ABSTRACT
The randomized METIMMOX trial (NCT03388190) examined if patients with previously untreated, unresectable abdominal metastases from microsatellite-stable (MSS) colorectal cancer (CRC) might benefit from potentially immunogenic, short-course oxaliplatin-based chemotherapy alternating with immune checkpoint blockade (ICB). Three of 38 patients assigned to this experimental treatment had metastases from BRAF-mutant MSS-CRC, in general a poor-prognostic subgroup explored here. The ≥70-year-old females presented with ascending colon adenocarcinomas with intermediate tumor mutational burden (6.2–11.8 mutations per megabase). All experienced early disappearance of the primary tumor followed by complete response of all overt metastatic disease, resulting in progression-free survival as long as 20–35 months. However, they encountered recurrence at previously unaffected sites and ultimately sanctuary organs, or as intrahepatic tumor evolution reflected in the terminal loss of initially induced T-cell clonality in liver metastases. Yet, the remarkable first-line responses to short-course oxaliplatin-based chemotherapy alternating with ICB may offer a novel therapeutic option to a particularly hard-to-treat MSS-CRC subgroup.
Introduction
Immune checkpoint blockade (ICB) is efficacious in patients with advanced colorectal cancer (CRC) that is microsatellite-instable/mismatch repair (MMR)-deficient.Citation1,Citation2 Also, a rare patient subgroup with pathogenic mutations in the exonuclease domain of polymerase ε (POLE) or δ1 (POLD1), associated with a hypermutated phenotype and mostly observed in microsatellite-stable (MSS)/MMR-proficient tumors,Citation3 shows ICB responsiveness.Citation4 The majority of patients, however, present MSS/MMR-proficient CRC with low response rates to ICB alone.Citation5 In patients with metastatic MSS-CRC given ICB, the presence of liver metastases was shown to be the most significant variable associated with rapid disease progression,Citation6 which may have reflected the de novo ICB resistance manifested by the targeted elimination of cytotoxic T cells in preclinical liver metastasis models and the generally low fraction of this specific immune-cell population in liver metastasis specimens from MSS-CRC patients.Citation7,Citation8
Moreover, among patients with MSS/MMR-proficient CRC, those with metastatic disease from BRAF-mutant tumors, comprising 14% of a population-based cohort,Citation9 have particularly poor prognosis with median overall survival of less than a year found in pooled data analysis.Citation10 While the median overall survival of first-line high-intensity chemotherapy containing both oxaliplatin and irinotecan was 13.6 months,Citation11 it was 18.3 months following a first-line three-drug combination of inhibitors directly targeting the intrinsically active tumor signaling pathways.Citation12 Median progression-free survival (PFS) was reported to 5 months or shorter for tumor signaling pathway inhibitors.Citation12,Citation13
We conducted the METIMMOX randomized phase 2 trial, in which the experimental-group patients received short-course oxaliplatin-based chemotherapy (the Nordic FLOX regimen) alternating with ICB (nivolumab) as a first-line treatment of unresectable infradiaphragmatic (liver, peritoneal, and/or nodal) metastases from MSS-CRC. The trial was negative with regard to the primary endpoint PFS, which for the intention-to-treat experimental-group patients (n = 38) was identical (9.2 months) with that for the control-group patients (n = 38) receiving FLOX alone.Citation14 Nevertheless, three experimental-group subjects had BRAF V600E disease, of whom all achieved complete response (CR) with PFS 20–35 months. Albeit a small number of cases, we present in-depth clinical and molecular characteristics that may elucidate the remarkable trajectories.
Materials and methods
Ethics approvals and consent to participate
Required approvals were given by the Regional Committee for Medical and Health Research Ethics of South-East Norway (2017/1850), the Norwegian Medicines Agency (17/12752), and the institutional review boards. The trial was conducted in accordance with Good Clinical Practice and the Helsinki Declaration. All patients provided written informed consent. The trial is registered with ClinicalTrials.gov, NCT03388190; by 2 January 2018.
