ABSTRACT
IL15 is a key cytokine for the activation and survival of anti-tumor effectors CD8+ T and NK cells. Recently published preclinical studies demonstrate that the therapeutic activity of IL15 requires conventional dendritic cells type 1 (cDC1). Radiotherapy cooperates with IL15 by enhancing cDC1 tumor infiltration via interferon type 1 activation.
Cytokines are key regulators of the immune response and among the first biologics to be tested for the ability to elicit anti-tumor immune responses.Citation1 Interleukin-15 (IL15) is a common gamma-chain cytokine that promotes the proliferation, survival and activation of natural killer (NK) and CD8+ T cells, and was ranked by Martin Cheever at the top of a list of immunotherapy agents with the potential to treat cancer.Citation2 Early phase clinical trials have shown that recombinant human IL15 (rhIL15) administered subcutaneously (s.c.) has good tolerability and activity, measured as significant NK and CD8+ T-cell expansion, but limited anti-cancer effects.Citation3
IL15 is produced as a heterodimer with the alpha chain of the IL15 receptor (IL15Rα), and it is this heterodimer that has biological activity,Citation4 prompting the development of similar constructs to increase the stability and activity of IL15 administered therapeutically. One such construct, het-IL15, was recently tested by Bergamaschi and colleagues in two different mouse models of carcinoma, colon MCA38 and lung TC1.Citation5 They observed an increased tumor infiltration, proliferation and survival of CD8+ T and NK cells in mice treated with intraperitoneal het-IL15 that was associated with a significantly slower tumor growth as compared to untreated mice. Further analyses confirmed a gene signature associated with activated, proliferative, cytotoxic lymphocytes in het-IL15-treated tumors with granzyme A and B among the most upregulated transcripts. In addition, NK and CD8+ T cells infiltrating the tumor of het-IL15-treated mice produced XCL1, a chemokine that drives conventional dendritic cells type 1 (cDC1) to the tumor.Citation6 Consistently, they observed an increased accumulation of cDC1, which are specialized in cross-presenting tumor antigens to CD8+ T cells.Citation6 The cDC1 recruited to the tumor, in turn, secreted CXCL9 and CXCL10 in response to het-IL15 and IFN-γ released by T and NK cells, attracting more effector T cells. Overall, this study suggests that het-IL15 boosts anti-tumor immune responses by amplification of a cycle of recruitment and activation of CD8+ T cells that hinges upon cDC1.
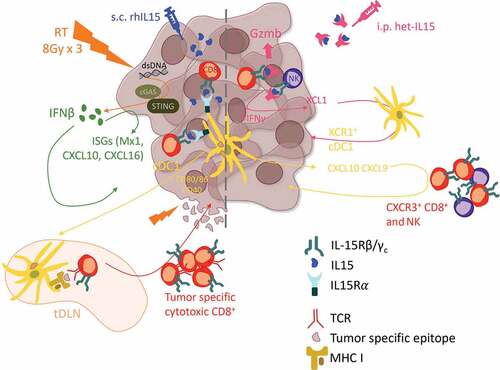
Our group tested rhIL15 administered subcutaneously in three mouse models of carcinoma, the TSA breast cancer, MCA38 colon cancer and LLC1 lung cancer and found that it did not have any anti-tumor effect.Citation7 The discrepancy with the results of Bergamaschi et al.,Citation5 may reflect the improved biological activity of IL15 administered as a heterodimer with IL15Rα chain. Additionally, it may be due to the delayed administration in our study (day 12 versus day 5 post-tumor inoculation in experiments performed by Bergamaschi et al), when the tumor microenvironment is well-established. The lack of clinical activity of single-agent IL15, even when given as a complex with the IL15Rα chain to patients with advanced cancer,Citation8 suggests that IL15 is unable by itself to overcome the immune suppression associated with tumor progression. However, we found that IL15 synergized with focal tumor radiotherapy leading to improved control and often complete regression of the irradiated tumor and protective memory responses in cured mice. The combination of IL15 and radiotherapy to one tumor also led to inhibition of a synchronous non-irradiated tumor (abscopal effect).Citation7 These responses were abrogated by CD8+ T cell depletion, and in the absence of cDC1, in Batf3-deficient mice. Similar to Bergamaschi et al.,Citation5 we observed an increase in cDC1 in the tumor of wild-type mice treated with IL15, but only when given together with radiation. Radiation itself increased cDC1 presence in the tumor, but the addition of IL15 significantly enhanced this effect, and increased the expression of costimulatory molecules CD80, CD86 and CD40 on intra-tumoral cDC1, and the priming of tumor-antigen specific CD8+ T cells in draining lymph nodes. Further investigation showed that cancer cell-intrinsic interferon type I (IFN-I) upregulation by radiation was required for the synergy of radiotherapy with IL15. Mice bearing the lung tumor LLC1, which expresses very low levels of the cytosolic DNA sensor cGAS and is thus unable to respond to radiation with increased IFNβ production, did not show any improvement in control of the irradiated tumor or survival when given IL15. Additionally, in TSA tumor-bearing mice the therapeutic synergy between IL15 and radiation was observed when radiation was given as 8 Gy doses on three consecutive days, a regimen that optimally activates IFN-I, but not as a single dose of 20 Gy, which is a poor inducer of IFN-I pathway in this model.Citation9 The increase in intra-tumoral cDC1 was abrogated by antibody-mediated blockade of the IFN-I receptor, consistent with the role of IFN-I in cDC1 recruitment.
