ABSTRACT
In recent years considerable progress has been made in the field of cancer immunotherapy whereby treatments that modulate the body's own immune system are used to combat cancer. This has the potential to not only elicit strong anti-cancer immune responses which can break pre-existing tolerance and help promote tumor regression, but could also induce immunological memory which may help prevent tumor recurrence. In order to ensure effective delivery of immunotherapeutic agents, such as vaccines, checkpoint inhibitors, chemotherapeutic agents and nucleic acids, a safe and effective delivery system is often required. One such approach is the use of multifunctional nanoparticles (NPs), such as liposomes, polymers, micelles, dendrimers, inorganic NPs, and hybrid NPs, which have the potential to combine the delivery of a diverse range of therapeutic immunomodulators thereby increasing the efficacy of tumor cell killing. This review focuses on recent progress in NP-mediated immunotherapy for the treatment of cancer.
Introduction
In recent years there has been considerable progress made in the prevention and treatment of cancer; nevertheless it remains one of the main causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer related deaths in 2012; a number expected to rise in the coming decades.Citation1 Cancer refers to a series of diseases characterized by the uncontrollable division of abnormal cells which may invade or spread to nearby healthy tissue and organs resulting in the formation of malignant tumors. Tumor specific antigens (TSA) are exclusively expressed on tumor cells and not on any other cells whereas tumor associated antigens (TAA) are endogenous antigens present on both tumor and normal cells. Although many of these antigens should be immunogenic, cancers that are detected clinically have evaded anti-tumor immune responses by a combination of immune evasion, induction of tolerance and downregulation of T cell signaling which has enabled tumors to progressively grow.Citation2 Even with improvement of patient's survival by conventional therapies (surgery, radiation, chemotherapy etc.), many types of cancer are still incurable or are associated with severe therapy-related adverse effects.Citation3,4 However, in recent years we have gained an increased knowledge of tumor immunology that has allowed the development of novel therapies with minimal toxicity.
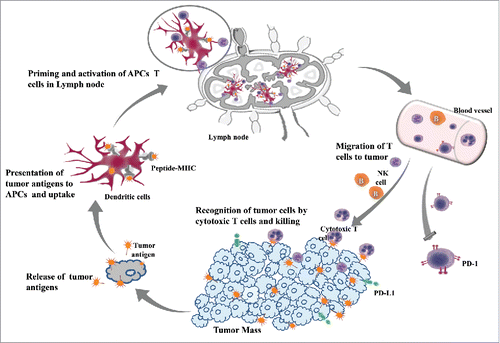
Immunotherapies against existing cancer either stimulate the activities of specific effector mechanisms of the immune system or counteract inhibitory or suppressive mechanisms. The generation of tumor-specific immunity involves multiple sequential immune actions: the release of antigens from tumor beds, presentation of tumor antigens by antigen-presenting cells (APCs), priming and activation of T cells by activated antigen-presenting cells (APCs), migration and infiltration of effector T cells back to the tumor, and finally the recognition and killing of tumor cells by effector T cells ().Citation5,6 It has been recognized that T-lymphocytes, particularly cytotoxic T Lymphocytes (CTL) that can selectively target and destroy malignant cells, play a pivotal role in immune cancer therapy. However, their isolated response is not sufficient enough to mount an effective stimulation and killing, but instead it is the cooperation between CTL, T helper (Th) cells, B cells and mature dendritic cells (DC) that has the potential to reduce the tumor mass and induce immunological memory to control tumor relapse.Citation7,8
A number of different immunotherapeutic approaches against cancer have been proposed. These can broadly be divided into active and passive immunotherapies. Active immunotherapies involve stimulation of the body's own immune response whether it be specifically via the administration of vaccines containing tumor-specific antigens and/or tumor-associated antigens or non-specifically, for example via the administration of cytokines such as IL-2 or IFN-α. In contrast, passive immunotherapy, for example via the administration of monoclonal antibodies or adoptive T cell transfer, targets the disease itself rather than activating the immune system to do so.Citation9,10
A number of novel immunotherapeutics against existing cancer have recently been approved which highlight the potential of these approaches. The first therapeutic cancer vaccine product, sipuleucel-T (Provenge®), a cell-based cancer immunotherapy for prostate cancer, was approved in the US in 2010, and greatly expedited the clinical development of other novel cancer immunotherapeutics.Citation11,12 More recently, an increasing number of monoclonal antibodies, including those termed checkpoint inhibitors which target the immune-inhibitory pathways activated by cancer cells, have now been approved. For example Ipilumumab (Yervoy), an anti-CTLA-4 monoclonal antibody (mAb) was approved by the US Food and Drug Administration (FDA) in 2011 as a first line therapy for melanoma patients with metastatic disease.Citation13
While the majority of cancer therapies have focused on the treatment of existing cancer, there have been some successes in the prevention of cancers, in particular those caused by certain viral infections such as the hepatitis B virus (HBV), a risk factor of hepatocellular cancer, and human papillomavirus (HPV), a risk factor of cervical cancer, for which vaccines are currently available. In Taiwan, where universal childhood HBV vaccination was introduced in 1984, there has been more than a 80% decline in liver cancer incidence rates among youth and young adults in the last 30 y.Citation14 While it is still too early to determine the effects of HPV vaccination on incidence of cervical cancer, results so far are very promising.Citation15
Despite these successes there still remain some major hurdles to the development of cancer immunotherapies. In many cases, response rates are low, responses are short-lived, side-effects can be severe and treatments can be prohibitively expensive.Citation13 In order to overcome this, a number of combinatorial approaches have been proposed including dual-checkpoint inhibitors, checkpoint inhibitors and agonists of co-stimulatory receptors, and checkpoint inhibitors and therapies that enhance tumor cell recognition or immunogenicity.Citation16,17 Another approach has been to enhance delivery of immune therapies, such as vaccine antigens and/or adjuvants, cytotoxic drugs, monoclonal antibodies, small interfering RNA (siRNA) etc., via the use of nanoparticles (NPs). Over the past 3 decades, there has been considerable advance in the use of NP delivery systems for cancer therapy and several NP-based compounds delivering encapsulated or conjugated cytotoxic drugs targeted to solid tumors have been approved by the FDA. There are multiple types of NPs used including lipid-based particles such as liposomes, lipoplexes and lipid NPs; polymeric NPs and micelles; pH and thermosensitive nanoparticles; and metal and inorganic NPs such as gold NPs.Citation18 Many of these particles act as a multi-functional drug delivery platform due to their ability to target delivery of tumor antigens and adjuvants,Citation19-22 enhance immune activation,Citation23-25 augment the efficacy of cell therapies,Citation26-28 accommodate multiple therapeutic moieties with different physicochemical properties, modify pharmacokinetics and pharmacodynamics of therapeutic agents,Citation29 and to improve the stability and resistance issues.Citation30
The first generation antitumor nanomedicines, such as DOXIL® and Abraxane®, relied on enhanced permeability and retention (EPR) effects arising due to the unique vascular characteristics of tumor tissue.Citation31 However, EPR effects are influenced by the interplay between the NP and tumor microenvironment which may vary considerably among different tumor types.Citation32 Therefore, EPR dependent passive targeting often results in unpredictable clinical outcomes, particularly in metastatic cancer, where tumor cells grow in different vascular beds.Citation33 Overall, clinical outcome therefore depends not only on the immunomodulatory effect of NPs but also their localization, retention, cell binding, internalization, and toxicity and this is in turn dependent on a number of factors including the NP composition, surface chemistry, biophysical properties, affinity to protein, and the route of administration.Citation34 One approach to help improve the efficacy of NP delivered therapies is through active targeting whereby NPs are associated with ligands selected to target surface molecules or receptors overexpressed on tumor cells.
