?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Spartalizumab (PDR001) is a humanized IgG4 monoclonal antibody targeting programmed cell death protein 1 (PD-1). We conducted a single-arm, phase 2 trial to investigate the efficacy and safety of spartalizumab in patients with refractory esophageal squamous cell carcinoma (ESCC). Patients with histologically confirmed ESCC who experienced disease progression after platinum-based chemotherapy received 300 mg of intravenous spartalizumab every three weeks until disease progression or occurrence of unacceptable toxicity. The primary endpoint was centrally assessed objective response according to the Response Evaluation Criteria in Solid Tumors, version 1.1. Adverse events were closely monitored throughout the study. From March 2020 through April 2021, 44 patients with ESCC were enrolled. Of the 44 patients, the objective response rate was 20.5% (95% confidence interval: 8.5–32.4). With a median follow-up of 10.9 months, median progression-free survival and overall survival were 3.2 months and 11.2 months, respectively. In addition, the median duration of response was 24.7 months. The most common grade 3 or 4 adverse event was grade 3 dysphagia (eight [18%] patients). Biomarker analyses explored programmed cell death ligand 1 and CD20 as potential predictive markers for PD-1 blockade. Spartalizumab showed promising activity with a manageable safety profile, indicating its potential as a new treatment option for patients with refractory ESCC.
Trial registration
The trial was registered at ClinicalTrials.gov under the identifier NCT03785496.
Introduction
Esophageal cancer ranks seventh in incidence and sixth in cancer-related mortality, with 544,000 deaths reported worldwide in 2020.Citation1 The most common histological subtype in Asia is squamous cell carcinoma (SCC), which accounts for approximately 90% of esophageal cancer cases.Citation2 Although neoadjuvant chemoradiation followed by surgery has moderately improved the survival rate of esophageal cancer,Citation3 over 30% of the patients still develop recurrence postoperatively.Citation4 Treatment options are limited for patients with metastatic esophageal squamous cell carcinoma (ESCC), highlighting the need for novel treatment strategies.
Immune checkpoint blockade has dramatically changed the treatment of melanoma and non-small cell lung cancer (NSCLC), and its efficacy is being explored in gastrointestinal cancer.Citation5,Citation6 Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on various immune cells, including activated T cells, regulatory T cells, and B cells.Citation7,Citation8 PD-1 interacts with programmed cell death ligand 1 (PD-L1) or programmed cell death ligand 2 (PD-L2) to downregulate effector T-cell responses and mediate immune tolerance.Citation9,Citation10 Monoclonal antibodies targeting PD-1 can restore effector T cell function and antitumor activity,Citation11 leading to clinical benefits in patients with advanced cancers.Citation12,Citation13 There is a growing trend to integrate immunotherapy with conventional chemotherapy to improve therapeutic outcomes. Currently, the combination of PD-1 inhibitor with cytotoxic chemotherapy is an established standard first-line treatment option for various tumor types including NSCLC, head and neck squamous cell carcinoma, and ESCC.Citation14–18
Spartalizumab (PDR001) is a humanized IgG4κ monoclonal antibody that binds to PD-1 with subnanomolar activity in vitro and blocks interaction with PD-L1/PD-L2 in cell-based assays.Citation19 Although limited efficacy was reported in a phase 1 trial targeting the heavily pretreated and heterogeneous population, on-treatment immune activation was observed via paired tumor biopsies of advanced or metastatic solid tumors.Citation19
Based on the rationale mentioned above, we conducted a multicenter, single-arm, phase 2 trial to evaluate the safety and efficacy of spartalizumab among recurrent or metastatic ESCC patients in Korea.
Materials and methods
Study design and participants
Eligible patients were diagnosed as having histologically confirmed stage IVB or recurrent ESCC refractory or intolerant to standard therapy. Patients were required to have an age of 20 years or older, Eastern Cooperative Oncology Group (ECOG) performance status of 0 through 2, a measurable lesion (based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1),Citation20 and adequate organ function starting therapy. Patients had no history of immune checkpoint inhibitor treatment and have experienced disease progression during or following prior platinum-based chemotherapy for metastatic disease or disease recurrence within six months after treatment with platinum-based chemotherapy with curative intent. Exclusion criteria included the presence of clinically significant concurrent malignancies interfering with the prognosis of ESCC, impaired cardiac or pulmonary function precluding major surgery, active autoimmune or infectious disease, ongoing systemic corticosteroid or other immunosuppressive therapy, pneumonitis or interstitial lung disease or active infection, and acute or chronic infection with hepatitis B or C virus. The complete inclusion and exclusion criteria are listed in the Supplementary methods. The study was approved by the Korean Cancer Study Group (KCSG) and the institutional ethics committees of each hospital. It was carried out strictly according to the principles of Good Clinical Practice and the Declaration of Helsinki. The KCSG study number was KCSG HN18–17, and the clinicaltrials.gov identifier number was NCT03785496. Additionally, this study (K-MASTER project 12; KM-12) was part of the K-MASTER project, a nationwide cancer genome screening project in Korea initiated in 2017.Citation21 Written informed consent was obtained from each participant. Each participant provided written consent after being fully informed.
Treatment
Eligible patients received spartalizumab intravenously at a dose of 300 mg in 30-minute infusions every three weeks in six-week cycles. Treatment continued until disease progression, death, intolerable toxic effects, withdrawal of consent, or an investigator’s decision to discontinue treatment. Dose modification was prohibited, but dose skipping was allowed if patients experienced grade 3 or worse treatment-related adverse events (TRAE) according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Treatment was discontinued if the investigator assessed the patient as having progressive disease according to RECIST version 1.1, worsening clinical symptoms, intolerable or life-threatening adverse events, or withdrew consent.
Assessments
The disease was assessed by computed tomography of the chest and abdominopelvic area at screening and every six weeks after that (Day 21[seven days] of every even-numbered cycle) until radiological disease progression.
End points
The primary endpoint was the centrally assessed objective response rate (ORR), which was defined as the proportion of patients whose best overall response was complete response or partial response according to the RECIST v1.1. Secondary endpoints were the depth of response (defined as the change in target-lesion size from baseline), best objective response, overall survival (OS, defined as the length of time from the start of treatment to death), progression-free survival (PFS, defined as the length of time from the start of treatment until the first evidence of disease progression or death), disease control rate (DCR), duration of response (DOR), and safety, assessed by the investigators.
