ABSTRACT
Transforming growth factor (TGF)-β1 is involved in the processes of airway inflammation and remodeling; however, its reported roles in asthma pathogenesis are controversial. We sought both to investigate the effects of active immunization targeting TGF-β1 on allergen-induced airway inflammatory responses and to evaluate its possible application for asthma treatment. BALB/c mice were immunized with a virus-like-particle (VLP) vaccine presenting a TGF-β1 peptide. For the preventive intervention of acute allergic airway inflammation, immunization was conducted before sensitization and challenges with ovalbumin (OVA), and for the therapeutic treatment of chronic inflammatory responses, immunization was initiated after inflammatory responses were established. Preventive immunization with VLPs led to increased proinflammatory IL-4, IL-13, and IL-33 levels in the bronchoalveolar lavage fluids (BALF) with no significant effects on lung tissue inflammation and airway goblet cell hyperplasia. Therapeutic treatment showed that at 24 h after the fourth 2-day challenge with OVA following 2 intraperitoneal sensitizations, airway subepithelial collagen deposition was significantly ameliorated in vaccinated mice, whereas the lung histology and cytokine profile in the BALF were not changed. In contrast, after a 4-week recovery from the last OVA challenge, the vaccinated mice's collagen deposition remained reduced, but they sustained lung-tissue inflammation and goblet-cell hyperplasia; elevated IL-13, TNF, and IFN-γ levels in the BALF; and increased airway resistance, tissue resistance, and tissue elastance. In a conclusion, the role of TGF-β1 is complicated in allergic airway inflammatory responses. It is important to make a careful assessment in accordance with specific disease conditions when targeting TGF-β1 for a therapeutic purpose.
Introduction
Transforming growth factor (TGF)-β1 is highly pleiotropic and multifunctional. Numerous studies have shown that TGF-β1 functions as an anti-inflammatory and immunosuppressive cytokine: it not only suppresses differentiation, proliferation, and the production and activity of cytokines but also promotes the apoptosis of various inflammatory cells.Citation1-5 In addition, it both promotes the generation of regulatory T cells (Tregs) and regulates their suppressive function.Citation6,7 Conversely, TGF-β1 also has pro-inflammatory effectsCitation1: it exerts potent chemoattractive activity and therefore contributes to the rapid accumulation of inflammatory cells at the inflammation site; it induces the differentiation of Th17 cells, which amplify inflammation both by secreting inflammatory cytokines such as IL-17 and by recruiting granulocytes.
TGF-β1 is also a master regulator of tissue fibrosis and remodeling. It promotes the expression of profibrotic mediators in various cell types.Citation8,9 Furthermore, it is a key component in inducing the epithelial-mesenchymal transition (EMT). In vitro studies have shown that TGF-β1 stimulates the proliferation of mesenchymal cells; moreover, it induces the differentiation of fibroblasts into myofibroblasts to synthesize extracellular matrix (ECM) proteins.Citation10 TGF-β1 also influences ECM component degradation by delicately balancing both MMP and tissue inhibitors of metalloproteinase activity.Citation11,12 Thus, TGF-β1 has the ability to elicit many of the structural alterations of airway remodeling.
TGF-β1 is believed to play an important role in airway inflammation and remodeling processes within the asthmatic lung.Citation8,13 Its gene polymorphisms have been associated with asthma susceptibility and development.Citation14,15 Epithelial cells isolated from asthmatic lungs have been shown to undergo EMT upon TGF-β1 stimulation.Citation16 Increased TGF-β1 mRNA and protein levels in either bronchial biopsy sections or the bronchoalveolar lavage fluids (BALF) have been observed in moderate to severe asthmatics in comparison to normal subjects or subjects with mild asthma.Citation9 Finally, in a cross-sectional sample of children with severe asthma,Citation17 both the levels of TGF-β1 expression and the number of TGF-β1 expressing cells showed a significant correlation not only with markers of airway remodelingCitation18 but also with airflow limitation. The above studies with clinical specimens suggest a direct role for TGF-β1 in airway remodeling. In mouse models, instillation into lungs and transgenic or adenoviral overexpression of TGF-β1 in the airway epithelium is sufficient to induce both airway collagen mRNA and protein deposition.Citation19 A SMAD3 knockout model has demonstrated that TGF-β1 contributes to the development of airway remodeling.Citation20 However, blocking TGF-β1 signaling with antibodies had varied outcomes, including reduced,Citation21,22 unaffectedCitation23,24 or increased pulmonary inflammatory responsesCitation25,26 and amelioratedCitation22,24 or unaffected airway remodeling.Citation27
Whereas TGF- β1 appears to be a potential target for the treatment of asthma, its roles in airway allergic inflammation and remodeling are controversial.Citation28 In our previous study, administration of a TGF-β1 peptide-based VLP vaccine significantly suppressed the development of colon fibrosis in a mouse model of chronic colitis.Citation29 In this study, employing the same vaccine, we sought to investigate the effects of the persistent intervention of TGF-β1 signaling through active immunization on allergen-induced chronic airway inflammation, remodeling and hyper-responsiveness.