Here, we review the current NP platform technologies for rational design of targeted next-generation carriers for cancer immunotherapy, ranging from antigen/adjuvant delivery vehicles to target professional antigen-presenting cells, to direct tumor antigen-specific T-lymphocyte-targeting compounds and their combinations thereof.
Immune regulation, tumor microenvironment and tumor evasion mechanisms
Cancer immunotherapy has made considerable progress in recent years largely due to an increase in our understanding of the complex tumor microenvironment (TME). The success of cancer immunotherapy has been dependent on strategies to optimize tumor-specific immune responses while overcoming tumor-evasion mechanisms. The key role that the immune system plays in tumor development is demonstrated by the association of tumor growth with chronic inflammation,Citation35 immune deficiencies such as malfunctions of IFN-γ, perforin, T cells and natural killer (NK) cells,Citation36,37 and an increased cancer occurrence in immune compromised or immunosuppressive recipients.Citation38 In the early stages of tumor growth, the presence of tumor cells can induce inflammatory signals leading to the recruitment and infiltration of immune cells such as NK cells, neutrophils, innate lymphoid cells, macrophages and DCs. This can lead to the direct killing of tumor cells by NK cells as part of the innate immunosurveillance process,Citation39,40 and also to the initiation of adaptive immune responses via APCs, in particular DCs, which process and present tumor derived antigens in the context of MHC class I molecules to activate CD8+ T cells. The subsequent release of IFN-γ may also impede angiogenesis and prevent tumor progression.Citation41 In some cases, tumor cells can be completely killed by such antitumor immune response and this may protect us from many early stage cancers. However, in many other cases this innate immunosurveillance is not sufficient and cancer cells do proliferate and form tumors. The TME is composed of a broad variety of cell types in addition to malignant cells, including lymphocytes, tumor associated macrophages, NK cells, DCs, myeloid-derived suppressor cells (MDSCs), the tumor vasculature endothelial cells, as well as fibroblasts, pericytes and sometimes adipocytes.Citation42 In addition, osteopontin, galectin-3, transforming growth factor-β and matrix metalloproteinases (MMP) are important secreted proteins closely associated with cancer development. The interaction among these cells, plus proteins, creates an extracellular matrix (ECM) which forms a physical skeleton to support the evolution and spreading of cancers. Moreover, cancer-associated fibroblasts are present in high numbers with an aberrant phenotype and have a potent effect on tumorigenesis and can impede anti-tumor responses. Furthermore, collagen deposition in the TME may also hinder T cell entry and in many patients, the tumor vasculature prevents the trafficking and function of T cells, particularly in those tumors which lack a type 1 interferon signature. The complexity of the TME therefore posts a significant challenge to the entry of adaptive immune cells into tumor sites.
Tumors form for a variety of reasons. Due to genetic instability, tumor cells constantly divide and can become less immunogenic over time so that they can evade immune elimination. Indeed, tumors can stay dormant without mass expansion for long periods of time until vasculature is established.Citation43 The continuous stimulation of innate receptors such as Toll-like receptors (TLRs) by necrotic cancer cells can create a chronic inflammatory condition that can promote immune tolerance, maintain tumor microenvironment, and promote tumor angiogenesis that actually supports tumor progression.Citation44 In addition, immunosuppressive cells in the TME such as CD4+/CD25+/FoxP+ regulatory T cells (Tregs) play a crucial role in tumor escape.Citation45,46 It has been demonstrated that blocking CD25+ by anti-CD25 mAb promotes tumor rejection.Citation47 Immune suppressive cytokines produced in the TME such as interleukin (IL)-10 and transforming growth factor-β (TGF-β), can facilitate the conversion of CD4+ T cells into Treg in situ, resulting in the direct inhibition of cytotoxic T lymphocyte proliferation and thereby contribute to tumor growth and progression.Citation48,49 Tumor cells can also recruit other inhibitory immune cells such as alternatively-activated macrophages (M1 and M2) and MDSCs to dampen cytotoxic functions of CTLs and to aid in tumor initiation, angiogenesis and metastasis.Citation50 Another mechanism by which tumors evade immune surveillance is by lowering the expression of major histocompatibility complex (MHC) Class I. As a result of this, tumor antigen expression is down regulated and effective killing of tumor by cytotoxic tumor infiltrating lymphocytes fails to occur.Citation51 Moreover, immunosuppressive enzymes like indoleamine 2,3-dioxygenase (IDO), arginase and nuclear factor kappa-B kinase may act on T cell tolerance leading to tumor cell proliferation.Citation52 Tumor cells also over express ligands that bind to inhibitory receptors and dampen T cell function within the TME. For example, programmed death-ligand 1 (PD-L1) is commonly expressed on the surface of macrophages and DCs and binds to programmed cell death protein 1 (PD-1) on activated T cells thereby modulating T cell response and minimizing over activation. However, PD-L1 is commonly overexpressed on tumor cells as a mechanism to evade detection and inhibit the immune response. Our understanding of inhibitory checkpoint molecules such as PD-1 and cytotoxic T lymphocyte associated protein 4 (CTLA-4) has led perhaps to the biggest breakthrough is cancer immunotherapy in recent years with the development of checkpoint inhibitors, which are mAbs targeting these receptors. Indeed, the FDA approved an anti-CTLA4 and an anti-PD1 antibody for the treatment of melanoma in 2011 and 2014, respectively, with subsequent approvals for other cancers whether used alone or in combination.Citation13,16
Use of NPs for targeted delivery
NPs were initially developed as a protective vehicle for incorporated material, such as antigens, proteins, peptides nucleic acids, etc., thereby allowing the use of lower doses and a more sustained release. However, by altering their biophysical features and through the incorporation of appropriate functional ligands, they can also be used to target distinct cell types such as tumor cells, DCs, T cells or the tumor microenvironment.
These advanced NP systems therefore provide a unique opportunity to develop therapeutic compounds that can more specifically promote tumor cell death and subsequent antitumor immunity.