Taken together, data from these studies support a model whereby IL15 activates and expands a preexisting effector population of CD8+ and NK cells which produce chemokines to attract cDC1 to the tumor. The latter, in turn, produce CXCL10 and amplify the cancer-immunity cycleCitation10 by attracting and activating more effector cells. In advanced tumors, when this effector response is largely suppressed, radiation is required to jumpstart the cancer-immunity cycle by inducing cancer cell-intrinsic IFN-I that attracts cDC1, and CXCL10 that attracts effector T cells (). Recruited cDC1, which express IL15Rα, can then cross-present tumor antigens released by radiation and trans-present IL15 resulting in activation of CD8+ T cells within the tumor as well as the draining lymph nodes. Thus, the ability of IL15 to foster the cross-talk of CD8+ T and NK cells with cDC1 appears to be a critical component of IL15-induced reprogramming of the tumor immune contexture toward tumor rejection.
Figure 1. Proposed model of the interactions between radiation and IL15 in the tumor microenvironment promoting anti-tumor immunity. (Left) In advanced tumors optimized radiation therapy regimens elicit cancer-intrinsic activation of the type I IFN pathway and tumor antigen exposure promoting the recruitment of cDC1 that cross-present tumor antigens and transpresent the subcutaneously administered recombinant IL15, resulting in cytotoxic CD8+ T cell infiltration, tumor control and establishment of anti-tumor effector and memory responses. (Right) In more immunogenic/less advanced tumors systemic treatment with biologically active IL15-IL15Rα dimers (het-IL15) expands and activates preexisting anti-tumor CD8+ T and NK cell, which infiltrate the tumor and secrete XCL1 to recruit cDC1. cDC1, in turn, promote further tumor infiltration by CD8+ T and NK cells.
It remains to be investigated if the key role of cDC1 identified in the preclinical studies will also be seen in patients treated with IL15. Several early trials are ongoing testing het-IL15 and another construct, ALT-803, as single agent (NCT03054909, NCT02452268, NCT02099539), whereas rh-IL15 is being tested in combination with other agents but not with radiotherapy (NCT03388632, NCT02689453, NCT03759184, NCT03905135, NCT04150562). Our results suggest that radiotherapy is a promising candidate for testing with IL15, but caution that the intrinsic tumor expression of cytosolic DNA sensors and the radiation dose and fractionation should be considered in the design of such studies.
Disclosure of potential conflicts of interest
The authors declared that no conflict of interest exists related to this work, but S.D. has received honorarium from Lytix Biopharma, EMD Serono, and Mersana Therapeutics for advisory service, and research grants from Lytix Biopharma and Nanobiotix. EGM has served as consultant or speaker for Roche, AstraZeneca, Clovis and Pharmamar, research funding from Roche and financial support from AstraZeneca, Roche, Pharmamar, Pfizer, Bristol Meyer Squibb and MSD.
Additional information
Funding
References
- Garcia-Martinez E, Smith M, Buque A, Aranda F, de la Pena FA, Ivars A, Canovas MS, Conesa MAV, Fucikova J, Spisek R, et al. Trial Watch: Immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology. 2018;7:e1433982. doi:10.1080/2162402X.2018.1433982.
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–2. doi:10.1111/j.1600-065X.2008.00604.x.
- Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, Sondel PM, Wakelee HA, Disis ML, Kaiser JC, et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin Cancer Res. 2018;24:1525–1535. doi:10.1158/1078-0432.CCR-17-2451.
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi:10.1016/j.tips.2011.09.004.
- Bergamaschi C, Pandit H, Nagy BA, Stellas D, Jensen SM, Bear J, Cam M, Valentin A, Fox BA, Felber BK, et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J Immunother Cancer. 2020;8:e000599. doi:10.1136/jitc-2020-000599.
- Bottcher JP, Reis ESC. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer. 2018;4:784–792. doi:10.1016/j.trecan.2018.09.001.
- Pilones KA, Charpentier M, Garcia-Martinez E, Daviaud C, Kraynak J, Aryankalayil J, Formenti SC, Demaria S. Radiotherapy cooperates with IL15 to induce antitumor immune responses. Cancer Immunol Res OnlineFirst June. 2020;12:2020. doi:10.1158/2326-6066.CIR-1119-0338.
- Margolin K, Morishima C, Velcheti V, Miller JS, Lee SM, Silk AW, Holtan SG, Lacroix AM, Fling SP, Kaiser JC, et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2018;24:5552–5561. doi:10.1158/1078-0432.CCR-18-0945.
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi:10.1038/ncomms15618.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi:10.1016/j.immuni.2013.07.012.