Results
Preventive experiments: immunization with VLPs increased proinflammatory cytokine levels in the BALF with no significant effects on lung-tissue inflammation
The animal experiment was conducted pursuant to the protocol (). TGF-β1-specific IgG responses were elicited through immunization with a VLP vaccine presenting a TGF-β1 antigenic peptide (). The cytokine levels in the BALF were measured with ELISAs. IL-4, IL-13, IL-10, and IL-33 were present at elevated levels, and IL-5, IFN-γ and MUC5AC levels did not clearly change in the vaccinated mice compared to the controls ().
Figure 1. Active immunization targeting TGF-β1 in an acute model of allergic airway inflammation (preventive experiments). (A) Protocol used for the animal experiments. (B) TGF-β1-specific IgG responses in the serum. The y-axis represents antibody titers, which was determined as the reciprocal of the highest dilution of the sample in which the OD405 value was twice of that of the corresponding control serum pooled from carrier immunized mice when its OD405 was around 0.10. (C) Cytokine levels in the BALF measured with ELISAs. (D) Semi-quantitative analyses of lung histology. Peribronchiolar and perivascular accumulation of inflammatory cells was assessed with H&E-stained sections using an indexed scale. Goblet-cell abundance was measured as the percentage of PAS-positive cells in the total airway epithelia of medium-sized airways. The data are expressed as score values. #P < 0.05, ##P < 0.01, ###P < 0.001, ns: not significant; N = 6/group
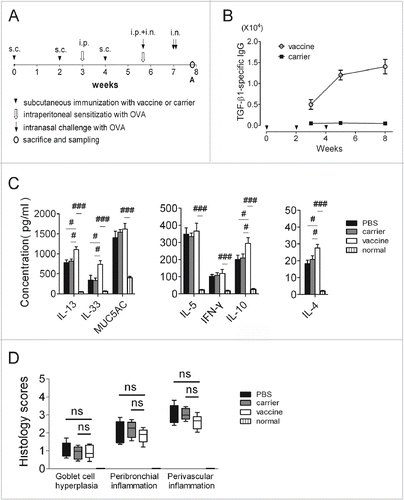
The formalin-fixed left lungs were subjected to tissue processing, slicing, and staining with H&E and PAS, and a semiquantitative analysis (including a scoring system) of immune cell infiltration was performed (). Mice in the PBS control group showed that sensitization and challenge with OVA led to severe immune-cell infiltration and significantly increased airway goblet-cell hyperplasia compared to the normal mice. Finally, there were no significant differences found between the vaccine group and the control groups.
Interventional experiments: Immunization with VLPs increased proinflammatory cytokine accumulation in the BALF and suppressed bodyweight gain at the endpoint, but it had no influence at the middle checkpoint
To investigate whether TGF-β1-specific IgG that is induced after inflammatory responses have been established can suppress the further development of chronic asthma, a study was performed according to the protocol shown in . We induced specific antibodies against TGF-β1, which were maintained at a high concentration throughout the experiments ().
Figure 2. The cytokine profile in the BALF was significantly affected in mice vaccinated at 4 weeks after discontinuation of OVA challenge (interventional experiments). (A) Protocol used for the animal experiments. (B) TGF-β1-specific IgG responses in the serum. (C) Cytokine levels in the BALF measured with ELISAs. (D) Changes in the bodyweight. The statistical analyses were performed with one-way ANOVA followed by Newman-Keuls multiple-comparisons test. #P < 0.05, ##p < 0.01, ###p < 0.001; N = 6/group at time point B, and N = 8/group at time point C
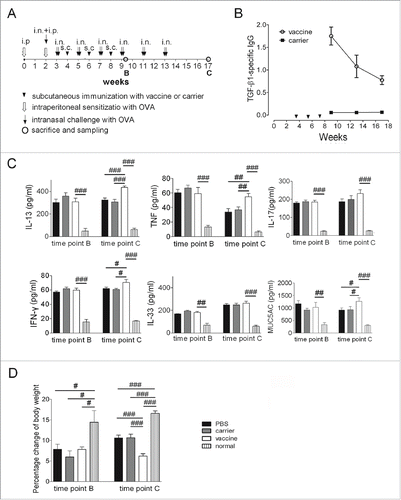
The cytokine concentrations in the BALF were measured with ELISA at time points B (24 h after the fourth 2-day challenge cycle of OVA) and C (4 weeks after discontinuing the OVA challenges). In the model mice (PBS group), the IL-33 concentration was elevated, and the TNF and MUC5AC levels were decreased dramatically at time point C compared to those at time point B, whereas IL-13, IL-17, and IFN-γ remained at similar levels (). Compared to the carrier and saline controls, the vaccinated mice had significantly higher concentrations of IL-13, IFN-γ, and MUC5AC at time point C, but no differences were observed at time point B (). The bodyweight data showed that OVA treatment induced significantly reduced bodyweight gain in the PBS, carrier and vaccine groups compared to the normal mice, and the weight gain in the vaccinated mice was significantly lower than in the PBS and carrier controls, indicating exacerbated airway inflammation and disease burdens ().
