ABSTRACT
Mucosal-associated invariant T (MAIT) cells constitute one of the most numerous unconventional T cell subsets, and are characterized by rapid release of Th1- and Th17-associated cytokines and increased cytotoxic functions following activation. MAIT cells accumulate in tumor tissue but show an exhausted phenotype. Here, we investigated if immune checkpoint blockade (ICB) with antibodies to PD-1 or PD-L1 affects the function of circulating MAIT cells from non-small cell lung cancer patients. ICB increased the proliferation and co-expression of the activation markers HLA-DR and CD38 on MAIT cells in most patients after the first treatment cycle, irrespective of treatment outcome. Furthermore, production of cytokines, especially TNF and IL-2, also increased after treatment, as did MAIT cell polyfunctionality. These results indicate that MAIT cells respond to ICB, and that MAIT cell reinvigoration may contribute to tumor regression in patients undergoing immune checkpoint therapy.
Introduction
Mucosal-associated invariant T (MAIT) cells constitute a distinct and numerous population of unconventional T cells in humans, with potent functions in both immunity to infections and tissue repair.Citation1 MAIT cells are characterized by their use of a semi-invariant T cell receptor (TCR) with a Vα7.2Jα33 alpha chain recognizing derivatives of vitamin B2, which are produced by bacteria and fungi and presented by the major histocompatibility complex class I-related protein 1 (MR1).Citation2 Upon TCR-mediated stimulation, MAIT cells rapidly secrete Th1 and Th17 associated cytokines, and can also kill antigen-presenting epithelial cells and tumor cells. Like other unconventional T cells, MAIT cells are also activated by cytokines like IL-12, IL-15, and IL-18 to secrete cytokines and cytotoxic effector molecules in a TCR-independent manner.Citation1
MAIT cells are detected in several types of epithelial tumors and are sometimes enriched in the tumor tissue.Citation3 Based on their cytotoxic potential and ability to produce Th1 cytokines, MAIT cells could easily be hypothesized to contribute to anti-tumor immunity. However, in several tissues, such as lungs, skin, and the female genital tract, there is also a prominent proportion of MAIT cells with a Th17-like cytokine repertoire,Citation4 which might indicate a tumor-promoting profile. The effects of MAIT cells in preclinical tumor models differ depending on context and antigen stimulation. Experiments performed in MR1-deficient mice, lacking MAIT cells, indicate a tumor-promoting function of MAIT cells. On the other hand, in vivo activation of MAIT cells by antigen administration strongly reduced tumor burden in several tumor models.Citation5–7
Tumor-infiltrating conventional T cells often show signs of functional exhaustion, typically manifested as reduced cytokine production upon stimulation. Exhaustion is induced by repeated stimulation in the tumor microenvironment and is a reversible state that is maintained by signaling by inhibitory receptors on the T cell.Citation8 The best characterized of these receptors is programmed cell death 1 (PD-1), that binds to ligands PD-L1 and PD-L2 during antigen presentation to the T cell and reduce signaling through the TCR and CD28.Citation9 The understanding of exhaustion mechanisms and signaling by inhibitory receptors has paved the way for the introduction of immune checkpoint blockade (ICB) immunotherapy, where blocking of PD-1 or PD-L1 has proven immensely useful for the treatment of tumors with high mutational burden like malignant melanoma and non-small cell lung cancer (NSCLC).Citation9 Still, the treatment response varies considerably, and less than half of the patients show a durable response to the treatment.Citation9
Tumor-infiltrating MAIT cells also show signs of exhaustion, both with regard to surface markers and reduced functionality.Citation10–12 MAIT cells from colon tumors also exhibit improved activation when stimulated in vitro in the presence of PD-1-blocking antibodies, indicating that they may also respond to ICB in vivo.Citation12 A recent study even suggested that MAIT cell frequencies and MAIT cell activation at the start of treatment correlate to patient response to ICB treatment in malignant melanoma.Citation13 Furthermore, single-cell mRNA analyses indicate that ICB in basal and squamous cell carcinoma results in MAIT cell activation and upregulation of effector functions in the tumors.Citation10 To further investigate if human MAIT cells are affected by ICB in vivo, and if MAIT cell function may influence treatment outcome, we analyzed MAIT cell frequencies, activation, and cytokine production in patients with NSCLC before and during the course of ICB. These studies show that circulating MAIT cells from NSCLC patients are invigorated by ICB treatment, which was evident from expression of activation markers, increased proliferation, and cytokine production.