Influence of biophysical features of NPs on tumor targeting
The uptake of TAAs, carried within NPs, by DCs is largely influenced by physicochemical properties of the NP including size, shape, surface charge and hydrophobicity. DCs preferentially engulf virus-sized particles (20 ∼200 nm) while macrophages (Mφ) preferentially uptake larger particles (0.5 ∼5 µm). For example, following intradermal administration to mice of small core-shell NPs (20, 45 or 100 nm) consisting of poly-ethylene glycol cross-linked to a core of polypropylene sulfide, particles with a size of 20 or 45 nm were retained in lymph nodes for up to 5 d.Citation53 An increase in NP-containing DCs was seen, suggesting these cells trafficked the NPs to the lymph nodes. Interestingly, NPs were internalized by up to 50 % of lymph node DCs without the use of a targeting ligand, indicating the key role of particle size.Citation53 Particle size will influence not only cellular uptake but also distribution, retention and clearance of NPs. Following subcutaneous, intradermal or intramuscular administration, the majority of small NPs (< 20 nm) will preferably drain to blood capillaries and subsequently be eliminated by the organism; NPs between 20 and 100 nm will penetrate the extracellular matrix, enter directly into the lymphatic vessels, travel to the lymphatic nodes where they will be taken up by immune cells, in particular DCs; larger NPs (> 100 nm) will most likely stay at the injection sites until they are captured by peripheral DC and then migrate to lymphatic nodes to initiate immune response.Citation54
However, due to their physical characteristics, NPs can also be internalized by scavenger cells, such as Mφ, which have impaired T cell priming capacity compared to DC. Kim et al. showed that, at least with NPs composed of poly-γ-glutamic acid and L-phenylalanine ethyl ester, small NPs having diameter in a range of approximately 30-130 nm were less accumulated into DCs in vitro, compared with larger NPs having diameter in range of 190-360 nm, although DC activation was strong with both types.Citation55 This suggests that DC activation occurs not only based on size-dependent uptake of the NPs, but also additional factors such as the interactions between the NPs and DCs, which may also be responsible for the induction of DC maturation.Citation55
The endocytosis by DCs of NPs is predominantly by the classic receptor-mediated pathway for NPs less than 150 nm, but by the caveolae-mediated pathway for smaller NPs in the range of 50-80 nm.Citation56 Studies found that the uptake of Simian Virus 40 (SV40), which is the most extensively studied ligand for caveolar endocytosis with a particle diameter of 50nm, occurs specifically by the caveolar pathway. Even when an excess of SV40 particles were incubated with cells, still less than 5% of internalized SV40 were found to pass through clathrin-coated pits and vesicles.Citation57 In contrast, when negative mutants of caveolin were expressed, internalization or infection was lost.Citation58
Since cell membranes are typically negatively charged, they attract positively charged NPs with high affinity. Therefore, while particles having diameters of less than 0.5 µm are usually optimal for DC uptake; larger positively charged particles can also be efficiently taken up.Citation59 Once internalized by APCs, cationic NPs are found in the perinuclear area, whereas negatively charged or neutral NPs tend to localize within lysosomes.Citation60 Thus, both the surface charge of NPs as well as the charge of the material to be delivered are clearly important considerations for influencing particle selection and their cellular uptake. For example, K-ras, the most common mutated oncogenic protein in solid tumors, has +8 surface charge. Therefore, a biodegradable negative charged polyester-based vector (BCPV) targeting to K-ras was used to effectively transport siRNA into pancreatic cancer cells, leading to apoptosis of tumor cells.Citation61 In contrast, cationic liposomes composed of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) were able to activate mouse bone marrow DCs (BMDCs) in a concentration-dependent manner resulting in the generation of reactive oxygen species (ROS) which led to activation of extracellular signal-regulated kinase (ERK) and p38, cytokine/chemokine production, and expression of the B7 costimulatory molecules CD80 and CD86. However, the presence of higher doses of DOTAP led to cytotoxicity and subsequent cell death,Citation62 highlighting the need for appropriate dosing levels when using cationic NPs.
Particle shape is another key parameter that can influence NP biodistribution, cellular uptake, and toxicity.Citation63 For example, non-spherical particles composed of diblock copolymers of poly(ethylene glycol) (PEG) and poly(ethylethylene) or biodegradable poly(ε-caprolactone) present as filamentous micelles,Citation64 showed a prolonged circulation time, increased accumulation in tumor tissue with a significantly greater anti-tumor effect than its spherical counterparts when used to deliver paclitaxel to A549 tumor-bearing mice.Citation64,65 Worm-shaped micelles made of polylactic acid (PLA) and inert block copolymer amphiphiles showed more effective penetration into nanoporous gel (a tissue model) than conventional 100 nm vesicles.Citation66
Innate immune activation can also be influenced by texture of microparticles. For example, polystyrene-block-poly(ethylene oxide) (PS-PEO) microparticles having a budding texture could be more readily phagocytosed, and induced a faster neutrophil recruitment to the injection site than smooth particles, leading to stronger IL-1β secretion through activation of the NLRP3 inflammasome.Citation67
The biodistribution, retention and clearance of NPs can be influenced by multiple properties including size and shape, charge, composition and presence of ligands. While certain NPs (typically > 400 nm) may accumulate in tumor sites as a result of extravasation from tumor vasculature enhanced by angiogenesis or via various targeting mechanisms,Citation68 other NPS will be phagocytosed and cleared from the bloodstream, in particular by the mononuclear phagocyte system (MPS) in the liver and spleen.Citation69 A better understanding of the impact that biophysical properties of NPs have on their biodistribution can directly impact their efficacy. Pegylated or STEALTH liposomes have been used for many years to improve circulation time and avoid the MPS. They can circulate for long periods as stable constructs and slowly extravasate into tumors, providing passive targeting to tumor tissue. More recently, mixed-charge gold NPs (AuNPs) have been shown to have a much longer blood half-life and accumulate far less in the liver and spleen than PEG-coated AuNPs which can result in higher accumulation and slower clearance in tumors. A number of other approaches including coating of AuNPs with biocompatible polymers have also been shown to improve the stability of the NPs and the payloads, improve biocompatibility and promote longer systemic circulation.
NPs target DCs
Dendritic cells have been called nature's adjuvant because they are the most potent antigen-presenting cells and are capable of inducing T cell responses in vivo without other adjuvants.Citation70 Therefore, DCs are central to the generation of antitumor responses and have been the target of immunotherapy for a number of years. The first approved autologous cellular immunotherapy, sipuleucel-T (Provenge®) for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate was approved by the FDA in 2010, based on an approximate 4 month median improvement in overall survival in Phase 3 trials.Citation71 Despite this success, there remains room for improvement in DC-based therapeutics and direct DC targeting in vivo using NPs may be one potential option.Citation72
Although small NPs (< 500 nm) can be efficiently internalized by local DCs, active targeting of designed NPs through molecular recognition, i.e., ligand-receptor interaction or antibody-antigen recognition, has been shown to be efficient in co-delivering Ags and other stimulatory molecules to specific DC population.Citation73,74 Particulate delivery systems including virus vectors, whole-cell vaccines, virosomes, immunostimulating complexes (ISCOMs), virus-like particles (VLPs) and other biodegradable nanocarriers can carry specific ligands and thereby increase interaction with target cells as compared to soluble antigens. For example, Cruz et al. investigated three distinct cell-surface receptors expressed on DCs as targets for pegylated poly(lactic-coglycolic acid) (PLGA) NPs: i) CD40, a TNF-α family receptor with known DC activating properties after binding to its specific ligand; ii) DEC-205, a C-type lectin receptor, and iii) CD11c, integrin receptor. These receptors were targeted by means of specific mAbs coupled to the NP, with co-encapsulation of OVA and TLR3/7 ligands. All three mAb-targeted NPs were efficiently internalized by DC in vitro compared to non-targeted NPs.
In addition, all targeted NPs could equally stimulate IL-12 production and induce strong proliferation and IFN-γ production by T cells in vitro. Moreover, all targeted NPs showed higher efficacy than non-targeted NP in stimulating antigen specific CD8+ T cell responses after subcutaneous administration to mice.Citation75-77
It is noteworthy that distinct immune responses can be induced by targeting different DC populations. For example, by using chimeric monoclonal antibodies to target antigen to the 2 major types of DCs found in spleen, it was shown that delivery of antigen to CD8+DEC205+ DCs led to preferential antigen presentation by MHC class I molecules, whereas targeting to CD8–33D1+ DCs led to presentation by MHC class II molecules.Citation78 Subsequent studies have used this approach of targeting different DC subpopulations in a number of different animal models. A similar approach may be feasible by using different functional groups to allow targeting to NPs to distinct DC subpopulations.