Privacy protection
The data generated in this study are subject to patient confidentiality in accordance with the General Data Protection Regulation of the European Union. Hence, each subject that is individually presented by their detailed disease course is de-identified by partitioning the clinical characteristics into thematic sections with random order of patient presentation. This procedure is pursuant to the information in the informed consent form.
Study design, procedures, and endpoints
Details are provided in Supplementary Methods. In brief, MSS-CRC patients with unresectable infradiaphragmatic metastases were randomly assigned to first-line treatment with the FLOX regimen (oxaliplatin 85 mg/m2 day 1 and bolus 5-fluorouracil 500 mg/m2 and folinic acid 100 mg days 1–2) Q2W (control group) or alternating 2 cycles each of FLOX Q2W and nivolumab (240 mg flat dose) Q2W (experimental group), with prespecified break periods (Supplementary Figure S1). Radiologic response assessment was done every 8 weeks with PFS as the primary endpoint. The intention-to-treat population consisted of patients who started the first therapy cycle. Because the first two therapy cycles were identical in the control and experimental groups (halfway toward the first radiographic reassessment), the per-protocol population included all subjects who adhered to treatment until this reassessment to enable objective comparison of the regimens.
Molecular procedures
Two of the molecular procedures reported here, comprising tumor DNA/RNA sequencing to determine somatic mutations and the tumor mutational burden (TMB), and the assessment of the dynamic plasma BRAF V600E variant-allele frequency (VAF), are both detailed in Supplementary Methods.
T-cell receptor (TCR) sequencing
For one study subject, the hsTCRB v4 immunoSEQ library preparation kit (Adaptive Biotechnologies) was used to generate TCR repertoire libraries from liver metastases at baseline (a biopsy from one of multiple metastases), from the first liver resection specimen (three samples from the two resected metastases), and from the second liver resection specimen (two samples from one of multiple resected metastases). The protocol used multiplex PCR with primers for all TCR-β gene V and J fragments, corrected for variability in primer efficiency. The protocol also included synthetic TCR templates and reference gene primers to facilitate accurate quantification of T cells in each sample. Pooled libraries were sequenced on the NextSeq 500/550 High Output (Illumina). TCR clonality was assessed using Hill diversity and evenness profiles, as previously described,Citation15 by which the clonality index was defined as 10 minus the area under the curve of each evenness profile. The Hill equation integrates classic clonality variables – species richness (α = 0), Shannon entropy (α = 1; from which Pielou’s evenness is derived), and Simpson’s index (α = 2) – into a unified framework.Citation16 The tracking of clones across repertoires was done using the Adaptive Biotechnologies analyzer 3.0 software (Adaptive Biotechnologies). Only repertoires with sequencing coverage >5× were included in the analysis. T-cell fractions were determined by dividing the number of detected, productive, rearranged sequences (as a proxy for the number of T cells) with the total number of genomes in the sample which was calculated by total amount of DNA/6.6 pg (mean DNA content/cell). The full procedure was recently described in detail.Citation15
Results
The patients with BRAF mutation
Thirteen individuals with BRAF-mutant MSS-CRC were enrolled between 31 August 2018 and 5 July 2021. The CONSORT diagram (Supplementary Figure S2), alongside details in Supplementary Results, accounts for the subject allocations and the intention-to-treat and per-protocol populations. For the present analysis, data cutoff was 15 August 2023 when the last patient with BRAF-mutant disease who completed the allocated treatment (11 of 13 intention-to-treat subjects; Supplementary Table S1) reached a prespecified endpoint. The eight control-group subjects had a median PFS of 4.0 months (95% confidence interval, 1.9–6.1) with partial response (PR) as best objective response occurring for only two patients. In contrast, all of the three experimental-group patients achieved CR with a median PFS of 33.0 months (minimum, 20.7; maximum, 35.0).
illustrates the course of study participation for each of the nine per-protocol subjects. Molecular tumor characteristics for the patients with PFS longer than 20 months are shown in Supplementary Tables S2–3. The six per-protocol control-group subjects are described in Supplementary Results.