Materials and methods
Patients and treatment
Thirty-five patients from a prospective study of patients with NSCLC undergoing ICB at the Sahlgrenska University Hospital were recruited for the study (7 with squamous cell carcinoma and 28 with adenocarcinoma). Inclusion criteria were stage 3 or 4 NSCLC treated with ICB alone or in combination with chemotherapy. Patients were selected from the larger cohort to obtain similar numbers of patients responding and not responding to the treatment. Additional patient information is presented in Supplementary Table S1. The study was performed according to the Declaration of Helsinki and approved by the Regional Board of Ethics in Medical Research in west Sweden (953/18). All patients gave a written informed consent before participation in the study.
Patients were treated with blocking antibodies to PD-1 (Pembrolizumab or Nivolumab) or PD-L1 (Durvalumab or Atezolizumab) every two (Nivolumab), three (Pembrolizumab and Atezolizumab), or four (Durvalumab) weeks until progression, unacceptable toxicity or up to a maximum of one (Durvalumab) or two (Pembrolizumab, Nivolumab, or Atezolizumab) years. Treatment cycles were scheduled according to the approved drug intervals and health conditions of the individual patients. In 23 of the patients, ICB was the first-line treatment, and in one of them, ICB was preceded by chemotherapy with Carboplatin and Pemetrexed. The remaining 11 patients received ICB and chemotherapy with Carboplatin and Pemetrexed in parallel. Patients were evaluated every 3 months by computed tomography scan, in line with the immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) algorithmCitation14 but assessed by an oncologist according to clinical judgment. The clinical response was defined as a persistent response at the 9 months follow-up and was divided into i) complete response, i.e., no measurable tumor, ii) partial response, decreased tumor size compared to baseline, iii) stable disease, and iv) progressive disease before or at 9 months. None of the patients achieved a complete response, but 13 of the patients (37%) experienced a partial response to the treatment and 8 patients (23%) had a stable disease, resulting in a disease control rate of 60%. Fourteen of the patients had progressive disease (40%) observed at 3 (n=5), 6 (n=8),or 9 months (n=1).
Venous blood was collected in EDTA-coated tubes at baseline (before treatment) and 2–4 weeks after the start of the treatment cycles depending on treatment interval (above). Whole blood was diluted in 10% DMSO and stored in liquid nitrogen until analysis as previously described.Citation15 This procedure preserves antigen expression and leukocyte ratios in the blood samples.
Only a limited amount (0.5–1 ml) of blood was available for these studies. Frequencies of MAIT cells among CD3+ cells could still be evaluated in all patients (n = 35). In contrast, unambiguous determination of MAIT cell phenotype could only be performed in a subset of patients, due to the low number of MAIT cells that could be retrieved. Thus, 14 patients were analyzed for MAIT cell activation markers and 9 for cytokine production before and after the first treatment cycle. Eight of these patients were analyzed for both activation markers and cytokines, one for only cytokines, and six for only activation markers. Information about these 15 patients is available in Supplementary Table S1. In addition, one patient had enough MAIT cells in the pre-treatment sample to allow analysis of activation markers, but not in the first post-treatment sample.
Lymphocyte isolation and stimulation
Blood was thawed and centrifuged twice to remove DMSO, and then used immediately for flow cytometry staining or stimulation. The number of live CD3+ cells retrieved from the samples varied between 2.9 × 103 and 1.8 × 105 (median 2.6 × 104). To assess production of cytokines and Granzyme B (GrB), cells were suspended in RPMI 1640 (GIBCO by Life TechnologiesTM) containing 10% fetal bovine serum (Sigma-Aldrich), 25 mM of hepes, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 292 μg/ml of L-glutamine (GIBCO), and 50 μg/ml of gentamicin (GIBCO) and stimulated with 50 ng/ml of PMA and 680 ng/ml of ionomycin calcium salt (Sigma-Aldrich) for 4 h, together with a protein transport inhibitor (BD Golgi stop, BD Biosciences).