It is worth noting that while DC targeting appears to be an attractive form of immunotherapy for cancer, DC malfunction and failure of DC maturation often occurs in cancer patients.Citation79 Indeed, it is believed that immature or partially matured DCs may induce T cell anergy or Treg expansion, resulting in antigen-specific tolerance.Citation80 The maturation of DCs is associated with increased expression of various surface markers including CD40, CD80, CD83, CD86 and MHC-I/-II and can be induced by inflammatory factors such as LPS, bacterial DNA or inflammatory cytokines such as TNF-α. LPS contamination in gold NPs has been shown to stimulate DC maturation, leading to an increased expression of co-stimulatory and MHC class I and II molecules and production of cytokines.Citation81 Signal transducer and activator of transcription 3 (STAT3) inhibits DC differentiation and when down-regulated can result in improved DC function.Citation82 Poly (ethylene glycol)-b-poly (L-lysine)-b-poly (L-leucine) (PEG-PLL-PLLeu) polypeptide micelles loaded with Poly I:C (PIC, a TLR3 agonist), STAT3 siRNA and OVA antigen could effectively overcome DC dysfunction in a murine B-16 melanoma model by deleting STAT3 gene in situ.Citation83 Similar results have been shown by other groups using NP based delivery of STAT3 siRNA alone or as part of an anti-cancer vaccine.Citation84,85
While the focus of cancer immunotherapy is on activation of DCs, for other therapeutic areas down-regulation of DCs is required. For example, in a murine autoimmune encephalomyelitis model, the administration of polystyrene or biodegradable PLGA microparticles (500-nm diameter) bearing encephalitogenic peptides induced long-term T-cell tolerance, highlighting the potential use of microparticles in autoimmune disease.Citation86 Therefore, it is important to note that DC targeting is not as simple as adding a DC targeting ligand to NPs, and that the polarization of DCs, either stimulation or suppressive, is essential to the success of immunotherapy. Thus, the challenge to achieve a desired immune outcome, particularly in an immune compromised population, remains. For cancer immunotherapy, the key to successful DC targeting is to select the appropriate DC surface target and the appropriate payload, whether be it antigen and adjuvant to elicit a protective and long-lasting immune response or mAbs or siRNA to downregulate inhibitory mechanisms.
NPs target tumor microenvironment
Tumor-associated macrophages (TAM), MDSCs and Tregs are a heterogeneous group of cells that are major components of the TME and play a critical role in enhancing tumor cell invasion and metastasis, promoting angiogenesis and extracellular matrix remodeling, while inhibiting the anti-tumor immune surveillance. Elimination or reprogramming of the immune suppressive TME is one of the major current challenges in immunotherapy of cancer.Citation57 Perhaps the most successful cancer immunotherapy to date has been immune checkpoint inhibition, which targets regulatory pathways in T cells to enhance antitumor immune responses. Four new immune checkpoint agents have now been approved by the US FDA for the treatment of melanoma. A mAb against CTLA-4 (ipilimumab) was approved in 2011, and 2 antibodies against PD-1 (pembrolizumab and nivolumab) were approved in 2014.Citation87 The therapeutic benefit of checkpoint inhibitors seems to be most optimal in patients with a pre-existing T-cell response against their tumor.Citation88 Li et al., used NPs, composed of poly(ethylene glycol)-block-poly(D,L-lactide) (PEG–PLA) that encapsulated CTLA-4 siRNA (siCTLA-4) and showed direct T cell activation both in vitro and in vivo. These cationic lipid-assisted PEG–PLA-based NPs efficiently delivered siRNA into T cells in vitro and significantly increased the percentage of anti-tumor CD8+ T cells, while also decreasing the ratio of CD4+ FOXP3+ Tregs among tumor infiltrating lymphocytes (TILs), resulting in inhibition of tumor growth and prolonged survival time.Citation89 Other approaches include the use of Papaya mosaic virus NPs (PapMV) as both an immunostimulatory molecule and as a vaccine platform to activate the innate immune response in an IFN-α-dependent manner. A synergistic effect of PapMV treatment in combination with PD-1 blockade was demonstrated in a murine melanoma model in triggering CD8+ T cells responses specific for the tumor antigens gp100 and TRP2, compared to anti-PD-1 treatment which when used alone, did not significantly increase tumor-specific T-cell numbers.Citation90 An increased therapeutic effect of anti-PD-1 blockade has been shown in a murine melanoma model when used in combination with immunostimulatory RNA, supporting the hypothesis that a synergistic therapeutic effect is likely the result of increased immune-cell infiltration and tumor-specific T-cell priming.Citation91
The tumor vasculature is abnormal in terms of its heterogeneous and chaotic branching structure, uneven vessel lumen, and leakiness. These features result in increased interstitial fluid pressure and uneven blood flow, oxygenation, nutrient and drug distribution which in turn increases hypoxia and promotes metastasis mediated via vascular endothelial growth factor (VEGF).Citation42 Although tumor vasculature is highly accessible and can therefore enhance delivery of drugs to tumors, most therapeutic agents, especially chemotherapy, have no intrinsic affinity for tumor vessels, and can cause severe drug resistance and systemic side effects. Vascular targeting using NPs is another inviting strategy for cancer therapy requiring effective margination to bring drugs/vaccines etc. to the tumor site. A number of TME targeting NP formulations have been designed for the prevention of angiogenesis, tumor growth and metastasis, for example by activating endogenous angiogenesis inhibitors via phage display,Citation58 silencing proangiogenic factors via delivery of CXCR4 antagonists using lipid-based NP,Citation92 or by directly destroying endothelial cells via active Integrin targeting.Citation93
Tumors possess a dense extracellular matrix (ECM) composed of fibrous proteins such as collagen and elastin, as well as a highly viscous polysaccharide-containing fluid.Citation94 NPs have been developed to target various ECM components thereby remodelling ECM dynamics and destroying the matrix needed for tumor growth. Several studies have utilized hyaluronan (HA)-conjugated nanocarriers to target CD44, an adhesion/homing molecule, which acts as receptor for glycosaminoglycan hyaluronan, one of the major components of the tumor extracellular matrix.Citation95 HA-conjugated NPs designed to target CD44 in the ECM have been used to deliver anti-cancer drugs (e.g., epirubicin, doxorubicin, paclitaxel, and mitomycin c), as well as siRNA to CD44 over-expressing cells.Citation95 Similarly, Tan et al. used gadolinium-containing dendrimers conjugated with a peptide (CLT-1), which bind specifically to fibronectin–fibrin complexes in the tumor ECM, to target breast tumor support structure and showed the potential of this approach for magnetic resonance cancer molecular imaging.Citation96 Several studies have used NPs coated with HA to target CD44 in combination with other targeting moieties as dual-functional drug carriers. For example, PLGA NPs conjugated with HA and anti-HER2/neu peptide (AHNP) have been used to deliver SN38, the active metabolite of the topoisomerase 1 inhibitor Irinotecan, and was shown to inhibit tumor growth in murine models to a greater extent than Irinotecan alone.Citation97 Indeed, even complex multi-functional drug carriers have now been proposed. For example, a multifunctional liposomal nanocarrier has been tested in vitro that combined: i) a nanoscale size for passive tumor targeting, ii) an anti-nucleosome mAb (mAb 2C5) for active targeting, iii) PEG tails to prevent non-specific interactions and prolong particle circulation time, iv) a matrix metalloprotease 2 (MMP2)-sensitive bond between PEG and lipid that is cleaved in the tumor by the highly expressed extracellular MMP2 to remove the PEG chains and v) a cell-penetrating peptide (TATp) that enhances intracellular delivery of the system after long-chain PEG removal and exposure of the previously hidden surface-attached TATp. This complex NP has been shown to enhance the targetability and internalization of nanocarriers in cancer cells.Citation98,99
T cell targeting