Figure 1. (a) Duration of study participation and efficacy assessment for the per-protocol BRAF-mutant population (experimental group (E), n = 3; control group (C), n = 6). Dynamics of plasma BRAF V600E DNA over the study treatment in (b) two experimental-group subjects and (c) three control-group subjects.
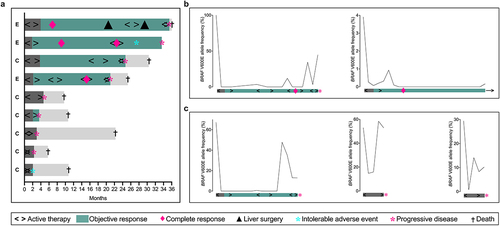
Circulating BRAF V600E dynamics
Five patients had plasma samples with measurable BRAF V600E VAF at baseline and could be followed for circulating tumor DNA dynamics. The experimental-group patient with shortest PFS (20.7 months) presented with 100% of the circulating BRAF as the V600E variant but experienced rapid and complete clearance of this allele. The same dynamics occurred again in the second and third treatment sequences, albeit from lower starting VAF measures. In the fourth sequence, the mutant-allele BRAF decreased after the initial FLOX cycles but then increased following the sequential nivolumab cycles, portending the ICB failure (, left panel). In contrast, the experimental-group subject with the longest PFS (35.0 months) had circulating BRAF V600E below the level of detection. The experimental-group patient with slightly shorter PFS (33.0 months) had low (3.9%) baseline BRAF V600E VAF, with durable clearance coinciding with the radiologic CR (, right panel). Interestingly, the single control-group long-term responder (male aged >70; PFS 24.4 months) also showed rapid and complete clearance (at radiographic PR) of the high (67.2%) baseline BRAF V600E VAF before a sharp increase when nearing treatment failure (, left panel). The other two control-group subjects with measurable plasma VAF for baseline BRAF V600E (none with objective response radiographically) had brief and transient responses (, middle and right panels).
The three experimental-group patients
The patients were ≥70-year-old females presenting with ascending colon adenocarcinomas (Supplementary Figures S3–4) with TMB 6.2–11.8 mutations per megabase and no POLD1/POLE mutations (Supplementary Tables S2–3). One patient had organ-infiltrating primary tumor (T4b), while the others had primaries confined to the bowel wall (T2).
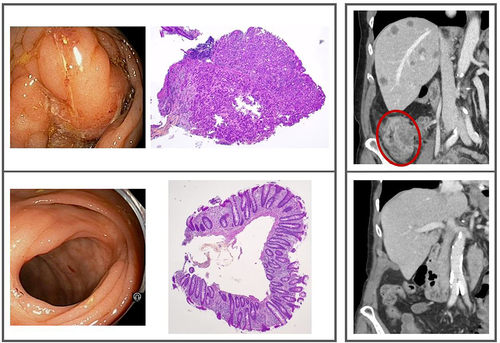
All patients experienced CR of the primary tumor in the first treatment break. The T4b subject had the CR status confirmed by endoscopy showing slight mucosal edema and scarring, from where biopsies proved restored mucosa with crypt branching and slight inflammation without residual tumor (, left panel). Despite the early CR, one T2 subject proceeded to subsequent right hemicolectomy. The surgical specimen had sporadic residual tumor glands embedded in fibrous scar tissue in the submucosa and bowel wall in addition to a restored mucosa (Supplementary Figure S4). The primary tumor CR status was durable for all subjects.
Figure 2. Left framed panel: The primary tumor site; baseline endoscopy and biopsy (top), endoscopy and biopsy at radiologic complete response (bottom). Right framed panel: The metastatic disease; the primary tumor (encircled) and multiple liver metastases at baseline (top), the ascending colon and liver at radiologic complete response (bottom). Further details are displayed in Supplementary Figures S3 and S5.
One patient (, second from the top; baseline plasma BRAF V600E VAF 3.9%), presenting with multiple peritoneal and para-aortal lymph node metastases, responded with CR in the first treatment break, which lasted almost a year before a new treatment sequence caused an immediate second CR. However, intolerable immune-mediated hepatotoxicity developed in the second break, making the patient ineligible for further study therapy when several liver metastases subsequently appeared (without radiologic evidence of the previous lymph node or peritoneal metastases), resulting in PFS 33.0 months. The patient proceeded to the FLOX regimen alone.