Flow cytometry analyses
Single-cell suspensions were stained with Fixable Viability Stain 575 V (BD HorizonTM) to exclude dead cells followed by staining of surface expressed proteins. For intracellular cytokine and GrB staining, cells were permeabilized with FIX&PERM® Cell Fixation and Permeabilization Kit (Nordic-MUbio) and for Ki67 staining with eBioscience™ Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher) followed by staining of the intracellular proteins. See Supplementary Table S2 for antibodies used. APC- and PE-labeled tetramers of MR1 presenting the MAIT cell antigen 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU) were kindly provided by the NIH tetramer core facility. Samples were acquired on a Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc.). Lymphocytes were identified by their forward and side scatter characteristics which were combined with staining for CD3 to detect T cells. Fluorescence minus one controls employing non-MAIT CD8+ T cells were used to determine positive staining. A cutoff of minimum 50 events was applied in all analyses of cell surface markers and effector molecules. Polyfunctionality was evaluated using SPICE6TM software, and the polyfunctionality index calculated as previously described.Citation16
Statistical analyses
Statistical analyses of paired data were performed using two-tailed Wilcoxon matched-pairs signed rank test and of unpaired data using Mann-Whitney test. Values of p < .05 were considered to be statistically significant. Graphpad Prism 9 software was used for all statistical evaluations.
Results
MAIT cell kinetics during ICB
MAIT cells were defined as CD8+ or double negative (CD4−CD8−) CD161hightet+ cells among the live CD3+ T cells (see Suppl. Fig. S1 for gating strategies). Before treatment, MAIT cell frequencies varied between 0.01 and 3.5% of all T cells (median 0.26%). This is somewhat lower than what has been reported for healthy individuals of the same age.Citation17 There were no significant differences in MAIT cell frequencies before treatment between the patients that responded to treatment and those that had progressive disease (Supplementary Figure S2). Likewise, there was no significant change in circulating MAIT cell frequencies following the first treatment cycle (Supplementary Figure S3). Seventeen of the patients could be followed throughout the 4 treatment cycles and in these patients, MAIT cell numbers did not change consistently during the later part of the treatment period either (Data not shown).
MAIT cell phenotype in untreated NSCLC patients
MAIT cell activation was evaluated by the ex vivo expression of CD25, CD69, HLA-DR, and CD38, and proliferation was assessed by Ki67 expression. In addition, PD-1 and CD39 were used to estimate activation and exhaustion. PD-1 is upregulated upon T cell stimulation, but is also maintained during exhaustion, usually together with CD39.Citation8 We were only able to obtain a small volume of blood from the patients in the study, and these analyses could be performed in 14 patients before and after the first treatment cycle, as the frequencies of MAIT cells that could be retrieved were otherwise too low to allow adequate detection of subsets. For Ki67 detection, only 10 patients were available for analysis.
A substantial proportion of MAIT cells expressed PD-1 before treatment in most of the patients (Supplementary Figure S4A), but PD-1 expression in MAIT cells was similar between the patients that would subsequently respond to treatment and in those who had progressive disease. CD39, on the other hand, was virtually absent from the PD-1-expressing MAIT cells in all of the patients (range 0–0.4%, median 0%). CD39 marks tumor-specific conventional CD8+ T cells, but is also expressed on terminally exhausted MAIT and conventional T cells together with PD-1,Citation12,Citation18–20 suggesting that PD-1 might be regarded as a marker of progenitor exhausted or possibly activated cells in the circulating MAIT cells. MAIT cell expression of the other activation markers varied at baseline, with the highest expression of CD69 and HLA-DR, and there was no significant difference with regard to any of these markers between responders and non-responders before the start of the treatment (Supplementary Figure S4B–F).
We conclude that MAIT cell frequencies, expression of PD-1, or other activation markers at the start of treatment do not correlate to patient response to ICB.
MAIT cell proliferation and activation following ICB treatment
To determine if MAIT cells can respond to ICB in vivo, we evaluated the expression of activation markers following treatment, using ex vivo PBMC without any intentional stimulation. Previous studies have indicated that the invigoration of circulating CD8+ conventional T cells in response to ICB can mainly be detected early during treatmentCitation21–24 and we thus focused on the potential response to the first treatment cycle. In conventional CD8+ T cells, invigoration can be assessed by the expression of Ki67, a marker of recent or ongoing cell division. Indeed, we could show that the expression of Ki67 by MAIT cells was increased following the first treatment cycle in all but one of the examined patients (p < 0.05; ).