Interventional experiments: Sustained lung-tissue inflammation and airway goblet-cell hyperplasia were elevated significantly in vaccinated mice
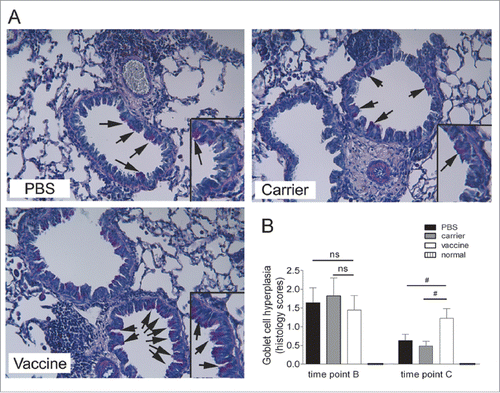
Increased goblet-cell abundance, a feature of airway remodeling, was analyzed using PAS-stained lung tissue sections. The results showed that mice receiving repeated OVA challenges had significant induced airway goblet-cell hyperplasia at time points B and C in comparison with the normal mice. Consistent with the increased MUC5AC concentration in the BALF detected by ELISA, semi-quantitative analyses of the PAS-stained sections with a scoring system showed that the goblet-cell abundance was significantly increased in the vaccinated mice at time point C in comparison with the carrier and saline groups; however, there was no significant difference found between the vaccine group and the controls at time point B. Representative pictures at time point C are shown ().
Figure 3. Sustained airway goblet cell hyperplasia was elevated significantly in mice vaccinated at 4 weeks after discontinuation of OVA challenge (interventional experiments). (A) Lung sections were stained with PAS, and the representative images are shown (original magnification, ×200). The inserts are powered magnifications showing goblet cells, and arrows point to goblet cells with pink color within the respiratory epithelium. (B) Semi-quantitative analysis. Goblet-cell abundance was measured as the percentage of PAS-positive cells in the total airway epithelia of medium-sized airways. The data are expressed as score values. The statistical analyses were performed with one-way ANOVA, followed by a Newman-Keuls multiple-comparison test. #P < 0.05; N = 6/group at time point B, and N = 8/group at time point C
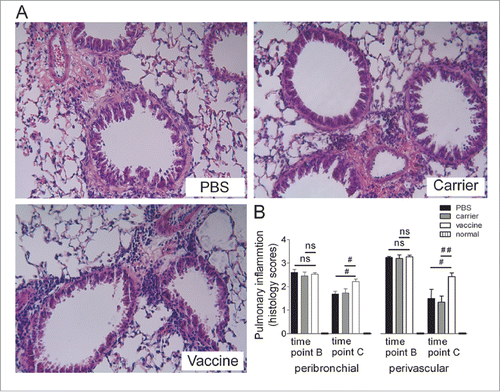
Lung-tissue inflammation was assessed by H&E staining, and the results showed significant inflammatory cell infiltration around the bronchial and vascular trees in all of the groups at time point B; this infiltration was dramatically reduced (but still present) at time point C. Semiquantitative analyses showed that significant differences were evident between the vaccine and the control groups at time point C, indicating that immunization significantly maintained the infiltration of inflammatory cells into the lung tissue even if acute inflammatory responses had clearly diminished in the control mice; however, vaccination did not produce visible effects on tissue inflammation at time point B when comparing the vaccine group with the carrier and saline controls (). Representative pictures at time point C are shown ().