Adoptive T cell therapy (ACT) is based on the isolation and ex vivo expansion of tumor specific T cells from fresh patient biopsy specimens typically in the presence of tumor antigen and IL-2. The tumor specific T cells are then infused into the same patient where they can target and kill tumor cells. A number of different forms of adoptive T cell therapy have been used for cancer treatment; culturing tumor infiltrating lymphocytes (TILs), isolating and expanding one particular T cell or clone, and using engineering T cells to recognize and attack tumors. While initial studies with ACT were limited by weak immune responses, attributed to the decline in viability and function of transplanted cells, insufficient expansion of transferred T cells, inefficient trafficking to tumor regions and dose-limiting toxicities,Citation100,101 considerable improvement in this technology have been made in recent years including the use of chimeric antigen receptors (CAR)-T cell technology whereby T cells are genetically engineered to express tumor-specific antigen receptors to enable targeting to tumor cells.Citation102-104
One promising approach to improve the efficacy of ACT is by combining CAR-T with blockade of co-inhibitory immune checkpoints such as anti-PD-1, anti-PD-L1 or anti-CTLA-4 monoclonal antibodies.Citation105,106,107 Another approach to enhance cell therapy is to modify the surfaces of therapeutic cells with adjuvant drug-loaded NPs. For example, liposome particles with encapsulated cytokines (IL-15, IL-21) or drugs (glycogen synthase kinase-3 β inhibitor TWS119) were coupled to the surface of living T cells via thiol-reactive maleimide headgroups on the lipid bilayer surface of the particles. These surface decorated NPs were non-toxic to their carrier T-cells, did not interfere with intrinsic cell function or migration patterns, yet gave their carrier cells substantially enhanced function using low drug doses that were ineffective when used alone by traditional systemic routes. Of note, after crossing the endothelial barrier, 83% (± 3%) of their original NP cargo was still physically attached to the carrier CD8+ T-cells.Citation27 Several other studies have demonstrated that T cells may serve as efficient delivery vehicles for the treatment of cancer. For example, gold NPs (AuNP) have been extensively studied for photothermal cancer therapy because AuNPs can generate heat upon near-infrared irradiation. When human T cells were loaded with 45 nm AuNPs and administered in a human tumor xenograft mouse model, 4-fold higher levels of AuNP were observed in tumors 24 hr post-injection compared to injection of free PEGylated AuNPs and overall NP biodistribution was also changed.Citation108 The cytotoxic antibiotic doxorubicin has also been delivered using Target MAG-doxorubicin NPs loaded onto T cells.Citation109
A selective drug carrier system involving antibody-coupled NPs is also an attractive drug-targeting system. Not only would they allow incorporation of cytotoxic antitumor drugs into the NP matrix, but they can also be routed by attachment of antibodies to a specific tumor cell type. This will allow a higher drug carrier capacity combined with improved specificity of drug targeting. Antibodies specific for the CD3 antigen on T cells were conjugated to the surface of gelatin NPs and were shown to be internalized into CD3-positive human T-cell leukemia cells and primary T lymphocytes. An uptake rate of ∼84% into T-cell leukemia cells was observed demonstrating the feasibility of receptor-mediated cellular uptake into a given target cell type.Citation110
Hybrid NPs
Hybrid NPs are typically composed of a synthetic polymer core with a lipoid shell. Originally developed to overcome the problem of delivering water-soluble drugs in hydrophobic lipid particles, they can be composed of a wide selection of biocompatible polymers and lipids, are easy to synthesize and possess the ability to deliver multiple therapeutic agents to the same target cell including both hydrophobic and hydrophilic molecules.Citation111 Co-delivery approaches include the delivery of multiple chemotherapeutic drugs, chemotherapeutic drugs and nucleic acids (e.g., DNA, RNAi, siRNA), chemotherapeutic drugs and mAbs, chemotherapeutic and epigenetic drugs.Citation112 In addition, as with other types of NPs, they can be surface coated with targeting moieties, to enable targeted drug delivery to tumor cells.
One of the tumor evasion mechanisms which tumor cells implement is the secretion of transforming growth factor-β (TGF-β), which restricts local tumor immune responses, limiting conventional cytokine therapy. In order to overcome this, a core-shell delivery platform was established combining features of both liposomal and solid cyclodextrin systems. This nanoscale liposomal polymeric gel was composed of drug-complexed cyclodextrins and cytokine-encapsulating biodegradable polymers, which enables sustained and simultaneous release of a TGF-β inhibitor and hydrophilic IL-2 to the TME, resulting in significantly delayed tumor growth and increased survival in a murine B16 melanoma model.Citation113 Such hybrid multifunctional drug-delivery systems offer the potential for combinatorial delivery and synergistic action in cancer therapy.
STAT3 inhibits DC differentiation and when downregulated can result in improved DC function.Citation82 It therefore imposes a major limitation for DC-based cancer therapies and becomes an attractive target for immunotherapeutic applications.Citation114 Activation of STAT3 in DCs can inhibit the expression of immunostimulatory molecules triggered by TLR ligands, such as cytosine-phosphate guanine oligonucleotides (CpG ODN), that regulate T cell activation.Citation115 Kim et al., designed an immunomodulatory hybrid nanoconjugate system aimed at concomitantly silencing STAT3 by siRNA and activating TLR9 by CpG ODN. Polymeric PLGA nanocomposites containing quantum dots (QDs) conjugated to CpG ODNs and STAT3 siRNA using a cleavable disulfide linker were able to simultaneously deliver STAT3 siRNA and CpG ODN to the target immune cells thereby inhibiting STAT3 and activating DCs by CpG ODNs giving synergistic antitumor effects in a murine B16 melanoma model.Citation115 Another similar approach was developed using PLGA NPs containing both siRNA for the knock-down of immune-suppressor gene STAT3 of DCs and imiquimod for the activation of DCs through TLR 7. Again, these NPs were efficiently taken up by DCs and various cytokines were expressed in matured DCs. Immunization of mice with these NP-treated DCs induced OVA-specific CTL activity in the EG7-OVA tumor model and tumor growth was efficiently delayed.Citation116
In an attempt to mimic the mechanism whereby immune effector cells crosslink surface receptors on target tumor cells and induce apoptosis and to circumvent the use of potentially toxic chemotherapeutic drugs, Chu et al. designed a biomimetic material platform composed of self-assembling hybrid nanoconjugates. This system comprised an anti-CD20 Fab’ antibody fragment, a pair of complementary phosphorodiamidate morpholino oligomers (MORF1 and MORF2), and a linear polymer (P) of N-(2-hydroxypropyl)methacrylamide (HPMA). Malignant CD20+ B-cells were first treated with anti-CD20 Fab’ conjugated to MORF1, resulting in the decoration of the cell surfaces with MORF1. Further addition of copolymer grafted with multiple copies of MORF2 resulted in MORF1 and MORF2 hybridization at the cell surface with concomitant CD20 cross-linking, which triggered cell apoptosis, resulting in improved long term survival in a murine model of human non-Hodgkin's lymphoma.Citation117 Although CD20 mAbs (e.g., obinutuzumab) can also induce direct apoptosis, this approach could also potentially reduce adverse effects.
Combination therapy
The strength of combinatorial approaches to cancer immunotherapy was demonstrated by the FDA's decision to grant accelerated approval to the combination of the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab to treat advanced melanoma cancer based on strong Phase 3 results.Citation118,119 In addition, a number of clinical studies have shown promising data with combinations of checkpoint inhibitors and agonists of costimulatory receptors such as CD70, 4-1BB (CD137/TNF superfamily 9), OX40 (CD134), combinations of checkpoint inhibitors and therapies that promote tumor cell recognition by T cells such as tumor vaccines and IDO inhibitors, and combinations of checkpoint inhibitors and therapies that enhance tumor immunogenicity such as inducers of ICD, oncolytic viruses and epigenetic therapy,Citation16 as well as the combination of different immunotherapies with conventional chemotherapy.Citation120 The use of NP technology may help some of these combinatorial approached achieve their full potential.