Figure 1. Activation markers in MAIT cells. Circulating MAIT cells were collected before and after the first treatment cycle with ICB and analyzed by flow cytometry for their expression of Ki67 (a), HLA-DR (b), CD38 (c), and co-expression of HLA-DR and CD38 (d). Left-hand graphs show all patients, and the right-hand graphs show patients grouped by treatment outcome. Symbols represent individual values. Bright green circles represent patients receiving ICB as their only treatment and dark green circles patients receiving ICB together with or after chemotherapy. Dot-plots show the expression of Ki67 and HLA-DR vs CD38 in MAIT cells gated as in Supplementary Figure S1 before and after the first treatment cycle. *p< .05, n=10–14.
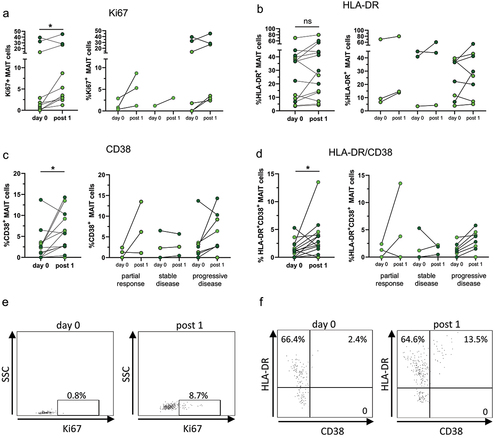
In addition to Ki67, there was also a significant increase in MAIT cell expression of CD38 following the first treatment cycle (p < .05) and a non-significant trend of increased HLA-DR expression in a majority (10 out of 14) of the analyzed patients. The combination of CD38 and HLA-DR has been used to identify conventional activated effector memory T cells,Citation25 and we found that the frequencies of MAIT cells co-expressing CD38 and HLA-DR were also significantly increased after ICB treatment (p < .05). The effect was similar in patients with a partial response and stable or progressive disease (). In contrast, expression of CD25 and CD69 did not change significantly in MAIT cells following the first treatment cycle in any of the patient groups (Supplementary Figure S5A-B). Furthermore, there were no differences when comparing treatment response with regard to expression of activation markers between patients receiving ICB treatment alone and those with combined chemotherapy and ICB treatment. A significant effect of ICB on MAIT cell activation markers was only seen after the first treatment cycle (Supplementary Figure S6). When the expression of the same markers by bulk non-MAIT CD8+ and CD4+ T cells from the same individuals was determined, a similar increase in CD38+, HLA-DR+ and co-expressing cells was seen (Supplementary Figure S7).
Taken together, these results show that MAIT cells are invigorated in response to ICB treatment, displaying both increased proliferation and expression of activation markers, and that this effect was independent of the subsequent response to therapy or the combination with chemotherapy.
MAIT cell cytokine and GrB production during ICB treatment
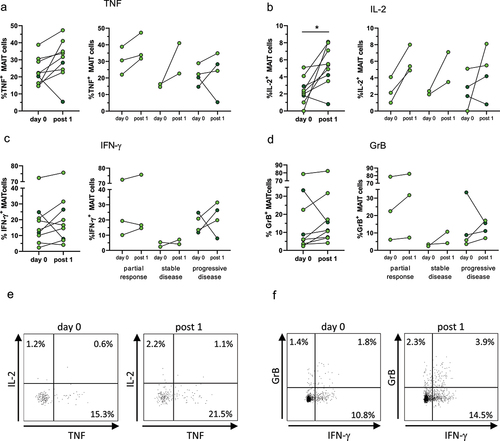
Previous studies in several tumor types have demonstrated that strong Th1 type responses correlate to a good patient prognosis, while Th17 type responses are more common in patients with progressive disease.Citation26 We therefore assessed the production of IFN-γ, TNF, IL-17, and IL-2 in MAIT cells before and after the first treatment cycle. In addition, MAIT cell cytotoxic potential was estimated by the production of GrB. Due to the limited amount of blood available, cytokine production could only be analyzed in 9 patients.
Polyclonal stimulation with PMA and Ionomycin induced substantial production of TNF, IFN-γ, and IL-2 in virtually all patients analyzed (). There was no difference in MAIT cell cytokine production between responders and non-responders before treatment. After the first treatment cycle, TNF and IL-2 were increased in all but one of the patients (p < .05 for IL-2), but there was no clear separation in the response patterns between responding and relapsing patients (). Frequencies of IFN-γ-producing MAIT cells, on the other hand, did not increase to the same extent after treatment (). In contrast to the other cytokines assessed, IL-17 production was almost absent in all patients (0–1.5% of MAIT cells, median 0; data not shown) and did not change by ICB treatment. GrB responses were not dramatically changed before and after treatment, but there was still a modest increase in GrB production in most patients when comparing the MAIT cells obtained after the first treatment cycle with those from the untreated patients (). There were no significant changes in MAIT cell cytokine production after the second to fourth treatment cycles (Supplementary Figure S6). Among bulk non-MAIT CD8+ and CD4+ T cells from the same individuals, there was a significant increase in TNF-production among CD8+ cells after the first treatment cycle, but not in any of the other analyzed cytokines (Supplementary Figure S8).