Figure 4. Sustained lung-tissue inflammation was significantly elevated in mice vaccinated at 4 weeks after discontinuation of the OVA challenge (interventional experiments). (A) Lung sections were stained with H&E, and the representative images are shown (original magnification, ×200). (B) Semi-quantitative analysis. Peribronchiolar and perivascular accumulation of inflammatory cells was assessed using an indexed scale. The data are expressed as score values. The statistical analyses were performed with one-way ANOVA followed by a Newman-Keuls multiple-comparison test. #P < 0.05, ##p < 0.01, ns: not significant; N = 6/group at time point B, and N = 8/group at time point C
Interventional experiments: Airway subepithelial collagen deposition was reduced significantly in the vaccinated mice
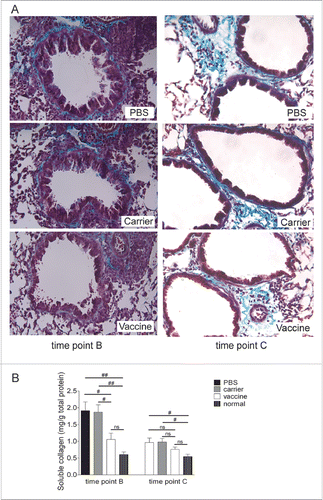
Extracellular matrix deposition, another feature of airway remodeling, was examined with Masson trichrome-stained lung tissue sections and quantitatively analyzed using a soluble collagen assay with lung tissue homogenates. Collagen distribution was visualized in the lung tissue, where collagen was stained blue and the nuclei were stained black with a red background. Trichrome staining revealed that significant collagen deposition was found in the airway subepithelial extracellular matrix. As shown (), 4 2-day challenge cycles of OVA induced significant collagen deposition around the bronchial and vascular tracts at time point B. This was also seen at time point C, 4 weeks after discontinuing the OVA challenges, although the strength and extent of collagen deposition were reduced. The collagen deposition in the lungs of the vaccinated mice was more moderate than in the controls, a finding that is supported by the result obtained from quantitative soluble collagen detection (). In summary, vaccination significantly decreased collagen deposition at time points B and C.
Figure 5. Airway subepithelial collagen deposition was reduced significantly in vaccinated mice (interventional experiments). (A) Lung sections were stained with Masson trichrome, and representative images are shown (original magnification, ×200). (B) A soluble collagen assay with lung tissue homogenates was performed for the quantitative detection of collagen deposition. The statistical analyses were performed with one-way ANOVA followed by a Newman-Keuls multiple-comparison test. #P < 0.05; N = 6/group at time point B, and N = 8/group at time point C
Interventional experiments: The vaccinated mice showed significant increases in airway resistance, tissue resistance, and tissue elastance at the endpoint
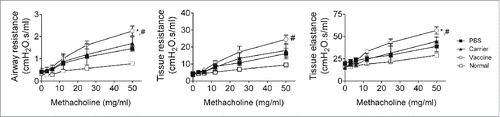
To assess the functional impact of the biomarkers of airway remodeling and inflammation, the airway and lung tissue mechanics were measured at time point C, when the acute inflammatory responses had declined (). The primary parameters of the respiratory mechanics measured included airway resistance, tissue resistance, and tissue elastance. All of the parameters were increased in the carrier and saline groups compared with the normal mice, indicating that sustained airway hyper-responsiveness was established by repeated OVA challenge and persisted at least 4 weeks after the allergen challenge was discontinued. Compared to the control groups, the vaccine group showed significant increases in allergen-augmented airway resistance, tissue resistance and tissue elastance at a dose of 50 mg/ml of methacholine stimulation (). The increases in lung mechanics found in the vaccinated mice are consistent with the increased sustained lung-tissue inflammation and elevated pro-inflammatory cytokines in the BALF, such as IL-13 and TNF, which are important in the development of airway hyper-responsiveness.
Figure 6. Vaccinated mice showed significant increases in airway resistance, tissue resistance, and tissue elastance at 4 weeks after discontinuation of OVA challenge (interventional experiments). Respiratory mechanic responses to inhaled methacholine were analyzed using low-frequency forced oscillation with a small-animal ventilator. The statistical analyses were performed with one-way ANOVA followed by a Newman-Keuls multiple-comparison test. *P < 0.05, between vaccine and carrier; #P < 0.05, between vaccine and carrier; N = 8/group
Discussion
Asthma is considered an allergic inflammatory airway disease, characterized with excessive Th2 responses. In this current study, the inflammatory markers and pathophysiology of the airway, including inflammatory cells and cytokines accumulation in BALF, lung tissue inflammation, airway goblet cell hyperplasia, lung collagen deposition, and airway mechanics, were carefully characterized. We examined the levels of Th2-associated cytokines including IL-4, IL-5, IL-10, IL13, and IL-33. In chronic and refractory asthma, Th1 and Th17 responses were also reported to play important roles for the maintenance and progress of airway inflammation and lung tissue remodeling, and thus IL-17, TNF and IFN-γ were selected to be measured for the interventional study in a chronic model. Mucus overproduction is a cardinal feature of asthma pathophysiology, and thus the level of mucus protein MUC5AC was detected to provide a quantitative support to the semi-quantitative analysis of goblet cells hyperplasia using lung tissue sections with PAS staining.