One patient (, fourth from the top; baseline plasma BRAF V600E VAF 100%) presented with metastases in most liver segments and several lymph node stations below and above the diaphragm (, right panel). She experienced early >90% shrinkage of the liver target lesions, followed by increasing lymph nodes and a second treatment sequence. In the second break, the liver target lesions were 97% reduced from baseline but lymph nodes at a previously unaffected site increased. Halfway into the third treatment sequence, CR occurred at all of the involved sites. A fourth treatment sequence, starting when previously unaffected lymph nodes increased, was immediately followed by adrenal gland and skeletal metastases (Supplementary Figure S5), resulting in PFS 20.7 months. The patient proceeded to second-line encorafenib and cetuximabCitation13 with rapid clinical deterioration.
One patient (, on the top; baseline plasma BRAF V600E VAF below the level of detection) presented with metastases affecting most liver segments (Supplementary Figure S6). She experienced early CR, lasting a year until a few liver metastases relapsed. These were resected, which were also subsequently relapsing multiple liver metastases that appeared as end-stage cancer. The patient reached PFS 35.0 months, of which active therapy had been given only 10 months. TCR sequencing of liver metastases, detailed below, illustrated the metastatic evolution.
Changes in T-cell clonal diversity and evenness
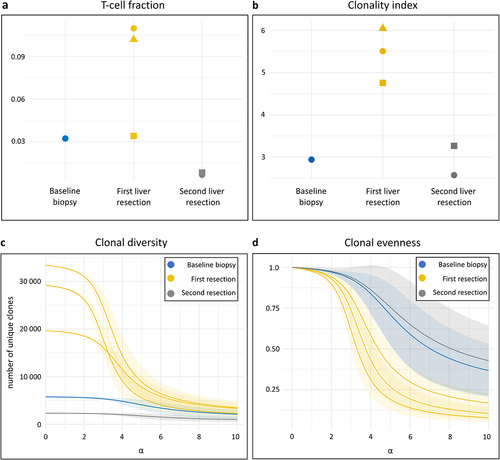
TCR sequencing was done on a baseline liver metastasis biopsy, three metastasis samples from the first resection, and two samples from the resected end-stage metastases. The mean T-cell fraction of 3.2% at baseline had increased to 8.2% at the first resection but fallen to 0.07% at the second (). The corresponding mean clonality indices (higher values implying more monoclonal repertoires) were 2.9, 5.5, and 2.9 (). In addition to the increased T-cell fraction, the primarily relapsed liver metastases exhibited a repertoire with notable skew toward highly abundant clones, implying an influx of clonally expanded tumor-specific T cells. Specifically, the Hill diversity profiles () revealed an absolute clonal diversity, averaging 27,423 clones, peaking in the primarily relapsed metastases, compared to 5,768 clones at baseline and 2,340 clones at end-stage (α = 0). When weighing clonal diversity by more abundant clones, however, the disparity over the disease course diminished (α = 10) with 3,029 clones at the first relapse, compared to 2,118 clones at baseline and 1,001 clones at end-stage. As illustrated by the Hill evenness profiles (), which are diversity profiles normalized against the number of unique clones, the clonal frequency distribution was more uneven (the most rapid decline at increasing α values) for the primarily relapsed liver metastases than at baseline and end-stage. This indicates that the intratumoral T cells in liver metastases responded with a transient increase of more monoclonal repertoires to the experimental METIMMOX treatment.