Figure 2. Cytokine production by MAIT cells. Circulating MAIT cells were collected before and after the first treatment cycle with ICB, activated by treatment with PMA and Ionomycin and analyzed by flow cytometry for their production of TNF (a), IL-2 (b), IFN-γ (c), and GrB (d). Left-hand graphs show all patients, and the right-hand graphs show patients grouped by treatment outcome. Symbols represent individual values. Bright green circles represent patients receiving ICB as their only treatment and dark green circles patients receiving ICB together with chemotherapy. Dot-plots show the expression of IL-2 vs TNF (e) and GrB vs IFN-γ (f) in MAIT cells gated as in Supplementary Figure S1 before and after the first treatment cycle. *p < .05, n=9.
Exhaustion results in a gradual loss of effector functions and polyfunctionality, i.e. the ability of a single cell to express several different effector molecules. To determine if this ability changed in circulating MAIT cells during ICB therapy, we calculated the polyfunctionality index for MAIT cells from the available donors before and after the first treatment cycle, including TNF, IL-2, IFN-γ, and GrB. These analyses showed an increase in the polyfunctionality index in all but one of the examined patients, which did not reach significance (). However, there was a significant increase (p < .05) in the frequencies of cells with 3 or 4 functions, i.e., production of 3 or 4 cytokines and/or GrB ().
Figure 3. Polyfunctionality in MAIT cells. Circulating MAIT cells were collected before and after the first treatment cycle with ICB and analyzed by flow cytometry for their production of TNF, IL-2, IFN-γ, and GrB. (a) Polyfunctionality index before and after the first treatment cycle. (b) Percentage of cells with 4, 3, 2, 1, or 0 functions before (pink and light grey circles) and after (red and dark grey circles) the first treatment cycle. Symbols represent individual values and the lines the median. Pink and red circles represent patients receiving ICB as their only treatment and light and dark grey circles patients receiving ICB together with chemotherapy. *p < .05, n=9.
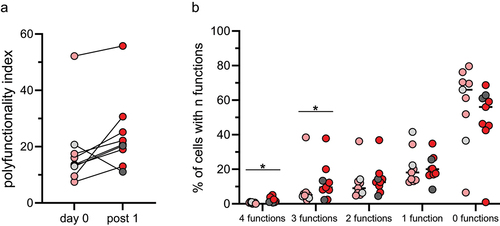
Taken together, we show that ICB increases MAIT cell anti-tumor effector functions and their polyfunctionality. This effect is evident both in patients that respond to the treatment and those that suffer from progressive disease.
Discussion
MAIT cells are abundant in human circulation and mucosal tissues, but circulating MAIT cell frequencies are often lower in cancer patients than in healthy donors. MAIT cells are also able to infiltrate tumor tissuesCitation3,Citation17,Citation27,Citation28 but there is no clear consensus on the role of MAIT cells in cancer progression. One could envision both a beneficial and unfavorable role due to their ability to secrete Th1 or Th17 type cytokines in the tumor microenvironment and their cytotoxic and wound healing potential. In the studies available so far, there is a correlation between higher intratumoral MAIT cell frequencies and a less favorable patient outcome in hepatocellular, colorectal, and squamous cell lung carcinoma, while the contrary was seen in cholangiocarcinoma, esophageal, and prostate adenocarcinoma.Citation3
MAIT cell function, not least cytokine production, varies in different tissues, with a clear Th1 bias in the circulation, gastrointestinal tract, and liver, while MAIT cells in the female genital tract and lungs are skewed toward IL-17 production.Citation4 In particular, MAIT cells that accumulate during bacterial lung infections are prominent producers of IL-17 and GM-CSF,Citation29 which both have tumor-promoting functions. MAIT cells are present in NSCLC tumors, and they were recently shown to have reduced Th1 cytokine and GrB production, but an increased IL-17-production in the tumors compared to the peritumoral lung tissue or blood.Citation10,Citation11,Citation27,Citation28 Furthermore, the tumor-infiltrating MAIT cells had a higher expression of exhaustion markers than cells in the adjacent unaffected tissue.Citation28 A recent study using a model of lung adenocarcinoma employing MR1-deficient mice showed that MAIT cells promote tumor progression, possibly due to their suppression of NK cell and CD8+ T cell cytotoxicity.Citation5 Other studies, however, demonstrated an anti-tumor effect of in vivo-activated MAIT cells in mouse models of lung metastases.Citation6,Citation7 Taken together, these studies indicate that a reinvigoration of MAIT cell function may not necessarily result in improved anti-tumor immunity, at least not in lung cancer, and that functional properties of tumor-infiltrating MAIT cells may not always be reflected in the circulation.