We employed a unique interventional strategy of active immunization targeting TGF-β1 in this study. This provided a distinct advantage over the passive administration of neutralizing antibodies used in the previous studies, i.e., only a brief immunization procedure can not only elicit a long-lasting specific antibody response but also provide persistent interventional effects.Citation29,30 TGF-β1 is critical in the process of tissue fibrosis through directly activating profibrotic cells and promoting mediators release, and therefore, this study investigated whether anti-TGF-β1 vaccination will suppress lung collagen deposition. However, TGF-β1 may affect indirectly lung fibrosis and tissue remodeling through modulating inflammatory responses. In the interventional experiments, 24 hours after the fourth OVA-challenge cycle, when airway inflammatory responses had been fully established, immunization with TGF-β1 vaccine did not result in significant changes in the BALF cytokine profile, lung-tissue inflammation, or airway goblet-cell hyperplasia. The results that there were no overall obvious changes in the inflammatory responses at this checkpoint may attribute to the dual anti- or pro- inflammatory effects of TGF-β1. It was reported that the anti-inflammatory effects of TGF-β1 are mediated by regulating lymphocyte homeostasis, inhibiting Th1 and Th2 cell responses, and promoting the differentiation of Treg cells.Citation31 Conversely, TGF-β1 also exerts potent chemoattractive activity and induces the differentiation of pro-inflammatory Th17 and Th9 cells, thus amplifying inflammation.Citation1 Another possible reason might be that the significantly elevated pro-inflammatory cytokines, such as IL-13, TNF and IL-5, played key roles in the development and maintenance of inflammation and therefore, neutralizing TGF-β1 did not have an obvious effect. In a study with an acute model while fibrosis is not detectable yet, prophylactic immunization was performed to show possible effects of targeting TGF-β1 on airway inflammatory responses. The results showed no obvious changes in lung histology assessment, although the cytokine levels in BALF were elevated in the immunized mice. The reasons may also attribute to the aforementioned dual roles of TGF-β1 in acute inflammatory responses. Notably, at the checkpoint of 24 hours after the fourth OVA-challenge cycle, subepithelial deposition of collagen was suppressed in the vaccinated mice. Our results are consistent with a previous report that therapeutic treatment of mice with anti-TGF-β1 antibodies significantly reduced peribronchiolar ECM deposition in the lung without affecting established airway inflammation and Th2 cytokine production.Citation24 The results strongly indicate that TGF-β1 does exert an important effect in repeated OVA challenge-induced airway subepithelial collagen deposition, and targeting TGF-β1 may provide the benefit of suppressing airway remodeling.
In studies in which TGF-β1 has been neutralized using antibodies in animal models of airway inflammation, outcomes have generally been examined immediately after the OVA challenge, when acute inflammation is at its most severe.Citation20,21,23-27 The results of these studies do not accurately reflect either the possible contributions of TGF-β1 to airway remodeling or the impact of airway remodeling on airway physiology. Therefore, in this study, we chose to evaluate a checkpoint at 4 weeks after the last OVA challenge, which allowed for the acute inflammatory responses to recover, and display the influences of suppressing TGF-β1 on allergen-induced persistent inflammatory responses and remodeling. The results showed that lung tissue inflammation (the inflammatory profile was more mononuclear in nature than in the acute studies) persisted in vaccinated mice and was significantly more severe than in the control mice. Moreover, this inflammation was accompanied by elevated levels of pro-inflammatory cytokines such as IL-13, IFN-γ, and TNF. The result of enhanced sustained inflammation in the vaccinated mice was distinctly different from that found in previous reports, in which inflammation was assessed 12 d after the final OVA challenge. In those reports, inhibition of TGF-β1 completely blocked the allergen-induced increase in monocytes/macrophages and reduced increases in eosinophils and lymphocytes.Citation22 In contrast, it was also reported that the abrogation of TGF-β1 or TGF-β1 signaling pathways exacerbated eosinophilic infiltration.Citation25-27 The lack of consistency between the studies is most likely due to differences in the models and approaches used to inhibit TGF-β1 activity. In accordance with the increased inflammation, important features of airway remodeling such as airway goblet-cell hyperplasia and excessive mucus production were significantly increased in the vaccinated mice. The enhanced goblet-cell hyperplasia is consistent with the increases of BALF MUC5AC and IL-13 which significantly induces mucus hypersecretion. One explanation for our results may be deduced that TGF-β1 exerted an overall anti-inflammatory effect during repeated OVA challenges by facilitating the generation of Treg cells and inhibiting the differentiation of Th2 and Th1 cells; suppressing TGF-β1 activity in vivo both impaired Treg cell induction and abolished tolerance, thus enhancing the airway and pulmonary inflammatory responses.