Biomarker analysis
Biopsy samples from the primary tumor were fixed in 10% formalin, embedded in paraffin, and cut into 4 μm-thick tissue sections for further analyses. Formalin-fixed, paraffin-embedded (FFPE) sections were transferred to adhesive slides and dried at 60°C for 30 minutes. The slides were incubated with primary antibodies at 4°C overnight. Immune cells within the tumor and surrounding stromal compartments were measured using immunohistochemistry (IHC) with a panel of six proteins (CD20, CD3, CD4, CD8, PD-1, FOXP3). Tertiary lymphoid structures (TLSs) were identified by clusters of CD20-positive B cells, encircled by accumulating T cells.Citation22 Patients were considered TLS-positive if at least one TLS was present within the tumor’s invasive margins. The following primary antibodies were used: rabbit PD-L1 monoclonal antibody (clone SP263, 1:100; Ventana Medical Systems, Tucson, USA), CD20 monoclonal antibody (L26, ready to use, Ventana Medical Systems, Tucson, USA), CD276 monoclonal antibody (6A1, 1:200; Abcam, Cambridge, UK), CD155 monoclonal antibody (D8A5G, 1:100; Cell Signaling Technology, Massachusetts, USA), rabbit CD4 monoclonal antibody (clone SP35, 1:100; Abcam, Cambridge, UK), rabbit CD8 monoclonal antibody (clone SP57, 1:100; Ventana Medical Systems), PD-1 monoclonal antibody (NAT105, 1:100; Cell Marque, USA), and mouse forkhead box P3 (FOXP3) monoclonal antibody (clone 263A/E7, 1:100; Abcam). After adding secondary antibodies and counterstaining with hematoxylin, immune detection was achieved using the DISCOVERY DAB Map Detection Kit (Ventana Medical Systems) according to the manufacturer’s instructions. The number and intensity of immunostaining cells were calculated using image analysis software (NuclearQuant, 3D HISTECH, Budapest, Hungary).
PD-L1 protein expression was determined by both tumor proportion score (TPS), defined as the number of PD-L1 membranous stained tumor cells divided by the total number of viable tumor cells, and combined positive score (CPS), defined as the number of PD-L1 staining cells (tumor cells, lymphocytes, and antigen-presenting cells) divided by the total number of viable tumor cells.
For CD3, CD4, CD8, CD20, PD-1, and FOXP3, immune cell density was calculated as the number of positively staining immune cells divided by the total annotation area. We established the cutoff of immune cell density for CD3, CD4, CD20, PD-1, and FOXP3 as their median values based on earlier studies.Citation22–24 We categorized patients with greater density values compared to the cutoff as “high” (330 cells/mm2 for CD3, 190 cells/mm2 for CD4, 18 cells/mm2 for CD20, 30 cells/mm2 for PD-1, 160 cells/mm2 for FOXP3, respectively) and the others as “low.” The cutoff cell density value of 400 cells/mm2 for CD8 was used based on earlier studies.Citation25
For CD276 IHC, patients were classified as negative if there was no observable staining in both the tumor and tumor-infiltrating lymphocytes. All others were classified as positive.
For poliovirus receptor (PVR, CD155) IHC, a histoscore (H-score) was calculated based on staining intensity (0, none; 1, weak; 2, intermediate; and 3, strong) and percentage of positive cell staining ranging from 0 to 300. To facilitate the stratification of patients according to the protein expression, we set the cutoff for expression of PVR in tumors as their median value and defined patients with higher H-score compared to the cutoff as “high” (H-score 60 for PVR) and the others as “low.”
Statistical analysis
To investigate the effectiveness of the treatment against a null hypothesis of a 10% objective response rate (ORR) and an alternative hypothesis of over 30% ORR, our study was designed with a Type I error rate of 0.05 and a Type II error rate of 0.10. Additionally, we aimed to examine the deviation from a 20% response rate in patients previously treated with platinum-based chemotherapy, using a one-sided alpha level of 0.05 for the primary analysis. Considering a 20% dropout and follow-up loss rate, we determined a total sample size of 44 patients to ensure the statistical validity and reliability of our findings.
All patients who received at least one dose of spartalizumab were included for analysis. The response rates (ORR and DCR) were determined by assessing the proportion of patients with RECIST-defined responses, accompanied by 95% confidence intervals (CI) calculated using normal approximation. For time-to-event endpoints (OS, PFS), median values and two-sided 95% CIs were estimated using the Kaplan-Meier method. Statistical analyses were performed using GraphPad Prism software version 8.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Patients and treatment
From March 2020 through April 2021, 49 patients were screened, and 44 eligible patients were enrolled. Patient demographic and baseline characteristics are displayed in . At the time of data cutoff (October 31, 2023), the median follow-up was 10.9 months (range, 0.8 to 41.3 months) with a median duration of treatment exposure of 3.2 months (range, 0.5 to 41.3 months), and four patients continued receiving spartalizumab treatment.
Table 1. Baseline patient characteristics.
Efficacy
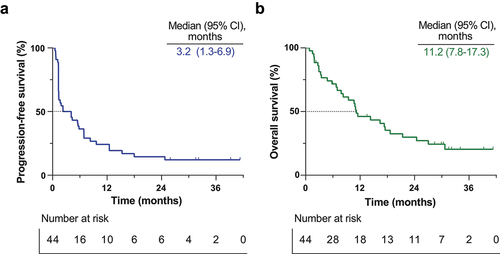
The ORR was 20.5% (95% CI: 8.5–32.4), with nine out of the 44 intent-to-treat population showing objective responses, according to the investigator’s assessment. Reductions in the sum of target lesion measurements were observed in 16 patients (36.4; 95% CI: 22.2–50.6, ). 25 patients achieved disease control (56.8%; 95% CI: 42.2–71.5). Median DOR was 23.4 months (95% CI: 4.4–30.2, Supplementary Fig. S1, ), with three patients showing persistent response at the data cutoff point (). In addition, there were six long-term survivors, including five persistent responders, who persisted in treatment for more than 24 months since receiving spartalizumab. In the overall intent-to-treat population, the median PFS was 3.2 months (95% CI: 1.3–6.9, , ), and the median OS was 11.2 months (95% CI: 7.8–17.3, , ), respectively. There was no significant difference in PFS or OS when analyzed by treatment line (Supplementary Fig. S2).