Figure 3. T-cell receptor (TCR) repertoire in sequential liver metastases. TCR sequencing was done on a baseline biopsy sample from one of multiple liver metastases, three samples from the two resected liver metastases that relapsed after the radiologic complete response, and two samples from the resected end-stage liver metastases. (a) T-cell fractions in the repertoire of each sample, stratified by sampling time. The mean number of TCR sequences detected was 22,185 at baseline, 82,263 at the first liver resection, and 7,252 at the second. (b) Repertoire clonality of each sample, stratified by sampling time. The clonality index is defined as 10 minus the area under the curve of the evenness profile, with higher values signifying more monoclonal repertoires. (c) Hill diversity profile and (d) Hill evenness profile for each repertoire, presented by mean (solid line) with 95% confidence interval (shaded area). The left part of the diversity curve (α = 0) represents the number of unique clones present in the repertoire. The further and more rapidly each curve declines at increasing α values, the more uneven is the clonal frequency distribution (more monoclonal repertoires). The evenness profiles represent the diversity profiles normalized by clonal richness in the repertoire.
Discussion
For BRAF-mutant metastatic CRC, several ongoing clinical trials that also include MSS/MMR-proficient disease evaluate combinations of RAF inhibitors with other molecularly targeted agents, some with the addition of oxaliplatin- or irinotecan-based chemotherapy or ICB.Citation17 Of note for the first-line METIMMOX trial, eligible patients had unresectable abdominal metastases from MSS-CRC, commonly considered unresponsive to ICB.Citation18 Moreover, the experimental-group patients received short-course oxaliplatin-based chemotherapy intended to invoke responsiveness to the sequential short-course ICB within a total sequence of only 4 months before treatment break, notably different from regimens given in other studies for this patient population. To our knowledge, the CR and PFS outcomes are unprecedented for BRAF V600E MSS-CRC. We have identified only one other reported case of this specific CRC entity responding with CR (to tumor signaling pathway inhibitors used at end-stage disease).Citation19
All three patients had right-sided primary tumors that disappeared and never reappeared. The tumors, devoid of POLD1/POLE mutations, were characterized by intermediate TMB, as such considered unresponsive to single-agent ICB.Citation20 One patient consistently experienced new metastatic progression at previously unaffected sites, also following CR. For her and one of the others, the disease when refractory to the METIMMOX regimen involved sanctuary organs solely. The third patient never experienced extrahepatic metastases. Instead, an intrahepatic tumor evolution was likely reflected in the subsequent loss by the end-stage metastases of the clonal T-cell expansion in the first metastases relapsing after long-lasting CR. We used the Hill equationCitation16 to create diversity and evenness profiles for the TCR data. Using single-point values like Shannon entropy and Pielou’s evenness for clonality would be challenging as they weight rare and common T-cell clones differently. Τhe choice of indices might therefore have led to qualitatively different results from the same clonal frequency data.Citation21
It was recently shown that the abundance and spatial distribution of different immune-cell subsets within MSS-CRC metastases exposed to neoadjuvant chemotherapy (treatment duration not reported) varied in an organ-specific manner; in liver metastases, activated T cells were enriched in the outer invasive margin, but not in the tumor core as seen in lung metastases and primary tumors.Citation22 Yet, we previously showed that neoadjuvant chemotherapy caused a transient increase in intratumoral T-cell density of MSS-CRC liver metastases,Citation23 a response retrieved in the case reported here by the expanded clonal TCR repertoires of the primarily relapsed liver metastases, lost shortly thereafter by the end-stage metastases.
We analyzed the dynamics of circulating BRAF V600E in an attempt to monitor the metastatic evolution, including emerging therapy resistance, in a noninvasive manner.Citation24,Citation25 Our observations echoed the recent findings for common CRC mutations, that both low baseline VAF and a rapid clearance from plasma are associated with improved clinical outcome in patients given standard chemotherapy regimens.Citation24 For patients treated with tumor signaling pathway inhibitors, a baseline plasma BRAF V600E VAF exceeding only 2% was a surrogate of high tumor burden, liver involvement, and consequently poor outcome.Citation26 With this in mind, it is noteworthy that the METIMMOX regimen redirected the disease course even for the patient with baseline plasma BRAF V600E 100% VAF and liver metastases at the initial presentation.
