ABSTRACT
Doxorubicin is a prototypical inducer of immunogenic cell death (ICD) that sensitizes to subsequent immunotherapy by PD-1 blockade. However, this systemic drug combination fails against glioblastoma, hidden behind the blood–brain barrier (BBB). A recent work delineates a biophysical method for BBB permeabilization that yields effective preclinical effects of chemoimmunotherapy.
Immunotherapy has achieved remarkable success across multiple cancers.Citation1 However, resistance remains frequent. Combining immunotherapy with agents that promote antitumor immune cell infiltration is a strategy to overcome resistance. Chemotherapeutic drugs inducing immunogenic cell death (ICD), notably anthracyclines like doxorubicin (DOX), are among these agents.Citation2 In preclinical studies, ICD-inducing interventions enhanced sensitivity to immune checkpoint inhibitors (ICIs), such as programmed cell death 1 (PDCD1, best known as PD-1)-blocking antibodies (αPD1).Citation3
Glioblastoma (GBM) is the most aggressive brain cancer. Immunotherapy for GBM is challenging due to the blood–brain barrier (BBB) and the immune-privileged status of the brain.Citation4,Citation5 These factors hinder drug delivery and immune cell trafficking, reducing treatment efficacy. Previous attempts to treat GBM with αPD1, either alone or with standard chemotherapy, or with liposome-embedded DOX plus an anti-angiogenic drug, failed in clinical trials.Citation6–8
A recent study by Arrieta et al. published in Nature Communications, exploited a novel strategy to transiently open the BBB utilizing low-intensity pulsed ultrasound (LIPU) combined with intravenously-administered microbubbles (MB). This technique aimed to enhance the delivery of therapeutic agents, specifically liposomal DOX, and αPD1, into the brain ().Citation9
Figure 1. Ultrasounds combined with administration of microbubbles facilitate the delivery of doxorubicin/anti-PD-1 to glioblastoma and improve therapeutic efficacy. LIPU/MB transiently opens the blood-brain barrier, facilitating the access of liposomal doxorubicin and anti-PD-1 to glioblastoma. Locally, concentration of the dual therapeutic agents stimulates IFNG production by cerebral myeloid cells and upregulation of MHC molecules by surrounding cells like malignant cells. This pro-inflammatory environment enhances the recognition of cancer cells by T lymphocytes. These latter show polyfunctionality, secreting both IFNG and TNF, improved antitumor activity, and persist in treated mice surviving the disease. IFNG, interferon-gamma; LIPU, low-intensity pulsed ultrasound; MB, microbubble; MHC, major histocompatibility complex; PD-1, programmed cell death 1; TNF, tumor-necrosis factor-alpha.
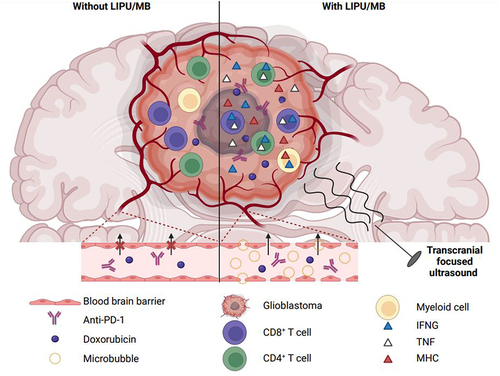
Four GBM patients received DOX/αPD1 treatment before surgery. DOX concentrations appeared twice higher in sonicated regions of resected tissues than non-sonicated areas. Consistently, a 4-fold increase in DOX concentrations was detected in brain tissues of naïve mice following LIPU/MB compared to controls. This demonstrated LIPU/MB’s ability to enhance drug penetration into the brain.Citation9
In clinical samples, enhanced DOX delivery led to higher expression of class-I and II antigen-presenting major histocompatibility complex (MHC) molecules in tumor cells. In contrast, temozolomide, a standard GBM treatment, did not stimulate MHC expression. The DOX treatment also facilitated recognition of murine glioma cells by CD8+ T cells, stimulating their activation and expansion, indicating the immunogenicity of DOX-accumulated GBM tissues.Citation9
In an intracranial murine GBM model, microglia and monocyte-derived macrophages produced more interferon-gamma (IFNG) following high-dose DOX treatment. This immunomodulatory effect included upregulation of surface MHC-I and CD274 (best known as programmed cell death 1 ligand 1, PD-L1), both IFNG-inducible genes. In GBM patients, microglial cells positive for IFNG and MHC-I were more abundant in post-treatment samples compared to pre-treatment tissues. Thus, LIPU/MB-mediated DOX delivery modulated the phenotype of myeloid cells constitutive of GBM microenvironment.Citation9
While LIPU/MB facilitated αPD1 brain penetration in mice, it was ineffective alone to treat GBM. In patient tissues, αPD1 was more concentrated in sonicated peritumoral areas. In the CT2A mouse model of intracranial GBM, combining αPD1 with liposomal DOX was more effective than either therapy alone, achieving 40% long-term survival. LIPU/MB further improved survival, reaching an 80% cure rate.Citation9
Mice cured of GBM were protected against tumor rechallenge, indicating immune memory establishment. Depleting microglia and bone marrow-derived macrophages impeded the antitumor activity of LIPU/MB-delivered liposomal DOX plus αPD1 and abrogated protection against tumor recurrence, supporting previously reported memory by cerebral myeloid cells.Citation9,Citation10
Liposomal DOX promoted accumulation of T cells co-producing IFNG and tumor-necrosis factor-alpha (TNF) in the brain of mice surviving orthotopic GBM. Sonication expanded polyfunctional CD4+ T cells without affecting CD8+ subsets. Depleting CD8+ T cells abrogated the therapeutic effect, highlighting their critical contribution to antitumor immunity induced by LIPU/MB-mediated liposomal DOX/αPD1 delivery. Correspondingly, GBM patients treated with this strategy exhibited higher IFNG production by tumor-infiltrating T lymphocytes than subjects without neoadjuvant treatment.