Figure 1. Tumor responses.
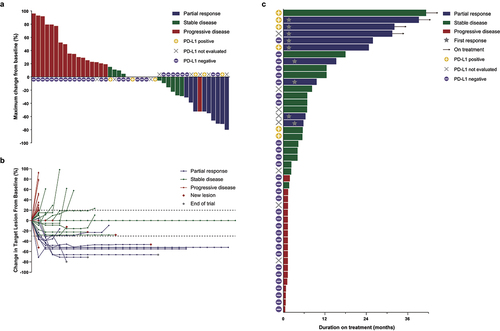
Table 2. Treatment efficacy.
Adverse events
Adverse events were reported in 38 (86%) out of 44 patients, with grade 3–5 events in 17 (39%, ) and serious adverse events in 17 (39%, Supplementary Table S1). The most common grade 3 or grade 4 adverse events were grade 3 dysphagia (eight [18%] patients), grade 3 lung infection (three [7%] patients), grade 4 hypercalcemia (two [5%] patients), and grade 3 hypoxia (two [5%] patients) (). The most common serious adverse events were dysphagia (25%), lung infection (11%), and fatigue (7%) (Supplementary Table S1). TRAEs were reported in 21 (48%) of 44 patients, with grade 3–5 events in 4 (9%) and serious treatment-related adverse events in 4 (9%). One patient had diabetic ketoacidosis, thrombocytopenia, and thrombosis deemed a treatment-related adverse event. There was one case each of aspiration pneumonia, colitis, and fever, all of whom recovered after antibiotics therapy ().
Table 3. Adverse events and treatment-related adverse events.
Treatment was interrupted six times in five patients (11%), all unrelated to treatment except for one case of grade 1 dermatitis with a probable causal relationship with the treatment. Treatment was discontinued in one patient (2%): a case of diabetic ketoacidosis, thrombocytopenia, and thrombosis, classified as TRAEs. One thromboembolic event led to mortality, and the causal relationship to spartalizumab could not be ruled out (Supplementary Table S1).
Exploratory analysis
To investigate the contribution of biomarker expression and immune cell composition of the tumor microenvironment on treatment efficacy, we categorized patients based on PD-L1 TPS (), PD-L1 CPS, density of CD3, CD4, CD8, CD20 (), PD-1, FOXP3 stained immune cells, CD276 tumor staining, and PVR H-score. PD-L1 positive patients (TPS ≥1%) showed superior prognosis compared to the PD-L1 negative patients (TPS <1%), both in terms of PFS (median PFS 33.0 vs 1.4 months, p = 0.0031, top) and OS (median OS unreached vs 7.8 months, p = 0.0042, Supplementary Fig. S3A). High expression of PD-L1 based on other cutoff values (TPS 10%, CPS 1, 10) also showed the same trend despite weak significance (Supplementary Fig. S3C-H).
Figure 3. Biomarker analysis.
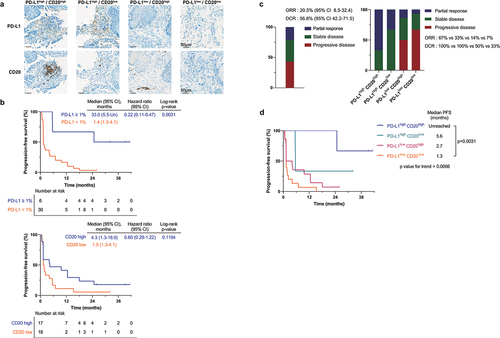
Furthermore, higher infiltration of CD20-positive immune cells displayed a trend toward improved prognosis, although statistically insignificant ( bottom, Supplementary Fig. S3B). This trend aligns with a prior study reporting a positive correlation between B cell infiltration and clinical benefit to PD-1 targeted immunotherapy in advanced ESCCCitation26 and different cancer types.Citation27
The ORR of 20.5% and DCR of 56.8% ( left, ) were subjected to further investigation based on biomarker expression. PD-L1 positive patients (TPS ≥1%) showed improved ORR (50 vs 10%, p = 0.0164, Supplementary Fig. S4A) and DCR (100 vs 43%, p = 0.0111, Supplementary Fig. S4A) in comparison to PD-L1 negative patients. The PD-L1 positive group with CPS ≥ 1 (29%, Supplementary Fig. S4B) and CD20 high group (24%, Supplementary Fig. S4C) had a higher ORR compared to the complementary group of CPS < 1 (5%, p = 0.0524, Supplementary Fig. S4B) and CD20 low group (11%, p = 0.3299, Supplementary Fig. S4C).
To further visualize the efficacy of spartalizumab based on immune checkpoint ligand expression and immune cell infiltration, we categorized patients into four groups:
High expression of PD-L1 (TPS ≥1%) and high density of CD20-positive immune cell infiltration (high/high)
High expression of PD-L1 and low density of CD20-positive immune cell infiltration (high/low)
Low expression of PD-L1 (TPS <1%) and high density of CD20-positive immune cell infiltration (low/high)
Low expression of PD-L1 and low density of CD20-positive immune cell infiltration (low/low).
For the four groups, the median PFS values were found to be unreached in PD-L1high CD20high, 5.6 months in PD-L1high CD20low, 2.7 months in PD-L1low CD20high, and 1.3 months in PD-L1low CD20low (). The median OS values were found to be unreached in PD-L1high CD20high, 21.6 months in PD-L1high CD20low, 9.5 months in PD-L1low CD20high, and 7.0 months in PD-L1low CD20low (Supplementary Fig. S5A). Similar trends were noticed when patients were categorized based on PD-L1 (CPS) and CD20-positive immune cell infiltration density (Supplementary Fig. S5B, S5C). In terms of ORR and DCR, the PD-L1high CD20high group presented higher ORR (67 vs 10%, p = 0.0109, right) and DCR (100 vs 33%, p = 0.0339, right) compared to the PD-L1low CD20low group.