Previous studies investigating circulating immune cells and their correlation to a good response to treatment have identified an increase in Ki67+CD8+ T cells expressing PD-1 following therapy, preferentially in responding NSCLC patients.Citation23 A similar increase in proliferating CD8+ T cells with an exhausted phenotype was also seen in patients with malignant melanoma after ICB treatment.Citation21,Citation22 In NSCLC patients, the proliferating CD8+ cells co-expressed PD-1, HLA-DR, and CD38, while PD-1+Ki67+ invigorated CD8+ T cells in malignant melanoma patients also expressed the exhaustion marker CD39. Importantly, the increased presence of these supposedly invigorated CD8+ T cell subsets in the circulation was seen very early (1–4 weeks) after the initiation of ICB, and the increase in proliferation appears to peak already 1 week after the start of the treatment.Citation24 Unfortunately, our study design did not allow evaluation of responses earlier than 2–4 weeks after treatment initiation. Therefore, the response we detect with regard to MAIT cell proliferation and activation may actually be underestimated. Nevertheless, we found compelling evidence for an invigoration of circulating MAIT cells in NSCLC patients after the first treatment cycle, which was manifested by increased frequencies of proliferating Ki67+ MAIT cells and HLA-DR+CD38+ cells. It should be noted, though, that there are still no studies to show that HLA-DR and CD38 expression directly correlates to MAIT cell activation as there is for conventional T cells.Citation25 We could also show an increase in MAIT cell cytokine production, more specifically TNF and IL-2, following treatment and increased GrB production. It is also interesting to note that responses were very similar in patients with a partial response to treatment and those with progressive disease.
Our results are partly similar to a recent study by Shi et al.Citation28 which do not show any change in activation markers, but an increased IFN-γ and GrB production and decreased IL-17 production in circulating MAIT cells from NSCLC patients responding to ICB. However, these responses were not compared in the same patients before and after treatment, but rather in treated and untreated individuals, which might explain the differences between the studies. Unfortunately, we could not monitor MAIT cell activation and effector functions in the tumors themselves, but a recent study showed increased HLA-DR expression along with increased expression of Granzymes in intratumoral MAIT cells from squamous cell carcinoma after ICB using single-cell mRNA sequencing.Citation10
In addition to the invigoration of circulating MAIT cells during treatment, we also investigated if MAIT cell frequency or functions could be used to predict the response to ICB. A previous study in melanoma patients show that MAIT cells with a high proportion of GrB-producing cells are enriched in the circulation of patients subsequently responding to ICB.Citation13 This finding was not reproduced in a second study with melanoma patients, but instead, an increase in circulating MAIT cell frequencies could be documented directly after the treatment, but only in responding patients.Citation30 High frequencies of circulating MAIT cells during treatment were also associated to a better overall survival in this melanoma cohort.Citation30 We could not document a similar increase in circulating MAIT cell frequencies during treatment in responding patients. Furthermore, there was no difference in MAIT cell frequencies before treatment between responding and non-responding patients in our study. These differences may be due to differences between patients with malignant melanoma and NSCLC, but also the relatively low number of patients in all the studies.
This is a single center study with a well-defined patient cohort. However, one limitation of the study is the small number of patients per treatment outcome. With a larger cohort, we may have been able to detect correlations between MAIT cell numbers or function and treatment outcome. Furthermore, the use of several PD-1/PD-L1 blocking antibodies with different administration intervals may also have caused some variation in the results that may have concealed genuine differences between patient groups. Along the same lines, inclusion of patients receiving chemotherapy in addition to ICB may also have generated variations that complicate the interpretation of the effect of ICB on MAIT cells.