The consensus molecular subtype classification of primary CRC tumors provided a gene expression-based framework for the role of the immune system by defining tumors enriched in immune response features as subtype-1.Citation27 Approximately half of BRAF-mutant MSS-CRC cases categorize to this particular subtype.Citation28,Citation29 In situ evaluation of BRAF-mutant primary tumors has shown high infiltration of cytotoxic T cells, even for MSS-CRC.Citation30 Of note, an impressive 45% response rate was observed in patients with treatment-refractory metastatic BRAF V600E MSS-CRC given nivolumab in combination with the signaling pathway inhibitors encorafenib and cetuximab.Citation31 The METIMMOX schedule with de-intensified chemotherapy might have augmented subtype-1 biological features also in abdominal metastases. However, the serial relapses suggest a metastatic evolution process that might have been averted, for example, by maintenance ICB during treatment breaks.
In summary, previously untreated patients with unresectable abdominal metastases from right-sided BRAF-mutant MSS-CRC, in general a poor-prognostic subgroup, achieved CR (including the primary tumor) and remarkably extended PFS to alternating short-course oxaliplatin-based chemotherapy and ICB. If confirmed in a larger patient cohort, the METIMMOX schedule may offer a novel treatment option for a poor-prognostic MSS-CRC subgroup.
Abbreviations
| CR | = | complete response |
| CRC | = | colorectal cancer |
| ICB | = | immune checkpoint blockade |
| MMR | = | mismatch repair |
| MSS | = | microsatellite-stable |
| PFS | = | progression-free survival |
| PR | = | partial response |
| TCR | = | T-cell receptor |
| TMB | = | tumor mutational burden |
| VAF | = | variant-allele frequency |
Suppl Figure S5.docx
Download MS Word (599.9 KB)Suppl Tables.docx
Download MS Word (21.3 KB)Suppl Results.docx
Download MS Word (20.8 KB)Suppl Methods.docx
Download MS Word (21.3 KB)Suppl Figure S3.docx
Download MS Word (2.4 MB)Suppl Figure S4.docx
Download MS Word (1.8 MB)Suppl Figure S6.docx
Download MS Word (582.1 KB)Suppl Figures S1 S2.docx
Download MS Word (110.3 KB)Acknowledgments
The authors are indebted to the Akershus University Hospital National Unit for Precision Medicine for the sequencing of tumor DNA/RNA.
Disclosure statement
AHR has received research support from Bristol-Myers Squibb (on behalf of Akershus University Hospital) and served on advisory board of Takeda. HMH has received personal honoraria from Bayer and Roche and served on advisory boards of AstraZeneca, Eisai, and InCyte. CK has served on advisory boards of AstraZeneca and Roche. HS has received personal honoraria from Ipsen, Pierre Fabre, Daiichi Sankyo, and SAM Nordic and served on advisory board of AAA Pharma. SM has served on advisory board of GSK. The other authors report there are no competing interests to declare.
Data availability statement
Requests for data can be made to the corresponding author. The data generated in this study are subject to patient confidentiality in accordance with the General Data Protection Regulation of the European Union, and the transfer of data or materials will require approval from the Data Privacy Officer at Akershus University Hospital and on some occasions from the Regional Committee for Medical and Health Research Ethics of South-East Norway. Any shared data will be de-identified.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2372886
Additional information
Funding
References
- André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–7. doi:10.1056/NEJMoa2017699.
- Lenz HJ, Van Cutsem E, Limon ML, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi:10.1200/JCO.21.01015.
- Bourdais R, Rousseau B, Pujals A, Boussion H, Joly C, Guillemin A, Baumgaertner I, Nuezillet C, Tournigand C. Polymerase proofreading domain mutations: new opportunities for immunotherapy in hypermutated colorectal cancer beyond MMR deficiency. Crit Rev Oncol Hematol. 2017;113:242–248. doi:10.1016/j.critrevonc.2017.03.027.
- Rousseau B, Foote MB, Maron SB, Diplas BH, Lu S, Argilés G, Cercek A, Diaz LA Jr. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med. 2021;384(12):1168–1170. doi:10.1056/NEJMc2031965.
- Guven DC, Kavgaci G, Erul E, Syed MP, Magge T, Saeed A, Yalcin S, Sahin IH. The efficacy of immune checkpoint inhibitors in microsatellite stable colorectal cancer: a systematic review. The Oncologist. 2024;29(5):e580–e600. doi:10.1093/oncolo/oyae013.