Additionally, positive staining of CD276 displayed a trend toward poor prognosis, although statistically insignificant (Supplementary Fig. S6A, S6B). Regarding response rate, the two groups had no difference in ORR. However, the CD276 negative group showed a significantly higher DCR (78%, Supplementary Fig. S4D) than the CD276 positive group (36%, p = 0.0135, Supplementary Fig. S4D). Once the patients were categorized into four groups according to PD-L1 (TPS) and CD276 expression, the PD-L1high CD276neg group presented higher ORR (33 vs 10%, p = 0.2837, Supplementary Fig. S4E) and DCR (100 vs 26%, p = 0.0137, Supplementary Fig. S4E) compared to the PD-L1low CD276pos group. In terms of survival, the PD-L1high CD276neg group presented elongated median PFS (Supplementary Fig. S7A, S7C) and median OS (Supplementary Fig. S7B, S7D) over the PD-L1low CD276pos for both PD-L1 (TPS) and PD-L1 (CPS). This trend aligns with prior studies reporting a negative correlation between CD276 expression and prognosis in non-small cell lung cancer.Citation28,Citation29
Different biomarkers displayed a possibility for predicting prognosis. Lower expression of PVR in cancer cells exhibited improved survival (Supplementary Fig. S8A, S8B), consistent with earlier studies in different cancer types.Citation30,Citation31 Increased infiltration of immune cells, irrespective of the biomarker, displayed a trend toward an improved prognosis. As earlier reports suggest, higher density of PD-1,Citation32 FOXP3,Citation33 CD3,Citation34 CD4,27 and CD825 positive immune cell infiltration exhibited improved efficacy against PD-1 blockade (Supplementary Fig. S8C-L). This tendency was repeatedly observed when we compared the density of biomarker-positive immune cells based on response (Supplementary Fig. S9). In case of TLS, ten cases were identified as TLS-positive. These patients exhibited a trend toward improvement in PFS, although OS did not differ (Supplementary Fig. S10A-B).
Furthermore, the expression of biomarkers in six patients who received treatment for more than 24 months was further explored. Excluding one patient without IHC data due to lack of FFPE, all five patients had a positive PD-L1 expression in CPS. Four patients had a positive PD-L1 expression in TPS (), while one patient with negative PD-L1 TPS expression exhibited extremely high infiltration of CD20-positive immune cells (1885.77 cells/mmCitation2 and was identified as TLS-positive (Supplementary Fig. S10C).
Discussion
In this multicenter, single-arm, phase 2 study, spartalizumab showed encouraging activity with a manageable safety profile in patients with advanced ESCC. Even though more than 30% of patients received spartalizumab as their 3rd line regimen, the central assessment based on RECIST version 1.1 revealed that 20.5% had an objective response, 56.8% achieved disease control, and target lesions decreased in 36.4% of patients. This encouraging activity was further underscored by a median DOR of 23.4 months in responding patients.
Despite recent advancements, patients with metastatic ESCC who progress after receiving first-line platinum-based doublet therapy face limited choices and a dismal outlook. Before the approval of nivolumab and pembrolizumab, there had been no established standard of care after first-line treatment, while taxanes and irinotecan were generally used.Citation35 Previously, two global trialsCitation36,Citation37 have evaluated the activity of PD-1 inhibitors in patients previously advanced or metastatic ESCC. As a monotherapy, the ORR was 17.2% and 14.3% in nivolumab and pembrolizumab, respectively.Citation36,Citation37 In addition, the phase III trials resulted in better OS and DOR compared to standard cytotoxic chemotherapy.Citation38,Citation39
The ORR of 20.5% and DCR of 56.8% imply that spartalizumab has the comparable ability to reduce tumor burden in ESCC as other immune checkpoint inhibitors previously reported in the palliative 2nd-line setting.Citation36,Citation37 This finding adds to the evidence for the efficacy of PD-1 blockade in ESCC.
The prolonged median DOR of 23.4 months in our study is comparable to or even superior to the global trials, with the KEYNOTE-180 study reporting a median DOR that was not reached (range, 1.9 to 14.4 months) and the ATTRACTION-1 study reporting a median DOR of 11.2 months.Citation40,Citation41 This finding could arise from the relatively high proportion of metachronous patients rather than de novo metastatic patients in our study group. Metachronous disease has shown an enhanced prognosis compared to de novo metastatic disease in different cancer types.Citation42–44 All five patients with prolonged response over 18 months had metachronous disease. This study demonstrates ten patients who continued treatment for more than 12 months, six of whom continued for more than 24 months. Notably, all five patients, except one without IHC data, showed positive expression of PD-L1 in CPS. Excluding one without IHC, four of the nine patients who received treatment for more than 12 months had a positive expression of PD-L1 in TPS. Additionally, four of the five patients with negative PD-L1 TPS exhibited a high CD20-positive immune cell infiltration density. These findings suggest that the combination of PD-L1 expression and CD20-positive immune cell infiltration may serve as a marker for treatment response and predict durable response.
The toxicity profile was consistent with that reported in other cancers, except for one fatal thromboembolic event.Citation19,Citation45–48 39% of patients experienced grade 3–5 adverse events, of which 9% were TRAEs. Treatment was interrupted six times in five patients (11%), with only one case of grade 1 dermatitis with a probable causal relationship with treatment and all others unrelated to treatment. In one case, treatment was discontinued due to a thromboembolic event that resulted in mortality. The patient had shown an excellent response to spartalizumab with an 80% reduction in target lesion, but a right ventricular thrombosis occurred that progressed despite anticoagulation treatment.