Our investigation of airway mechanics showed that airway resistance, tissue elastance and tissue resistance were elevated in the vaccinated mice. Additionally, our study showed that collagen deposition was reduced in the vaccinated mice. Similar results were reported previously by Alcorn et al.Citation23 The results contrast with the well-accepted notion that collagen deposition results in tissue remodeling that both limits airway contraction and causes airway hyper-responsiveness.Citation32 As a possible explanation, it has been proposed that fibrotic remodeling of the airway wall may actually reduce AHR by stiffening the airway and making it more resistant to constrictionCitation33; conversely, our results may simply indicate the combined effects of reduced airway responses due to the ameliorated remodeling and elevated airway responses caused by sustained inflammation that are evidenced by the increased infiltration of inflammatory cells and BALF cytokines.
Although the inflammatory markers and pathophysiology of the airway were carefully characterized in this current study, the detailed mechanisms underlying the lung pathophysiology (e.g., how the immunization targeting TGF-β1 may modulate the Treg, Th1, Th2, Th17, and Th9) remain to be further studied. In addition, mice in this study were challenged with OVA which is not a natural allergen, and repeated challenges with OVA caused mice an obvious immune tolerance, which may affect the lung pathophysiological mechanisms and the appropriate assessment on interventional effects of targeting TGF-β1. Furthermore, the study was performed in a murine model of asthma, which may have limitations for the interpretation of the data to clinical application considering the differences of airway structure, immune system, and disease inducement, etc.
The results presented here indicate that the role of TGF-β1 is complicated in allergic airway inflammatory responses, likely due to varied immune microenvironments. Targeting TGF-β1 with a vaccine may ameliorate airway collagen deposition but exacerbate sustained airway inflammation, mucus production and airway mechanics. We clearly showed that the vaccine intervened successfully TGF-β1 activities and it could be applied effectively for mechanism studies in animal models. While the applicability of this approach in clinic appeared to be controversial, which was indicated in murine models of asthma in our current and the other studies,Citation21-27 the actual prospective of the vaccine may need to be assessed in the models of larger animals such as primates and even human clinical studies. And, the strategy is likely to be practicable for some other diseases, for which fibrosis process or immune suppression rather than inflammation is critical in pathogenesis. For example, the TGF-β1 vaccine may be potent to modulate immunosuppressive tumor microenvironment and enhance specific anti-tumor cellular immune. In summary, the vaccine itself has been proven to be working and effective from the view of its capability of intervening TGF-β1 activities in this current study; the further development of this vaccine is probably to identify the applicable diseases and disease conditions; while both TGF-β1 and its antagonists are considered potent clinical treatments for some severe human diseases such as asthma, the safety issues of these strategies must be seriously considered.
Materials and methods
Mice
Female BALB/c mice (6- to 8-wk-old) (SCXK [jing] 2012– 0001; 16–18 g), purchased from Vital River Laboratory Animal Technology Co., Ltd., were raised and maintained in the Central Animal Care Services of the Institute of Medical Biology, CAMS, and PUMC, under specific-pathogen-free (SPF) conditions. All of the animal experiment protocols were approved by the Institute's Animal Ethics Committee.
Immunization, sensitization and challenge
Preparation of the vaccine was performed as described in our previously published papers.Citation29,34 Briefly, an antigenic peptide from mouse TGF-β1 (66–74 amino acids: QHNPGASAS) was inserted between 78–79 amino acids in the immunodominant epitope of HBcAg (1–149 amino acid, GenBank accession number: GQ377581) through gene engineering methods. The recombinant proteins were expressed in E. coli DH5α cells, and VLPs were purified using 40% ammonium sulfate precipitation and a combined chromatography procedure consisting of Sepharose CL-4B (Sigma-Aldrich) and Ceramic Hydroxyapatite columns (Bio-Rad). The protocols for the animal studies are shown (). In the preventive study, 4 groups of mice were included. Group 1, vaccine (n = 6): immunized subcutaneously (s.c.) with 50 µg of vaccine, then subjected to intraperitoneal (i.p.) sensitization and intranasal (i.n.) challenge with OVA (Sigma-Aldrich, grade IV); group 2: carrier (n = 6): immunized s.c with 50 µg of carrier (HBcAg VLPs without the epitope inserted) and sensitized/challenged with OVA; group 3: phosphate buffered saline (PBS, 0.02 M sodium phosphate buffer, 0.15 M NaCl, pH 7.2) (n = 6): administered s.c. with PBS and sensitized/challenged with OVA; group 4, normal control (n = 6): mice were not administered any treatment. In the interventional study, 4 groups of mice (n = 14/each group) were included, including the vaccine, carrier, PBS and normal control groups. The mice were immunized after acute inflammatory responses were established. At the checkpoints, mice were subjected to heart puncture for blood sampling under anesthetization with isoflurane, and then euthanized by cervical dislocation.
