y, Wu M, Malladi VS, Huang W, Walker W, et al. Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity. J Immunother Cancer. 2019;7:32. doi:10.1186/s40425-019-0504-5.
- Ma Z, Lian J, Yang M, Wuyang J, Zhao C, Chen W, Liu C, Zhao Q, Lou C, Han J, et al. Overexpression of Arginase-1 is an indicator of poor prognosis in patients with colorectal cancer. Pathol Res Pract. 2019;215:152383. doi:10.1016/j.prp.2019.03.012.
- Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5:101. doi:10.1186/s40425-017-0308-4.
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi:10.1038/nri2567.
- Kosaka N, Kogure A, Yamamoto T, Urabe F, Usuba W, Prieto-Vila M, Ochiya T. Exploiting the message from cancer: the diagnostic value of extracellular vesicles for clinical applications. Exp Mol Med. 2019;51:31. doi:10.1038/s12276-019-0219-1.
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi:10.1038/nature15756.
- Dorayappan KDP, Wallbillich JJ, Cohn DE, Selvendiran K. The biological significance and clinical applications of exosomes in ovarian cancer. Gynecol Oncol. 2016;142:199–205. doi:10.1016/j.ygyno.2016.03.036.
- Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, Wolinska E, Gaj P, Grazul M, Pilch Z, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun. 2019;10:3000. doi:10.1038/s41467-019-10979-3.