In a first-in-human phase I trial, spartalizumab was evaluated in patients with multiple types of tumors, including sarcoma, renal cell carcinoma, NSCLC, and melanoma, with one case of esophageal cancer.Citation19 The trial assessed the safety and preliminary efficacy of spartalizumab in treating these advanced solid tumors. The trial results showed that spartalizumab was well-tolerated without any major adverse events reported. The recommended phase 2 dosing regimens were established as 400 mg every four weeks or 300 mg every three weeks. Encouragingly, clinical activity was observed in patients with melanoma, renal cell carcinoma, and the lone esophageal cancer case. Spartically treated responders displayed on-treatment increases in PD-L1 expression and CD8+ tumor-infiltrating lymphocytes, suggestive of an induced immune response. Furthermore, recent investigations highlight the potential of spartalizumab in combination with ieramilimab, a LAG-3 inhibitor, for treating various advanced solid malignancies,Citation49,Citation50 opening avenues for combination therapy.
Studies conducted on ESCC have shown a correlation between PD-L1 expression and the efficacy of PD-1 inhibitor therapy, similar to what has been observed in other tumor types like NSCLC.Citation51 ESCC patients with higher PD-L1 levels tend to respond better to PD-1 blockade therapies, with pembrolizumab showing superior outcomes in PD-L1 positive patients compared to those with negative PD-L1 expression (ORR: 13.8% versus 6.3%).Citation37 Notably, while using pembrolizumab did not yield an overall survival benefit compared to conventional chemotherapy in all patients, the most substantial survival benefits were seen in patients with a PD-L1 CPS ≥ 10 in the phase III trial.Citation39 In case of nivolumab, antitumor activity was observed regardless of PD-L1 status, although responses were enriched in patients with PD-L1 positive tumors.Citation36 Nonetheless, greater clinical benefits were observed in patients with PD-L1 expression of 1% or more, as opposed to those with less than 1% (HR: 0.69 versus 0.84).Citation38 In the current study, patients with a PD-L1 TPS of 1% or higher exhibited an ORR of 50% and a DCR of 100%, whereas patients with a PD-L1 TPS of less than 1% had an ORR of 10% and a DCR of 43%. Patients with positive or high PD-L1 expression had better prognoses regarding both PFS and OS than patients with negative or low PD-L1 expression. However, not all patients with positive or high PD-L1 expression responded to spartalizumab, suggesting that while PD-L1 expression is an important predictive biomarker, it is not the only factor determining response to PD-1 inhibitors. Furthermore, the predictive utility of PD-L1 CPS regarding treatment response is limited compared to PD-L1 TPS.
To further elucidate the potential mechanisms underlying the clinical efficacy of spartalizumab in ESCC, we selected a panel of markers (CD3, CD4, CD8, CD20, PD-1, FOXP3) based on previous literature to comprehensively assess the immune microenvironment of ESCC and its potential association with response to spartalizumab.Citation52,Citation53 These markers have been extensively studied in various tumor types and have been shown to have prognostic and/or predictive value in the context of immunotherapy.Citation25–27,Citation54,Citation55 Our results showed that patients with abundant CD20-positive immune cell infiltration were associated with improved activity of the PD-1 inhibitors compared to low CD20-positive immune cell infiltrating patients, even in the low PD-L1 subgroup. The high PD-L1 expression and high CD20-positive immune cell infiltrating subgroup displayed the best survival outcomes, with the median PFS and OS unreached at the time of analysis. Although a limited number of patients were categorized into this subgroup (n = 3), all patients showed a preeminent response and maintained therapy for more than 24 months, including two patients continuing spartalizumab treatment at the time of data cutoff. Our results align with the emerging picture of tumor-infiltrating B lymphocyte’s ability to form and sustain immunologically hot tumor microenvironments and promote antitumor immunity.Citation53 Studies have demonstrated that an increase in CD20-positive B lymphocyte infiltration within the tumors can predict the effectiveness of immunotherapy in ESCC and other cancers, including breast cancer, melanoma, and head and neck squamous cell carcinoma.Citation14,Citation22,Citation27,Citation56 High CD20-positive immune cell infiltration in combination with PD-L1 expression could act as a predictive biomarker for immunotherapy response and selection of subgroups that do not develop resistance.
Furthermore, we conducted an in-depth exploration of the role of cell surface markers beyond PD-L1 and identified a correlation between CD276 expression and tumor progression. Our results showed the presence of CD276 expression in the tumor tissue of ESCC samples, which is consistent with previous research.Citation57,Citation58 It is valuable to note that positive CD276 expression in patients with low PD-L1 was associated with lower activity of the PD-1 inhibitors compared to negative CD276 expression. In addition, our findings indicate a diminished clinical benefit of spartalizumab in ESCC patients exhibiting positive CD276 expression. When combined with PD-L1 expression, patients with low PD-L1 and positive CD276 expression had the worst survival compared to other subgroups. However, assessing the activity of spartalizumab in patients with high PD-L1 and positive CD276 expression was challenging due to the limited number of patients in this subgroup (n = 3). CD276, also known as B7-H3, is a member of the B7 family, which has been recognized for its potential therapeutic impact on malignancies due to its crucial role in controlling the T-cell immune response.Citation57 CD276 was first introduced in 2001 and is essential for cell motility, invasion, and proliferation in cancer. Its expression has been linked to poor survival in various types of cancer and may be a promising therapeutic target for malignant tumors.Citation59,Citation60 Our data suggest that the therapeutic activity of PD-1 blockade is relatively limited in high CD276-expressing tumors in ESCC and that a dual blockade of PD-1 and CD276 may be a promising therapeutic approach. Currently, targeted CD276 inhibitors including monoclonal antibodies and radioimmunotherapy, are showing promising preliminary clinical results.Citation61 Enoblituzumab, a monoclonal antibody targeting CD276, is being tested in clinical trials alongside anti-PD-1 therapy.Citation62 Additionally. omburtamab, a radiolabeled monoclonal antibody aimed at CD276, is under investigation for intrathecal use in treating neurological cancers.Citation63
Immunotherapy has emerged as a promising treatment option for various cancers and has demonstrated efficacy in improving long-term survival in a subset of patients. While traditional clinical trials have used ORR as the primary endpoint to evaluate the efficacy of immunotherapy, it is increasingly recognized that the percentage of long-term responders may be a more clinically meaningful measure of efficacy.Citation64 While ORR can provide valuable information regarding the initial tumor response to therapy, it may not accurately reflect the long-term benefits of treatment. The percentage of long-term responders measures the proportion of patients who achieve a durable response to immunotherapy, defined as a response lasting for a specified period (usually at least six or 12 months). This measure considers that some patients may initially progress or experience stable disease but ultimately achieve a durable response and long-term survival. Durable responses may persist for many years and thus are an essential measure of efficacy in immunotherapy.