Bronchoalveolar lavage fluid (BALF) collection and differential cell counts
The BALF was collected with 3 repeated washes of the excised lungs using 1 ml PBS. After cytospinning, the slides were stained with HEMA 3 (Fisher Diagnostics, Pittsburg, PA, USA). Differential cell counts were performed according to standard hematological procedures. At least 400 cells were counted from each preparation.
Cytokines and antibody measurements with enzyme-linked immunosorbence assay (ELISA)
Cytokine levels in the BALF supernatants and TGF-β1-specific IgG were assayed using ELISA, either as previously describedCitation29,30,34 or by following the manufacturer's instructions (PharMingen, San Diego, CA).
Histological assessment
The left lungs were fixed in formalin, then embedded in paraffin and sectioned. Specimens were stained either with hematoxylin and eosin (H&E) or with periodic acid Schiff (PAS). As previously described,Citation30 peribronchiolar and perivascular inflammation in the H&E-stained slides were assessed using an indexed scale: 0 = normal; 1 = infrequent inflammatory cells; 2 = a ring of inflammatory cells 1 cell layer deep; 3 = a ring of inflammatory cells 2 - 4 cells deep; 4 = a ring of inflammatory cells of > 4 cells deep. Goblet-cell abundance was measured as the percentage of PAS-positive cells in the total airway epithelia of medium-sized airways. Collagen deposits were detected by staining the sections with Masson's trichrome solution.
Soluble collagen assay
The right lungs were homogenized in 0.5 M acetic acid containing 1 mg of pepsin (at a concentration of 10 mg of tissue/5 ml of acetic acid solution). The resulting mixture was then incubated and stirred for 24 h at 4°C. The total soluble collagen content of the mixture was determined using a Sircol Collagen Assay Kit (Biocolor ltd., Carrickfergus, Antrim, UK), following the manufacturer's instructions. The collagen content was normalized to the total protein concentration.
Analysis of airway and lung-tissue mechanics
Airway reactivity was measured using a flexiVent small-animal ventilator (Scireq Respiratory Equipment Inc., Montreal, PQ, Canada). As described previously,Citation30 the mice were anesthetized and then received serial dilutions of methacholine, which were nebulized through a cannula inserted in the trachea. The respiratory mechanics were assessed using a preset flexiVent Prime-8 low-frequency forced oscillation protocol.
Statistical analysis
Differences between experimental groups were assessed by one-way analysis of variance (ANOVA), followed by the Newman-Keuls multiple-comparison test (GraphPad Prism 3.03, GraphPad Software, Inc., San Diego, CA, USA). The values are reported as the mean ± standard error of the mean.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by grants from the Yunnan Provincial Science and Technology Department (2011FA023 and 2013IA005) and the National Natural Science Foundation of China (81072399).
References
- Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy 2012; 67:1193-202; PMID:22913656; http://dx.doi.org/10.1111/j.1398-9995.2012.02880.x
- Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem 2010; 147:781-92; PMID:20410014; http://dx.doi.org/10.1093/jb/mvq043
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007; 26:579-91; PMID:17481928; http://dx.doi.org/10.1016/j.immuni.2007.03.014
- Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol 2007; 19:55-62; PMID:17137775; http://dx.doi.org/10.1016/j.coi.2006.11.008
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998; 16:137-61; PMID:9597127; http://dx.doi.org/10.1146/annurev.immunol.16.1.137
- Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol 2012; 4:29-37; PMID:22158907; http://dx.doi.org/10.1093/jmcb/mjr033
- Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol 2008; 38:915-7; PMID:18395859; http://dx.doi.org/10.1002/eji.200738111
- Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol 2011; 44:127-33; PMID:20525803; http://dx.doi.org/10.1165/rcmb.2010-0027TR
- Fattouh R, Jordana M. TGF-beta, eosinophils and IL-13 in allergic airway remodeling: a critical appraisal with therapeutic considerations. Inflamm Allergy Drug Targets 2008; 7:224-36; PMID:19075788; http://dx.doi.org/10.2174/187152808786848388
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010; 298:L715-31; PMID:20363851; http://dx.doi.org/10.1152/ajplung.00361.2009
- Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy 2005; 4:177-81; PMID:15853739; http://dx.doi.org/10.2174/1568010053586246