Based on the different correlations between MAIT cell frequencies and patient outcome in different tumor types, the function of intratumoral MAIT cells may vary between different tumor types. Thus, the effect of MAIT cell invigoration may also vary between tumors with different characteristics and localization. We believe that a more detailed and contextual understanding of MAIT cell function in different types of tumors is necessary both to predict their role in disease progression and to use MAIT cells for immunotherapy purposes. Our study shows that circulating MAIT cells respond to ICB with increased activation and cytokine production in patients with NSCLC, indicating that PD-1 signaling is important for maintaining exhaustion not only in conventional T cells, but also in MAIT cells from cancer patients.
Abbreviations
| 5-OP-RU | = | 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil |
| GrB | = | Granzyme B |
| ICB | = | Immune checkpoint blockade |
| MAIT cell | = | Mucosal-associated invariant T cell |
| MR1 | = | major histocompatibility complex class I-related protein 1 |
| NSCLC | = | non-small cell lung cancer |
| PD-1 | = | programmed cell death 1 |
| PD-L1 | = | PD-ligand 1 |
| TCR | = | T cell receptor |
Suppl material 240112.docx
Download MS Word (930.8 KB)Acknowledgments
We would like to thank all patients for their participation in this study. Study nurse Christina Krångh Turesson is also gratefully acknowledged for her help.
Tetramers of MR1 were kindly provided by the NIH tetramer core facility. The MR1 tetramer technology was developed jointly by Dr. James McCluskey, Dr. Jamie Rossjohn, and Dr. David Fairlie. The material was produced by the NIH Tetramer Core Facility and permitted to be distributed by the University of Melbourne.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Disclosure statement
M Quiding Järbrink has received consultancy fees from Biomunex Pharmaceuticals.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2312631
Additional information
Funding
References
- Provine NM, Klenerman P. MAIT cells in health and disease. Annu Rev Immunol. 2020;38(1):203–8. doi: 10.1146/annurev-immunol-080719-015428.
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605.
- O’Neill C, Cassidy FC, O’Shea D, Hogan AE. Mucosal associated invariant T cells in cancer-friend or foe? Cancers Basel. 2021;13(7):1582. doi: 10.3390/cancers13071582.
- Dias J, Boulouis C, Sobkowiak MJ, Lal KG, Emgard J, Buggert M, Parrot T, Gorin JB, Leeansyah E, Sandberg JK. Factors Influencing Functional Heterogeneity in Human Mucosa-Associated Invariant T Cells. Front Immunol. 2018;9:1602. doi: 10.3389/fimmu.2018.01602.
- Yan J, Allen S, McDonald E, Das I, Mak JYW, Liu L, Fairlie DP, Meehan BS, Chen Z, Corbett AJ, et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov. 2020;10(1):124–141. doi: 10.1158/2159-8290.CD-19-0569.
- Petley EV, Koay HF, Henderson MA, Sek K, Todd KL, Keam SP, Lai J, House IG, Li J, Zethoven M, et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun. 2021;12(1):4746. doi: 10.1038/s41467-021-25009-4.
- Ruf B, Catania VV, Wabitsch S, Ma C, Diggs LP, Zhang Q, Heinrich B, Subramanyam V, Cui LL, Pouzolles M, et al. Activating mucosal-associated invariant T cells induces a broad antitumor response. Cancer Immunol Res. 2021;9(9):1024–1034. doi: 10.1158/2326-6066.CIR-20-0925.
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37(1):457–495. doi: 10.1146/annurev-immunol-041015-055318.
- Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, Liu J, Subudhi SK, Poon C, Gant KL, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186(8):1652–1669. doi: 10.1016/j.cell.2023.03.006.
- Yao T, Shooshtari P, Haeryfar SMM. Leveraging public single-cell and bulk transcriptomic datasets to delineate MAIT cell roles and phenotypic characteristics in human malignancies. Front Immunol. 2020;11:1691. doi: 10.3389/fimmu.2020.01691.
- Li S, Simoni Y, Becht E, Loh CY, Li N, Lachance D, Koo SL, Lim TP, Tan EKW, Mathew R, et al. Human tumor-infiltrating MAIT cells display hallmarks of bacterial antigen recognition in colorectal cancer. Cell Rep Med. 2020;1(3):100039. doi: 10.1016/j.xcrm.2020.100039.