In recent years, immunotherapy has exhibited promising outcomes as part of the treatment armamentarium of ESCC.Citation65,Citation66 The United States Food and Drug Administration (US FDA) has approved anti-PD-1 therapy combined with chemotherapy as the first-line treatment of patients with advanced or metastatic ESCC.Citation67 Pembrolizumab and nivolumab in combination with chemotherapy have shown superior survival benefits over chemotherapy alone for treatment-naïve patients with advanced or metastatic ESCC.Citation17,Citation18 Several trials are currently ongoing to assess the efficacy of immunotherapy in ESCC, including neoadjuvant or adjuvant therapy as well as palliative therapy.Citation6,Citation68 The encouraging results of our trial contribute to the increasing body of evidence regarding the efficacy and limitation of anti-PD1 monotherapy in ESCC and highlight the potential for combining spartalizumab with chemotherapy or other immune-modulating drugs in various therapeutic settings for ESCC.
This study has several potential limitations. Firstly, the present study is a single-arm trial, lacking a comparator group, which poses a significant challenge in evaluating the efficacy of spartalizumab in advanced ESCC patients. Secondly, since the present study was conducted at multiple centers within a single country, it limits the ability to assess the efficacy of the treatment in different ethnic populations. Moreover, the limited number of subgroups evaluated in the study makes it difficult to generalize the findings and conclude that patients with high PD-L1 expression and CD20-positive immune cell infiltration respond well to spartalizumab. In addition, we acknowledge that our IHC-based methodology has inherent limitations in capturing the full complexity of the immune landscape and the determinants of immunotherapy response. In particular, our study did not assess global gene expression profiles, utilize advanced imaging techniques, or evaluate well-established predictive signatures such as the interferon-gamma response signature.Citation69 The incorporation of these complementary approaches in future studies could provide valuable additional insights into the mechanisms of action of spartalizumab and help to refine our understanding of the key immunological drivers of response and resistance. Despite these limitations, we believe that our study provides important proof-of-concept for the efficacy and safety of spartalizumab in advanced ESCC and lays the foundation for future biomarker-driven trials. By integrating our findings with those of other ongoing studies and leveraging cutting-edge translational research techniques, we hope to accelerate the development of personalized immunotherapy strategies that can improve outcomes for patients with this challenging disease.
In conclusion, spartalizumab has demonstrated promising efficacy with a favorable DOR and a manageable safety profile. Our findings provide preliminary evidence of a potential new treatment option for patients with refractory ESCC.
List of abbreviations
CI, confidence interval; CPS, combined positive score; DCR, disease control rate; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; ESCC, esophageal squamous cell carcinoma; FFPE, formalin-fixed, paraffin-embedded; FOXP3, forkhead box P3; H-score, histoscore; IHC, immunohistochemistry; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PD-L2, programmed cell death ligand 2; PFS, progression-free survival; PVR, poliovirus receptor; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score; TRAE, treatment-related adverse event
Author’s contributions
Conceptualization: BCC. Investigation: All authors. Formal analysis: DKL. Writing – original draft: DKL and MHH. Writing – review & editing: All authors. Supervision: BCC and MHH.
Availability of data and material
All data relevant to the study are included in the article or supplemental information.
Ethics approval and consent to participate
The study was approved by the Korean Cancer Study Group (KCSG) and the institutional ethics committees of each hospital and was carried out strictly to the principles of Good Clinical Practice and the Declaration of Helsinki.
Supplementary Figure_revised.eps
Download EPS Image (21 MB)Supplementary data_revised.docx
Download MS Word (33.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2371563
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–12. doi:10.3322/caac.21660. PMID: 33538338.
- Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi:10.1053/j.gastro.2017.08.023. PMID: 28823862.
- van Hagen P, Hulshof MC, van Lanschot Jj, Steyerberg EW, van Berge Henegouwen Mi, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088. PMID: 22646630.
- Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J. et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer. 2020;138:113–121. doi:10.1016/j.ejca.2020.08.002. PMID: 32877795.
- Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol. 2015;6(5):561–569. doi:10.3978/j.issn.2078-6891.2015.037. PMID: 26487950.
- Thuss-Patience P, Stein A. Immunotherapy in squamous cell cancer of the esophagus. Curr Oncol. 2022;29:2461–2471. doi:10.3390/curroncol29040200. PMID: 35448174.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704. doi:10.1146/annurev.immunol.26.021607.090331. PMID: 18173375.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239. PMID: 22437870.
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027. PMID: 11015443.
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi:10.1038/85330. PMID: 11224527.
- Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–1234. doi:10.1093/intimm/dxm091. PMID: 17898045.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643. PMID: 26412456.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi:10.1056/NEJMoa1503093. PMID: 25891173.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF. et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005. PMID: 29658856.
- Borghaei H, O’Byrne KJ, Paz-Ares L, Ciuleanu TE, Yu X, Pluzanski A, Nagrial A, Havel L, Kowalyszyn RD, Valette CA. et al. Nivolumab plus chemotherapy in first-line metastatic non-small-cell lung cancer: results of the phase III CheckMate 227 Part 2 trial. ESMO Open. 2023;8(6):102065. doi:10.1016/j.esmoop.2023.102065. PMID: 37988950.
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., Psyrri A, Basté N, Neupane P, Bratland Å. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi:10.1016/s0140-6736(19)32591-7. PMID: 31679945.
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/s0140-6736(21)01234-4. PMID: 34454674.
- Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A. et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi:10.1056/NEJMoa2111380. PMID: 35108470.
- Naing A, Gainor JF, Gelderblom H, Forde PM, Butler MO, Lin CC, Sharma S, Ochoa de Olza M, Varga A, Taylor M. et al. A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti–PD-1 antibody, in patients with advanced solid tumors. J Immunother Cancer. 2020;8(1):e000530. doi:10.1136/jitc-2020-000530. PMID: 32179633.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026. PMID: 19097774.