- Wang C, Sandhu J, Ouyang C, Ye J, Lee PP, Fakih M. Clinical response to immunotherapy targeting programmed cell death receptor 1/Programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. Vol. 4. JAMA Netw Open; 2021. p. e2118416. doi:10.1001/jamanetworkopen.2021.18416.
- Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–164. doi:10.1038/s41591-020-1131-x.
- Ho WW, Gomes-Santos IL, Aoki S, Datta M, Kawaguchi K, Talele NP, Roberge S, Ren J, Liu H, Chen IX, et al. Dendritic cell paucity in mismatch repair–proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy. Proc Natl Acad Sci USA. 2021;118(45):e2105323118. doi:10.1073/pnas.2105323118.
- Aasebø KØ, Dragomir A, Sundström M, Mezheyeuski A, Edqvist PH, Eide GE, Ponten F, Pfeiffer P, Glimelius B, Sorbye H. Consequences of a high incidence of microsatellite instability and BRAF-mutated tumors: a population-based cohort of metastatic colorectal cancer patients. Cancer Med. 2019;8(7):3623–3635. doi:10.1002/cam4.2205.
- Cohen R, Liu H, Fiskum J, Adams R, Chibaudel B, Maughan TS, Van Cutsem E, Venook A, Douillard JY, Heinemann V, et al. BRAFV600E mutation in first-line metastatic colorectal cancer: an analysis of individual patient data from the ARCAD database. J Natl Cancer Inst. 2021;113(10):1386–1395. doi:10.1093/jnci/djab042.
- Cremolini C, Antoniotti C, Stein A, Bendell J, Gruenberger T, Rossini D, Masi G, Ongaro E, Hurwitz H, Falcone A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020;38:3314–3324.
- Van Cutsem E, Taieb J, Yaeger R, Yoshino T, Grothey A, Maiello E, Elez E, Dekervel J, Ross P, Ruiz-Casado A, et al. ANCHOR CRC: results from a single-arm, phase II study of exchorafenib plus binimetinib and cetuximab in previously untreated BRAFV600E-mutant metastatic colorectal cancer. J Clin Oncol. 2023;41:2628–2637.
- Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi:10.1056/NEJMoa1908075.
- Ree AH, Šaltytė Benth J, Hamre HM, Kersten C, Hofsli E, Guren MG, Sorbye H, Johansen C, Negård A, Bjørnetrø T, et al. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer—the randomised METIMMOX trial. Br J Cancer. [2024 Apr 25]. 130(12):1921–1928. doi:10.1038/s41416-024-02696-6.
- Høye E, Dagenborg VJ, Torgunrud A, Lund-Andersen C, Fretland ÅA, Lorenz S, Edwin B, Hovig E, Fromm B, Inderberg EM, et al. T cell receptor repertoire sequencing reveals chemotherapy-driven clonal expansion in colorectal liver metastases. Gigascience. 2022;12:giad032. doi:10.1093/gigascience/giad032.
- Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427–432. doi:10.2307/1934352.
- Ros J, Rodríguez-Castells M, Saoudi N, Baraibar I, Salva F, Tabernero J, Élez E. Treatment of BRAF-V600E mutant metastatic colorectal cancer: new insights and biomarkers. Expert Rev Anticancer Ther. 2023;23(8):797–806. doi:10.1080/14737140.2023.2236794.
- Fakih M, Wang C, Sandhu J, Ye J, Egelston C, Li X. Immunotherapy response in microsatellite stable metastatic colorectal cancer is influenced by site of metastases. Eur J Cancer. 2024;196:113437. doi:10.1016/j.ejca.2023.113437.
- Piringer G, Decker J, Trommet V, Kühr T, Heibl S, Dörfler K, Thaler J. Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line treatment in a patient with advanced BRAFV600E mutated, microsatellite-stable colon cancer: a case report and literature review. Front Oncol. 2023;13:1166545. doi:10.3389/fonc.2023.1166545.