- Rodin W, Sundstrom P, Ahlmanner F, Szeponik L, Zajt KK, Wettergren Y, Bexe Lindskog E, Quiding Jarbrink M. Exhaustion in tumor-infiltrating Mucosal-Associated Invariant T (MAIT) cells from colon cancer patients. Cancer Immunol Immunother. 2021;70(12):3461–3475. doi: 10.1007/s00262-021-02939-y.
- De Biasi S, Gibellini L, Lo Tartaro D, Puccio S, Rabacchi C, Mazza EMC, Brummelman J, Williams B, Kaihara K, Forcato M, et al. Circulating mucosal-associated invariant T cells identify patients responding to anti-PD-1 therapy. Nat Commun. 2021;12(1):1669. doi: 10.1038/s41467-021-21928-4.
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litiere S, Dancey J, Chen A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8.
- Langenskiold C, Mellgren K, Abrahamsson J, Bemark M. Determination of blood cell subtype concentrations from frozen whole blood samples using TruCount beads. Cytometry B Clin Cytom. 2018;94(4):660–666. doi: 10.1002/cyto.b.21390.
- Larsen M, Sauce D, Arnaud L, Fastenackels S, Appay V, Gorochov G, Hoshino Y. Evaluating cellular polyfunctionality with a novel polyfunctionality index. PLoS One. 2012;7(7):e42403. doi: 10.1371/journal.pone.0042403.
- Loh L, Gherardin NA, Sant S, Grzelak L, Crawford JC, Bird NL, Koay HF, van de Sandt CE, Moreira ML, Lappas M, et al. Human mucosal-associated invariant T cells in older individuals display expanded TCRalphabeta clonotypes with potent antimicrobial responses. J Immunol. 2020;204(5):1119–1133. doi: 10.4049/jimmunol.1900774.
- Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9(1):2724. doi: 10.1038/s41467-018-05072-0.
- Chow A, Uddin FZ, Liu M, Dobrin A, Nabet BY, Mangarin L, Lavin Y, Rizvi H, Tischfield SE, Quintanal-Villalonga A, et al. The ectonucleotidase CD39 identifies tumor-reactive CD8+ T cells predictive of immune checkpoint blockade efficacy in human lung cancer. Immunity. 2023;56(1):93–106.e6. doi: 10.1016/j.immuni.2022.12.001.
- Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PloS Pathog. 2015;11(10):e1005177. doi: 10.1371/journal.ppat.1005177.
- Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y.
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079.
- Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114.
- Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH, Ahn JS, Cheon J, Min YJ, Park SH, et al. The first-week proliferative response of peripheral blood PD-1(+)CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25(7):2144–2154. doi: 10.1158/1078-0432.CCR-18-1449.
- Kestens L, Vanham G, Gigase P, Young G, Hannet I, Vanlangendonck F, Hulstaert F, Bach BA. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6(8):793–797. doi: 10.1097/00002030-199208000-00004.
- Chraa D, Naim A, Olive D, Badou A. T lymphocyte subsets in cancer immunity: friends or foes. J Leukoc Biol. 2019;105(2):243–255. doi: 10.1002/JLB.MR0318-097R.
- Ouyang L, Wu M, Zhao J, Jiang L, Shen Z, Cheng X, Wang W, Wu X, Cao X, Weng X. Mucosal-associated invariant T cells reduce and display tissue-resident phenotype with elevated IL-17 producing capacity in non-small cell lung cancer. Int Immunopharmacol. 2022;113(Pt B):109461. doi: 10.1016/j.intimp.2022.109461.
- Shi L, Lu J, Zhong D, Song M, Liu J, You W, Li WH, Lin L, Shi D, Chen Y. Clinicopathological and predictive value of MAIT cells in non-small cell lung cancer for immunotherapy. J Immunother Cancer. 2023;11(1):e005902. doi: 10.1136/jitc-2022-005902.
- Meierovics AI, Cowley SC. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med. 2016;213(12):2793–2809. doi: 10.1084/jem.20160637.
- Vorwald VM, Davis DM, Van Gulick RJ, Torphy RJ, Borgers JS, Klarquist J, Couts KL, Amato CM, Cogswell DT, Fujita M, et al. Circulating CD8(+) mucosal-associated invariant T cells correlate with improved treatment responses and overall survival in anti-PD-1-treated melanoma patients. Clin Trans Immunol. 2022;11(1):e1367. doi: 10.1002/cti2.1367.