- Park KH, Choi JY, Lim AR, Kim JW, Choi YJ, Lee S, Sung JS, Chung HJ, Jang B, Yoon D. et al. Genomic landscape and clinical utility in Korean advanced pan-cancer patients from prospective clinical sequencing: K-MASTER program. Cancer Discov. 2022;12(4):938–948. doi:10.1158/2159-8290.Cd-21-1064. PMID: 34862196.
- Gavrielatou N, Fortis E, Spathis A, Anastasiou M, Economopoulou P, Foukas GRP, Lelegiannis IM, Rusakiewicz S, Vathiotis I, Aung TN. et al. B-cell infiltration is associated with survival outcomes following programmed cell death protein 1 inhibition in head and neck squamous cell carcinoma. Ann Oncol. 2024;35(4):340–350. doi:10.1016/j.annonc.2023.12.011. PMID: 38159908.
- Noble F, Mellows T, McCormick Matthews LH, Bateman AC, Harris S, Underwood TJ, Byrne JP, Bailey IS, Sharland DM, Kelly JJ. et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother. 2016;65(6):651–662. doi:10.1007/s00262-016-1826-5. PMID: 27020682.
- Chen K, Zhu Z, Zhang N, Cheng G, Zhang F, Jin J, Wu J, Ying L, Mao W, Su D. Tumor-Infiltrating CD4+ lymphocytes predict a favorable survival in patients with operable esophageal squamous cell carcinoma. Med Sci Monit. 2017;23:4619–4632. doi:10.12659/msm.904154. PMID: 28949934.
- Lee J, Kim B, Jung HA, La Choi Y, Sun JM. Nivolumab for esophageal squamous cell carcinoma and the predictive role of PD-L1 or CD8 expression in its therapeutic effect. Cancer Immunol Immunother. 2021;70(5):1203–1211. doi:10.1007/s00262-020-02766-7. PMID: 33123755.
- Guo JC, Hsu CL, Huang YL, Lin CC, Huang TC, Wu IC, Lin CY, Lien MY, Kuo HY, Cheng AL. et al. B cells in tumor microenvironment associated with the clinical benefit to programmed cell death protein-1 blockade therapy in patients with advanced esophageal squamous cell carcinoma. Front Oncol. 2022;12:879398. doi:10.3389/fonc.2022.879398. PMID: 35847892.
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi:10.1038/s41586-019-1922-8. PMID: 31942075.
- Zhang C, Hao X. Prognostic significance of CD276 in non-small cell lung cancer. Open Med (Wars). 2019;14(1):805–812. doi:10.1515/med-2019-0076. PMID: 31737785.
- Li F, Chen H, Wang D. Silencing of CD276 suppresses lung cancer progression by regulating integrin signaling. J Thorac Dis. 2020;12(5):2137–2145. doi:10.21037/jtd.2020.04.41. PMID: 32642118.
- Lee BR, Chae S, Moon J, Kim MJ, Lee H, Ko HW, Cho BC, Shim HS, Hwang D, Kim HR. et al. Combination of PD-L1 and PVR determines sensitivity to PD-1 blockade. JCI Insight. 2020;5(14). doi:10.1172/jci.insight.128633. PMID: 32554931.
- Liu WF, Quan B, Li M, Zhang F, Hu KS, Yin X. PVR—A prognostic biomarker correlated with immune cell infiltration in hepatocellular Carcinoma. Diagn (Basel). 2022;12(12):2953. doi:10.3390/diagnostics12122953. PMID: 36552960.
- Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD. et al. Tumor immune profiling predicts response to anti–PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):3447–3452. doi:10.1172/jci87324. PMID: 27525433.
- Yang L, Zhao Q, Wang X, Pilapong C, Li Y, Zou J, Jin J, Rong J. Investigation on the regulatory T cells signature and relevant Foxp3/STAT3 axis in esophageal cancer. Cancer Med. 2022;12(4):4993–5008. doi:10.1002/cam4.5194. PMID: 36226375.
- Jesinghaus M, Steiger K, Slotta-Huspenina J, Drecoll E, Pfarr N, Meyer P, Konukiewitz B, Bettstetter M, Wieczorek K, Ott K. et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget. 2017;8(29):46756–46768. doi:10.18632/oncotarget.18606. PMID: 28657901.
- Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: The challenges and opportunities for the next decade. Front Oncol. 2020;10:1727. doi:10.3389/fonc.2020.01727. PMID: 33014854.
- Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S. et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18(5):631–639. doi:10.1016/s1470-2045(17)30181-x. PMID: 28314688.
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT. et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–550. doi:10.1001/jamaoncol.2018.5441. PMID: 30570649.
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi:10.1016/s1470-2045(19)30626-6. PMID: 31582355.
- Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH. et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi:10.1200/jco.20.01888. PMID: 33026938.
- Kato K, Kojima T, Hochhauser D, Bennouna J, Hollebecque A, Lordick F, Kim S-B, Tajika M, Kim HT, Park H. et al. Pembrolizumab in previously treated metastatic esophageal cancer: Longer term follow-up from the phase 2 KEYNOTE-180 study. J Clin Oncol. 2019;37:4032–4032. doi:10.1200/JCO.2019.37.15_suppl.4032.
- Satoh T, Kato K, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Iwasa S, Muro K. et al. Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07). Esophagus. 2021;18(4):835–843. doi:10.1007/s10388-021-00850-0. PMID: 33993388.
- Gibson AJW, Li H, D’Silva A, Tudor RA, Elegbede AA, Otsuka S, Bebb DG, Cheung WY. Comparison of clinical characteristics and outcomes in relapsed versus de novo metastatic non–small cell lung cancer. Am J Clin Oncol. 2019;42(1):75–81. doi:10.1097/coc.0000000000000483. PMID: 30211724.
- Moore S, Leung B, Wu J, Ho C. Survival Implications of De novo versus recurrent metastatic non–small cell lung cancer. Am J Clin Oncol. 2019;42(3):292–297. doi:10.1097/coc.0000000000000513. PMID: 30608237.