- Watelet JB, Bachert C, Claeys C, Van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy 2004; 59:54-60; PMID:14674934; http://dx.doi.org/10.1046/j.1398-9995.2003.00364.x
- Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 2008; 121:560-70; quiz 71–2; PMID:18328887; http://dx.doi.org/10.1016/j.jaci.2008.01.031
- Ierodiakonou D, Postma DS, Koppelman GH, Gerritsen J, ten Hacken NH, Timens W, Boezen HM, Vonk JM. TGF-beta1 polymorphisms and asthma severity, airway inflammation, and remodeling. J Allergy Clin Immunol 2013; 131:582-5; PMID:23111237; http://dx.doi.org/10.1016/j.jaci.2012.08.013
- Zhang Y, Zhang J, Huang J, Li X, He C, Tian C, Peng C, Guo L, Xiao Y, Fan H. Polymorphisms in the transforming growth factor-beta1 gene and the risk of asthma: A meta-analysis. Respirology 2010; 15:643-50; PMID:20409029; http://dx.doi.org/10.1111/j.1440-1843.2010.01748.x
- Camara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair 2010; 3:2; PMID:20051102; http://dx.doi.org/10.1186/1755-1536-3-2
- Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol 2012; 129:388-96, 96 e1–8; PMID:22206775; http://dx.doi.org/10.1016/j.jaci.2011.11.037
- Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodelling of the airway wall in bronchial asthma. Thorax 1998; 53:21-7; PMID:9577517; http://dx.doi.org/10.1136/thx.53.1.21
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004; 200:377-89; PMID:15289506; http://dx.doi.org/10.1084/jem.20040104
- Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J Immunol 2007; 178:7310-6; PMID:17513781; http://dx.doi.org/10.4049/jimmunol.178.11.7310
- Leung SY, Niimi A, Noble A, Oates T, Williams AS, Medicherla S, Protter AA, Chung KF. Effect of transforming growth factor-beta receptor I kinase inhibitor 2,4-disubstituted pteridine (SD-208) in chronic allergic airway inflammation and remodeling. J Pharmacol Exp Ther 2006; 319:586-94; PMID:16888081; http://dx.doi.org/10.1124/jpet.106.109314
- Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. Tgf-Beta isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS One 2010; 5:e9674; PMID:20300191; http://dx.doi.org/10.1371/journal.pone.0009674
- Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG. Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease. Am J Respir Crit Care Med 2007; 176:974-82; PMID:17761617; http://dx.doi.org/10.1164/rccm.200702-334OC
- McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol 2005; 174:5774-80; PMID:15843580; http://dx.doi.org/10.4049/jimmunol.174.9.5774
- Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, Iwamoto I. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med 2000; 192:151-8; PMID:10899902; http://dx.doi.org/10.1084/jem.192.2.151
- Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur J Immunol 2005; 35:198-206; PMID:15593298; http://dx.doi.org/10.1002/eji.200425209
- Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC Jr, Lonning S, Gauldie J, et al. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med 2008; 177:593-603; PMID:18174546; http://dx.doi.org/10.1164/rccm.200706-958OC
- Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor beta and severe asthma: a perfect storm. Respir Med 2014; 108:1409-23; PMID:25240764; http://dx.doi.org/10.1016/j.rmed.2014.08.008
- Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL, Ma A, Zhou G, Qing G, Peng Z. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis 2010; 16:1040-50; PMID:19924805; http://dx.doi.org/10.1002/ibd.21167
- Ma Y, Halayko AJ, Basu S, Guan Q, Weiss CR, Ma AG, HayGlass KT, Becker AB, Warrington RJ, Peng Z. Sustained suppression of IL-13 by a vaccine attenuates airway inflammation and remodeling in mice. Am J Respir Cell Mol Biol 2013; 48:540-9; PMID:23470628; http://dx.doi.org/10.1165/rcmb.2012-0060OC
- Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity 2009; 31:438-49; PMID:19766086; http://dx.doi.org/10.1016/j.immuni.2009.08.007
- Royce SG, Cheng V, Samuel CS, Tang ML. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol 2012; 351:167-75; PMID:22266540; http://dx.doi.org/10.1016/j.mce.2012.01.007
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol (1985) 2003; 95:426-34; PMID:NOT_FOUND; http://dx.doi.org/10.1152/japplphysiol.00159.2003
- Long Q, Huang W, Yao Y, Yang X, Sun W, Jin X, Li Y, Chu X, Liu C, Peng Z, et al. Virus-like particles presenting interleukin-33 molecules: immunization characteristics and potentials of blockingIL-33/ST2 pathway in allergic airway inflammation. Hum Vaccin Immunother 2014; 10:2303-11; PMID:25424936; http://dx.doi.org/10.4161/hv.29425