These findings by Arrieta and colleagues suggest that LIPU/MB-mediated drug delivery systems could significantly improve GBM treatment outcomes and potentially extend to other intracranial cancers limited by the BBB and local immunosuppression. Continued research and clinical trials will be essential to optimize this procedure for becoming the standard-of-care in managing GBM and other challenging cancers.
Acknowledgments
J.G.P. is supported by the SIRIC Cancer Research and Personalized Medicine (CARPEM); Multi-Organism Institute (ITMO) Aviesan Cancer (National Alliance for Life Sciences and Health), Institut National du Cancer (INCa), and Fondation pour la Recherche Médicale (FRM). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR-22-CE14-0066 VIVORUSH, ANR-23-CE44-0030 COPPERMAC, ANR-23-R4HC-0006 Ener-LIGHT); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Joint Programme on Rare Diseases (EJPRD) Wilsonmed; European Research Council Advanced Investigator Award (ERC-2021-ADG, Grant No. 101052444; project acronym: ICD-Cancer, project title: Immunogenic cell death (ICD) in the cancer-immune dialogue); The ERA4 Health Cardinoff Grant Ener-LIGHT; European Union Horizon 2020 research and innovation programs Oncobiome (grant agreement number: 825410, Project Acronym: ONCOBIOME, Project title: Gut OncoMicrobiome Signatures [GOMS] associated with cancer incidence, prognosis and prediction of treatment response, Prevalung (grant agreement number 101095604, Project Acronym: PREVALUNG EU, project title: Biomarkers affecting the transition from cardiovascular disease to lung cancer: toward stratified interception), Neutrocure (grant agreement number 861878: Project Acronym: Neutrocure; project title: Development of “smart” amplifiers of reactive oxygen species specific to aberrant polymorphonuclear neutrophils for treatment of inflammatory and autoimmune diseases, cancer, and myeloablation); National support managed by the Agence Nationale de la Recherche under the France 2030 programme (reference number 21-ESRE-0028, ESR/Equipex+ Onco-Pheno-Screen); Hevolution Network on Senescence in Aging; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology ANR-18-IDEX-0001; a Cancer Research ASPIRE Award from the Mark Foundation; PAIR-Obésité INCa_1873, the RHUs Immunolife and LUCA-pi (ANR-21-RHUS-0017 and ANR-23-RHUS-0010, both dedicated to France Relance 2030); Seerave Foundation; SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris Cité ANR-18-IDEX-0001. The views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union, the European Research Council or any other granting authority. Neither the European Union nor any other granting authority can be held responsible for them.
Disclosure statement
J.G.P. is the inventor of patents covering the diagnosis, prognosis, and treatment of cancers, including patents licensed to Turnstone Biologics and Therafast Bio. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds, and Rejuveron Life Sciences. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. GK’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk, and Roche, was on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Funding
References
- Kroemer G, Chan TA, Eggermont AMM, Galluzzi L. Immunosurveillance in clinical cancer management. CA Cancer J Clin. 2024;74(2):187–3. doi:10.3322/caac.21818.
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi:10.1084/jem.20050915.
- Galluzzi L, Guilbaud E, Schmidt D, Kroemer G, Marincola FM. Targeting immunogenic cell stress and death for cancer therapy. Nat Rev Drug Discov. 2024;23(6):445–460. doi:10.1038/s41573-024-00920-9.
- Agosti E, Zeppieri M, De Maria L, Tedeschi C, Fontanella MM, Panciani PP, Ius T. Glioblastoma immunotherapy: a systematic review of the present strategies and prospects for advancements. Int J Mol Sci. 2023;24(20):24. doi:10.3390/ijms242015037.
- Sferruzza G, Consoli S, Dono F, Evangelista G, Giugno A, Pronello E, Rollo E, Romozzi M, Rossi L, Pensato U, et al. A systematic review of immunotherapy in high-grade glioma: learning from the past to shape future perspectives. Neurol Sci. 2024;45(6):2561–2578. doi:10.1007/s10072-024-07350-w.
- Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O, et al. Effect of Nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi:10.1001/jamaoncol.2020.1024.
- Lim M, Weller M, Idbaih A, Steinbach J, Finocchiaro G, Raval RR, Ansstas G, Baehring J, Taylor JW, Honnorat J, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. doi:10.1093/neuonc/noac116.
- Kasenda B, Konig D, Manni M, Ritschard R, Duthaler U, Bartoszek E, Bärenwaldt A, Deuster S, Hutter G, Cordier D, et al. Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO Open. 2022;7(1):100365. doi:10.1016/j.esmoop.2021.100365.
- Arrieta VA, Gould A, Kim KS, Habashy KJ, Dmello C, Vazquez-Cervantes GI, Palacín-Aliana I, McManus G, Amidei C, Gomez C, et al. Ultrasound-mediated delivery of doxorubicin to the brain results in immune modulation and improved responses to PD-1 blockade in gliomas. Nat Commun. 2024;15(1):4698. doi:10.1038/s41467-024-48326-w.
- Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LM, Wild K, Skodras A, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556(7701):332–338. doi:10.1038/s41586-018-0023-4.