- Miotke L, Nevala-Plagemann C, Ying J, Florou V, Haaland B, Garrido-Laguna I. Treatment outcomes in recurrent versus de novo metastatic pancreatic adenocarcinoma: a real world study. BMC Cancer. 2022;22(1):1054. doi:10.1186/s12885-022-10130-4. PMID: 36224524.
- Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA. et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol. 2020;38:2620–2627. doi:10.1200/jco.19.02727. PMID: 32364844.
- Even C, Wang HM, Li SH, Ngan RK, Dechaphunkul A, Zhang L, Yen CJ, Chan PC, Chakrabandhu S, Ma BBY. et al. Phase II, Randomized Study of Spartalizumab (PDR001), an Anti–PD-1 Antibody, versus Chemotherapy in Patients with Recurrent/Metastatic Nasopharyngeal Cancer. Clin Cancer Res. 2021;27(23):6413–6423. doi:10.1158/1078-0432.Ccr-21-0822. PMID: 34433653.
- Minami H, Doi T, Toyoda M, Imamura Y, Kiyota N, Mitsuma A, Shimokata T, Naito Y, Matsubara N, Tajima T. et al. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci. 2021;112(2):725–733. doi:10.1111/cas.14678. PMID: 33031626.
- Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, Halperin DM, Li D, Tafuto S, Raj N. et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. 2021;28(3):161–172. doi:10.1530/erc-20-0382. PMID: 33480358.
- Schöffski P, Tan DSW, Martín M, Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, Kyi C, Esaki T, Prawira A, Akerley W. et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer. 2022;10(2):e003776. doi:10.1136/jitc-2021-003776. PMID: 35217575.
- Lin C-C, Garralda E, Schöffski P, Hong DS, Siu LL, Martin M, Maur M, Hui R, Soo RA, Chiu J. A phase 2, multicenter, open-label study of anti-LAG-3 ieramilimab in combination with anti-PD-1 spartalizumab in patients with advanced solid malignancies. OncoImmunology. 2024;13(1):2290787. doi:10.1080/2162402X.2023.2290787.
- Zhou YX, Chen P, Sun YT, Zhang B, Qiu MZ. Comparison of PD-1 Inhibitors in patients with advanced esophageal squamous cell carcinoma in the second-line setting. Front Oncol. 2021;11:698732. doi:10.3389/fonc.2021.698732. PMID: 34621668.
- Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Baba H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111(9):3132–3141. doi:10.1111/cas.14541. PMID: 32579769.
- Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer. 2022;22(7):414–430. doi:10.1038/s41568-022-00466-1. PMID: 35393541.
- Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, Validire P, Ingels A, Cathelineau X, Fridman WH. et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120(1):45–53. doi:10.1038/s41416-018-0327-z. PMID: 30413828.
- Wang J, Gong R, Zhao C, Lei K, Sun X, Ren H. Human FOXP3 and tumour microenvironment. Immunology. 2023;168(2):248–255. doi:10.1111/imm.13520. PMID: 35689826.
- Hu Q, Hong Y, Qi P, Lu G, Mai X, Xu S, He X, Guo Y, Gao L, Jing Z. et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat Commun. 2021;12(1):2186. doi:10.1038/s41467-021-22300-2. PMID: 33846305.
- Liu S, Liang J, Liu Z, Zhang C, Wang Y, Watson AH, Zhou C, Zhang F, Wu K, Zhang F. et al. The Role of CD276 in Cancers. Front Oncol. 2021;11:654684. doi:10.3389/fonc.2021.654684. PMID: 33842369.
- Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, Ferrone S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin Cancer Res. 2021;27(5):1227–1235. doi:10.1158/1078-0432.Ccr-20-2584. PMID: 33051306.
- Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi:10.1016/j.lungcan.2006.05.012. PMID: 16782226.
- Katayama A, Takahara M, Kishibe K, Nagato T, Kunibe I, Katada A, Hayashi T, Harabuchi Y. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol. 2011;38(5):1219–1226. doi:10.3892/ijo.2011.949. PMID: 21344157.
- Feustel K, Martin J, Falchook GS. B7-H3 Inhibitors in Oncology Clinical Trials: A Review. J Immunother Precis Oncol. 2024;7(1):53–66. doi:10.36401/jipo-23-18. PMID: 38327753.
- Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, Lou Y, Hauke R, Vogelzang N, Pz D. et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022;10(4):e004424. doi:10.1136/jitc-2021-004424. PMID: 35414591.
- Modak S, Zanzonico P, Grkovski M, Slotkin EK, Carrasquillo JA, Lyashchenko SK, Lewis JS, Cheung IY, Heaton T, LaQuaglia MP. et al. B7H3-Directed Intraperitoneal Radioimmunotherapy with Radioiodinated Omburtamab for desmoplastic small round cell tumor and other peritoneal tumors: Results of a phase I study. J Clin Oncol. 2020;38:4283–4291. doi:10.1200/jco.20.01974. PMID: 33119478.
- Anagnostou V, Yarchoan M, Hansen AR, Wang H, Verde F, Sharon E, Collyar D, Chow LQM, Forde PM. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23(17):4959–4969. doi:10.1158/1078-0432.Ccr-16-3065. PMID: 28864724.
- Reiter JG, Makohon-Moore AP, Gerold JM, Heyde A, Attiyeh MA, Kohutek ZA, Tokheim CJ, Brown A, DeBlasio RM, Niyazov J. et al. Minimal functional driver gene heterogeneity among untreated metastases. Science. 2018;361(6406):1033–1037. doi:10.1126/science.aat7171. PMID: 30190408.
- Balmain A. The critical roles of somatic mutations and environmental tumor-promoting agents in cancer risk. Nat Genet. 2020;52(11):1139–1143. doi:10.1038/s41588-020-00727-5. PMID: 33106632.
- Twomey JD, Zhang B. Cancer Immunotherapy Update: FDA-Approved checkpoint inhibitors and companion diagnostics. Aaps J. 2021;23(2):39. doi:10.1208/s12248-021-00574-0. PMID: 33677681.
- Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi:10.1056/NEJMoa2032125. PMID: 33789008.
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V. et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/jci91190. PMID: 28650338.