- Vegivinti CTR, Gomez CG, Syed M, Ferrell M, Cheng S, Singhi A, Saeed A, Sahin IH. The role of immune checkpoint inhibitors for patients with advanced stage microsatellite stable colorectal cancer and high tumor mutation burden: quantity or quality? Expert Opin Biol Ther. 2023;23(7):595–601. doi:10.1080/14712598.2023.2226327.
- Greiff V, Bhat P, Cook SC, Menzel U, Kang W, Reddy ST. A bioinformatic framework for immune repertoire diversity profiling enables detection of immunological status. Genome Med. 2015;7(1):49. doi:10.1186/s13073-015-0169-8.
- Ye J, Guo W, Wang C, Egelston CA, D’Apuzzo M, Shankar G, Fakih MG, Lee PP. Peritumoral immune-suppressive mechanisms impede intratumoral lymphocyte infiltration into colorectal cancer liver versus lung metastases. Cancer Res Commun. 2023;3(10):2082–2095. doi:10.1158/2767-9764.CRC-23-0212.
- Dagenborg VJ, Marshall SE, Yaqub S, Grzyb K, Boye K, Lund-Iversen M, Høye E, Berstad AE, Fretland ÅA, Edwin B, et al. Neoadjuvant chemotherapy is associated with a transient increase of intratumoral T-cell density in microsatellite stable colorectal liver metastases. Cancer Biol Ther. 2020;21(5):432–440. doi:10.1080/15384047.2020.1721252.
- Urbini M, Marisi G, Azzali I, Bartolini G, Chiadini E, Capelli L, Tedaldi G, Angeli D, Canale M, Molinari C, et al. Dynamic monitoring of circulating tumor DNA in patients with metastatic colorectal cancer. JCO Precis Oncol. 2023;7(7):e2200694. doi:10.1200/PO.22.00694.
- Roazzi L, Patelli G, Bencardino KB, Amatu A, Bonazzina E, Tosi F, Amoruso B, Bombelli A, Mariano S, Stabile S, et al. Ongoing clinical trials and future research scenarios of circulating tumor DNA for the treatment of metastatic colorectal cancer. Clin Colorectal Cancer. 2024;Feb16:S1533–0028(24)00006–9. doi:10.1016/j.clcc.2024.02.001.
- Ros J, Matito J, Villacampa G, Comas R, Garcia A, Martini G, Baraibar I, Saoudi N, Salvà F, Martin Á, et al. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann Oncol. 2023;34(6):543–552. doi:10.1016/j.annonc.2023.02.016.
- Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi:10.1038/nm.3967.
- Kopetz S, Murphy DA, Pu J, Ciardiello F, Desai J, Grothey A, Van Cutsen E, Wasan HS, Yaeger R, Yoshino T, et al. Molecular correlates of clinical benefit in previously treated patients (pts) with BRAF V600E-mutant metastatic colorectal cancer (mCRC) from the BEACON study. J Clin Oncol. 2021;39(15_suppl):3513. doi:10.1200/JCO.2021.39.15_suppl.3513.
- van der Pol Y, Yilma B, Morris VK, Chao C, Harris M, Guinney J, Kopetz S. Molecular characterization of microsatellite stable (MSS) colorectal cancer (CRC) patients with a BRAF V600E mutation. Cancer Res. 2024;84(6_Supplement):5056. doi:10.1158/1538-7445.AM2024-5056.
- Edin S, Gylling B, Li X, Stenberg Å, Löfgren-Burström A, Zingmark C, van Guelpen B, Ljuslinder I, Ling A, Palmqvist R. Opposing roles by KRAS and BRAF mutation on immune cell infiltration in colorectal cancer – possible implications for immunotherapy. Br J Cancer. 2024;130(1):143–150. doi:10.1038/s41416-023-02483-9.
- Morris VK, Parseghian CM, Escano M, Johnson B, Raghav KPS, Dasari A, Huey R, Overman MJ, Willis J, Lee MS. et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J Clin Oncol. 2022;40(suppl):12. doi:10.1200/JCO.2022.40.4_suppl.012.