In an attempt to increase the efficacy of chemotherapeutic approaches without causing systemic toxicity due to high dose levels required, combinatory chemo-immunotherapeutic approaches have been evaluated. Sequential delivery of paclitaxel (PTX) and TLR9 agonists (CpG ODNs) plus siRNA to silence IL-10 using 2 types of PLGA NPs has shown promising results in murine tumor models. It is hypothesized that the primary injection of PTX caused tumor cell death and the subsequent release of large amounts of antigenic debris resulted in vaccination in situ. The released tumor-associated antigen was thought to subsequently be internalized by tumor-recruited bone marrow-derived dendritic cells (BMDCs). The secondary injection with immunomodulating CpG ODN-encapsulated in PLGA NPs and IL-10 siRNA-encapsulated PLGA NPs triggered the activation and migration of BMDCs to the tumor-draining lymph nodes. As a result, tumor growth was inhibited and animal survival rate was increased.Citation121
To date, the main limitation of immunotherapy is the lack of reduction of tumor load in clinical patients. Additionally, tumor progression and metastasis usually occurs, frequently after a slightly extended period of remission. Targeted therapy appears to obtain a higher response rate in a greater number of patients. Nevertheless, despite a lower overall response rate, responses to immunotherapy, tend to be more durable than those seen with targeted therapy.Citation122-125 Therefore, it is attractive to combine immunotherapy with targeted therapy to try to achieve sustained anti-tumor responses with a long-lasting tumor remission. A number of tumor antigen targets have been identified, for example, overexpressed proteins on tumor cells (e.g., human epidermal growth factor receptor 2 protein in breast cancer (HER-2)), mutant proteins that drive cancer progression (e.g., an altered form of BRAF in melanomas) and fusion genes that produce fusion proteins (e.g., BCR-ABL fusion protein in leukemia cells).Citation123 The folate receptor has also been recognized as a tumor marker as it is overly overexpressed on the surface of different cancers making it a good target for folate nanoconjugates.Citation126 For example, various folic acid functionalized polyethylenimine (PEI) polymers were evaluated to deliver PD-L1 siRNA to SKOV-3-Luc epithelial ovarian cancer cells. Folic acid modified PEI polymers increased siRNA uptake into SKOV-3-luc cells and decreased non-specific uptake into monocytes resulting in enhanced T cell killing.Citation127 In many cases, although tumor cells exhibit abnormal antigens due to their genetic defects, the immune responses to these tumor antigens is not strong because they are recognized as self. One way to overcome this is by the use of effective delivery sytems. For example, archaeosomes, which are liposome like vesicles made with polar membrane lipids of Archaea, possess receptor-targeting sugar headgroups which can facilitate targeted and more efficient uptake of archaeosome vesicles by macrophages.Citation128 Archaeosome have been shown to overcome immunologic tolerance to melanoma self antigens and effectively evoke CD8+ T cells.Citation129 The use of mAbs, Ab fragments (Fab, single chain variable fragment, scFv) and single domain antibodies, (sdAbs) can also act as highly specific probes when they are attached to NPs to aid in targeted delivery of various antitumor cytotoxic agents.Citation130,131 Therefore, overall combinatorial approaches including antibody targeting, chemotherapy and immunotherapy are growing in popularity due to their ability to combine specific targeting to cancer cells and potent antitumor effects.Citation130,131
Conclusion
The goal of the current review was to highlight the potential application of NPs in the field of immunotherapy. Great efforts have been applied to the development of novel vaccine adjuvants and nanoparticulate delivery systems using our improved knowledge of cancerous cellular dynamics and the interactive mechanism between NPs and tumors. Current nanotechnology allows for the simultaneous delivery of synergistic drug combinations and the engagement of the patient's innate and adaptive immune systems to combat cancer. By rationally designing NPs based on active targeting of immune cells, solid tumors and the tumor microenvironment, an improved efficacy can be achieved. The application of nanotechnology to cancer immunotherapy has already produced some exciting results in controlling tumor growth and holds even greater promise for cancer patients in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization. Cancer. Fact Sheet Number 297:2015; available at: http://www.who.int/mediacentre/factsheets/fs297/en/
- Vigneron N. Human tumor antigens and cancer immunotherapy. BioMed Res Int 2015; 2015:948501; PMID:26161423; http://dx.doi.org/10.1155/2015/948501
- Gatenby RA. A change of strategy in the war on cancer. Nature 2009; 459:508-9; PMID:19478766; http://dx.doi.org/10.1038/459508a
- Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharmaceutical Design 2010; 16:3-10; PMID:20214614; http://dx.doi.org/10.2174/138161210789941847
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1-10; PMID:23890059; http://dx.doi.org/10.1016/j.immuni.2013.07.012
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/10.1038/nature10673
- Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, Florindo HF, Barata TS. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Frontiers Chem 2014; 2:105; PMID:25505783; http://dx.doi.org/10.3389/fchem.2014.00105
- Corrigan-Curay J, Kiem HP, Baltimore D, O'Reilly M, Brentjens RJ, Cooper L, Forman S, Gottschalk S, Greenberg P, Junghans R, et al. T-cell immunotherapy: looking forward. Mol Therapy 2014; 22:1564-74; PMID:25186558; http://dx.doi.org/10.1038/mt.2014.148
- Lam SS, Zhou F, Hode T, Nordquist RE, Alleruzzo L, Raker J, Chen WR. Advances in strategies and methodologies in cancer immunotherapy. Discov Med 2015; 19:293-301; PMID:25977192
- Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol 2010; 2010:1–14; PMID:20706612; http://dx.doi.org/10.1155/2010/596432
- So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Exp Rev Anticancer Therapy 2006; 6:1163-7; PMID:17020451; http://dx.doi.org/10.1586/14737140.6.9.1163
- Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunotherapy 2013; 62:137-47; PMID:22865266; http://dx.doi.org/10.1007/s00262-012-1317-2
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016; 14:73; PMID:27151159; http://dx.doi.org/10.1186/s12916-016-0623-5
- Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. Jama 2013; 310:974-6; PMID:24002285; http://dx.doi.org/10.1001/jama.2013.276701
- Drolet M, Benard E, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565-80; PMID:25744474; http://dx.doi.org/10.1016/S1473-3099(14)71073-4
- Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukocyte Biol 2016; 100(2):275-90; PMID:27256570
- Kleponis J, Skelton R, Zheng L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol Med 2015; 12:201-8; PMID:26487965
- Pillai G. Nanomedicines for cancer therapy: An update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci 2014; 1:1-13; http://dx.doi.org/10.1166/jpsp.2014.1001
- Kapadia CH, Tian S, Perry JL, Luft JC, DeSimone JM. Reduction sensitive PEG hydrogels for co-delivery of antigen and adjuvant to induce potent CTLs. Mol Pharmaceutics 2016; 13(10):3381-3394; PMID:27551741
- Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Controlled Release 2013; 168:179-99; PMID:23524187; http://dx.doi.org/10.1016/j.jconrel.2013.03.010
- Jia F, Liu X, Li L, Mallapragada S, Narasimhan B, Wang Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J Controlled Release 2013; 172:1020-34; PMID:24140748; http://dx.doi.org/10.1016/j.jconrel.2013.10.012
- Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008; 26:5046-57; PMID:18680779; http://dx.doi.org/10.1016/j.vaccine.2008.07.035
- Kreutz M, Giquel B, Hu Q, Abuknesha R, Uematsu S, Akira S, Nestle FO, Diebold SS. Antibody-antigen-adjuvant conjugates enable co-delivery of antigen and adjuvant to dendritic cells in cis but only have partial targeting specificity. PloS One 2012; 7:e40208; PMID:22808118; http://dx.doi.org/10.1371/journal.pone.0040208
- Sur A, Pradhan B, Banerjee A, Aich P. Immune activation efficacy of indolicidin is enhanced upon conjugation with carbon nanotubes and gold nanoparticles. PloS One 2015; 10:e0123905; PMID:25876153; http://dx.doi.org/10.1371/journal.pone.0123905
- Rahimian S, Fransen MF, Kleinovink JW, Christensen JR, Amidi M, Hennink WE, Ossendorp F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J Controlled Release 2015; 203:16-22; PMID:25660830; http://dx.doi.org/10.1016/j.jconrel.2015.02.006
- Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Therapy 2010; 18:1650-6; PMID:20606648; http://dx.doi.org/10.1038/mt.2010.136
- Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 2010; 16:1035-41; PMID:20711198; http://dx.doi.org/10.1038/nm.2198
- Gao H, Zhang Q, Yang Y, Jiang X, He Q. Tumor homing cell penetrating peptide decorated nanoparticles used for enhancing tumor targeting delivery and therapy. Int J Pharmaceutics 2015; 478:240-50; PMID:25448586; http://dx.doi.org/10.1016/j.ijpharm.2014.11.029
- Comenge J, Sotelo C, Romero F, Gallego O, Barnadas A, Parada TG, Dominguez F, Puntes VF. Detoxifying antitumoral drugs via nanoconjugation: the case of gold nanoparticles and cisplatin. PloS One 2012; 7:e47562; PMID:23082177; http://dx.doi.org/10.1371/journal.pone.0047562
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Delivery Rev 2013; 65:1866-79; PMID:24120656; http://dx.doi.org/10.1016/j.addr.2013.09.019
- Rink JS, Plebanek MP, Tripathy S, Thaxton CS. Update on current and potential nanoparticle cancer therapies. Curr Opin Oncol 2013; 25:646-51; PMID:24097107; http://dx.doi.org/10.1097/CCO.0000000000000012
- Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Delivery Rev 2014; 66:2-25; PMID:24270007; http://dx.doi.org/10.1016/j.addr.2013.11.009
- Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano 2015; 9:16-30; PMID:25469470; http://dx.doi.org/10.1021/nn5062029
- Jiao Q, Li L, Mu Q, Zhang Q. Immunomodulation of nanoparticles in nanomedicine applications. BioMed Res Int 2014; 2014:426028; PMID:24949448
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:12490959; http://dx.doi.org/10.1038/nature01322
- van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med 1996; 184:1781-90; PMID:8920866; http://dx.doi.org/10.1084/jem.184.5.1781
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 2000; 191:661-8; PMID:10684858; http://dx.doi.org/10.1084/jem.191.4.661
- Chew V, Toh HC, Abastado JP. Immune microenvironment in tumor progression: characteristics and challenges for therapy. J Oncol 2012; 2012:608406; PMID:22927846; http://dx.doi.org/10.1155/2012/608406
- Pross HF, Lotzova E. Role of natural killer cells in cancer. Natural Immunity 1993; 12:279-92; PMID:8257832
- Weigelin B, Krause M, Friedl P. Cytotoxic T lymphocyte migration and effector function in the tumor microenvironment. Immunol Letters 2011; 138:19-21; PMID:21333682; http://dx.doi.org/10.1016/j.imlet.2011.02.016
- Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res 2003; 63:4095-100; PMID:12874012
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012; 125:5591-6; PMID:23420197; http://dx.doi.org/10.1242/jcs.116392
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Letters 2010; 294:139-46; PMID:20363069; http://dx.doi.org/10.1016/j.canlet.2010.03.004
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer 2009; 9:57-63; PMID:19052556; http://dx.doi.org/10.1038/nrc2541
- Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 2012; 13:e32-42; PMID:22225723; http://dx.doi.org/10.1016/S1470-2045(11)70155-3
- Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res 2008; 14:1032-40; PMID:18281535; http://dx.doi.org/10.1158/1078-0432.CCR-07-2056
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res 1999; 59:3128-33; PMID:10397255
- Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 2006; 66:5527-36; PMID:16740684; http://dx.doi.org/10.1158/0008-5472.CAN-05-4128
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295-307; PMID:16557261; http://dx.doi.org/10.1038/nri1806
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008; 8:618-31; PMID:18633355; http://dx.doi.org/10.1038/nrc2444
- Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukocyte Biol 2002; 71:907-20; PMID:12050175
- Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ 2006; 13:738-47; PMID:16485028; http://dx.doi.org/10.1038/sj.cdd.4401877
- Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Controlled Release 2006; 112:26-34; PMID:16529839; http://dx.doi.org/10.1016/j.jconrel.2006.01.006
- Correia-Pinto JF, Csaba N, Alonso MJ. Vaccine delivery carriers: insights and future perspectives. Int J Pharmaceutics 2013; 440:27-38; PMID:22561794; http://dx.doi.org/10.1016/j.ijpharm.2012.04.047
- Kim H, Uto T, Akagi T, Baba M, Akashi M. Amphiphilic poly(Amino Acid) nanoparticles induce size-dependent dendritic cell maturation. Adv Functional Materials 2010; 20:3925-31; http://dx.doi.org/10.1002/adfm.201000021
- Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic 2002; 3:311-20; PMID:11967125; http://dx.doi.org/10.1034/j.1600-0854.2002.30501.x
- Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunol 2013; 138:105-15; PMID:23216602; http://dx.doi.org/10.1111/imm.12036
- Ren SX, Ren ZJ, Zhao MY, Wang XB, Zuo SG, Yu F. Antitumor activity of endogenous mFlt4 displayed on a T4 phage nanoparticle surface. Acta pharmacologica Sinica 2009; 30:637-45; PMID:19417736; http://dx.doi.org/10.1038/aps.2009.44
- Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharmaceutics 2005; 298:315-22; PMID:15961266; http://dx.doi.org/10.1016/j.ijpharm.2005.03.035
- Yue ZG, Wei W, Lv PP, Yue H, Wang LY, Su ZG, Ma GH. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011; 12:2440-6; PMID:21657799; http://dx.doi.org/10.1021/bm101482r
- Yang C, Hu R, Anderson T, Wang Y, Lin G, Law WC, Lin WJ, Nguyen QT, Toh HT, Yoon HS, et al. Biodegradable nanoparticle-mediated K-ras down regulation for pancreatic cancer gene therapy. J Mater Chem B 2015; 3:2163-72; http://dx.doi.org/10.1039/C4TB01623H
- Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Controlled Release 2008; 130:22-8; PMID:18554742; http://dx.doi.org/10.1016/j.jconrel.2008.05.005
- Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PL, Hammond PT, Yang YY. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Delivery Rev 2011; 63:1228-46; PMID:21777633; http://dx.doi.org/10.1016/j.addr.2011.06.016
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2007; 2:249-55; PMID:18654271; http://dx.doi.org/10.1038/nnano.2007.70
- Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, Discher DE. Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol Pharmaceutics 2009; 6:1343-52; PMID:19249859; http://dx.doi.org/10.1021/mp900022m
- Kim Y, Dalhaimer P, Christian DA, Discher DE. Polymeric worm micelles as nano-carriers for drug delivery. Nano Technol 2005; 16:S484-91; PMID:21727469; http://dx.doi.org/10.1088/0957-4484/16/7/024
- Vaine CA, Patel MK, Zhu J, Lee E, Finberg RW, Hayward RC, Kurt-Jones EA. Tuning innate immune activation by surface texturing of polymer microparticles: the role of shape in inflammasome activation. J Immunol 2013; 190:3525-32; http://dx.doi.org/10.4049/jimmunol.1200492
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 1998; 95:4607-12; PMID:9539785; http://dx.doi.org/10.1073/pnas.95.8.4607
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological Rev 2001; 53:283-318; PMID:11356986
- Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol 1996; 24:859-62; PMID:8690042
- Gulley JL, Mulders P, Albers P, Banchereau J, Bolla M, Pantel K, Powles T. Perspectives on sipuleucel-T: Its role in the prostate cancer treatment paradigm. Oncoimmunology 2016; 5:e1107698; PMID:27141392; http://dx.doi.org/10.1080/2162402X.2015.1107698
- D HY, Appel S. Current status and future perspectives of dendritic cell-based cancer immunotherapy. Scandinavian J Immunol 2013; 78:167-71; PMID:23672402; http://dx.doi.org/10.1111/sji.12060
- Calixto G, Bernegossi J, Fonseca-Santos B, Chorilli M. Nanotechnology-based drug delivery systems for treatment of oral cancer: a review. Int J Nanomed 2014; 9:3719-35; PMID:25143724; http://dx.doi.org/10.2147/IJN.S61670
- Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG. Targeting nanoparticles to dendritic cells for immunotherapy. Meth Enzymol 2012; 509:143-63; PMID:22568905; http://dx.doi.org/10.1016/B978-0-12-391858-1.00008-3
- Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Lowik CW, Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J Controlled Release 2014; 192:209-18; PMID:25068703; http://dx.doi.org/10.1016/j.jconrel.2014.07.040
- Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, de Gruijl T, Lowik C, Oostendorp J, van der Burg SH, et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015; 40:88-97; PMID:25465442; http://dx.doi.org/10.1016/j.biomaterials.2014.10.053
- Saluja SS, Hanlon DJ, Sharp FA, Hong E, Khalil D, Robinson E, Tigelaar R, Fahmy TM, Edelson RL. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. Int J Nanomed 2014; 9:5231-46; PMID:25419128
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007; 315:107-11; PMID:17204652; http://dx.doi.org/10.1126/science.1136080
- Chen K, Wang JM, Yuan R, Yi X, Li L, Gong W, Yang T, Li L, Su S. Tissue-resident dendritic cells and diseases involving dendritic cell malfunction. Int Immunopharmacol 2016; 34:1-15; PMID:26906720; http://dx.doi.org/10.1016/j.intimp.2016.02.007
- Hardin JA. Dendritic cells: potential triggers of autoimmunity and targets for therapy. Annals Rheumatic Dis 2005; 64 Suppl 4:iv86-90; PMID:16239396
- Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Letters 2006; 6:1682-6; PMID:16895356; http://dx.doi.org/10.1021/nl060860z
- Brady MT, Miller A, Sait SN, Ford LA, Minderman H, Wang ES, Lee KP, Baumann H, Wetzler M. Downregulation of signal transducer and activator of transcription 3 improves human acute myeloid leukemia-derived dendritic cell function. Leukemia Res 2013; 37:822-8; PMID:23628554; http://dx.doi.org/10.1016/j.leukres.2013.04.002
- Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, Cai L, Ma Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials 2015; 38:50-60; PMID:25457983; http://dx.doi.org/10.1016/j.biomaterials.2014.10.050
- Das J, Das S, Paul A, Samadder A, Bhattacharyya SS, Khuda-Bukhsh AR. Assessment of drug delivery and anticancer potentials of nanoparticles-loaded siRNA targeting STAT3 in lung cancer, in vitro and in vivo. Toxicol Letters 2014; 225:454-66; PMID:24440344; http://dx.doi.org/10.1016/j.toxlet.2014.01.009
- Heo MB, Cho MY, Lim YT. Polymer nanoparticles for enhanced immune response: combined delivery of tumor antigen and small interference RNA for immunosuppressive gene to dendritic cells. Acta Biomaterialia 2014; 10:2169-76; PMID:24394635; http://dx.doi.org/10.1016/j.actbio.2013.12.050
- Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol 2012; 30:1217-24; PMID:23159881; http://dx.doi.org/10.1038/nbt.2434
- Romano E, Romero P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J Immunother Cancer 2015; 3:15; PMID:25901287; http://dx.doi.org/10.1186/s40425-015-0059-z
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015; 523:231-5; PMID:25970248; http://dx.doi.org/10.1038/nature14404
- Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ, Xia JX, Zhu YH, Wang J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Controlled Release 2016; 231:17-28
- Lebel ME, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Letters 2016; 16:1826-32; PMID:26891174; http://dx.doi.org/10.1021/acs.nanolett.5b04877
- Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov 2014; 4:674-87; PMID:24589924; http://dx.doi.org/10.1158/2159-8290.CD-13-0458
- Liu JY, Chiang T, Liu CH, Chern GG, Lin Ts T, Gao DY, Chen Y. Delivery of siRNA using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol Ther 2015; 23:1772-82; PMID:26278330; http://dx.doi.org/10.1038/mt.2015.147
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10:9-22; PMID:20029421; http://dx.doi.org/10.1038/nrc2748
- Kanapathipillai M, Brock A, Ingber DE. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv Drug Delivery Rev 2014; 79-80:107-18; PMID:24819216; http://dx.doi.org/10.1016/j.addr.2014.05.005
- Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 2011; 278:1429-43; PMID:21362138; http://dx.doi.org/10.1111/j.1742-4658.2011.08071.x
- Tan M, Wu X, Jeong EK, Chen Q, Lu ZR. Peptide-targeted Nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging. Biomacromolecules 2010; 11:754-61; PMID:20131758; http://dx.doi.org/10.1021/bm901352v
- Yang Z, Luo H, Cao Z, Chen Y, Gao J, Li Y, Jiang Q, Xu R, Liu J. Dual-targeting hybrid nanoparticles for the delivery of SN38 to Her2 and CD44 overexpressed human gastric cancer. Nanoscale 2016; 8:11543-58; PMID:27203688; http://dx.doi.org/10.1039/C6NR01749E
- Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis 2015; 2:26-34; PMID:26097889; http://dx.doi.org/10.1016/j.gendis.2014.12.002
- Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano 2012; 6:3491-8; PMID:22409425; http://dx.doi.org/10.1021/nn300524f
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007; 117:1466-76; PMID:17549249; http://dx.doi.org/10.1172/JCI32446
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12:6106-15; PMID:17062687; http://dx.doi.org/10.1158/1078-0432.CCR-06-1183
- Almasbak H, Aarvak T, Vemuri MC. CAR T cell therapy: A game changer in cancer treatment. J Immunol Res 2016; 2016:5474602; PMID:27298832; http://dx.doi.org/10.1155/2016/5474602
- Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunological Rev 2014; 257:56-71; PMID:24329789; http://dx.doi.org/10.1111/imr.12132
- Turtle CJ, Maloney DG. Clinical trials of CD19-targeted CAR-modified T cell therapy; a complex and varied landscape. Exp Rev Hematol 2016; 9(8):719-21; PMID:27322438
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013; 19:5636-46; PMID:23873688; http://dx.doi.org/10.1158/1078-0432.CCR-13-0458
- Kodumudi KN, Siegel J, Weber AM, Scott E, Sarnaik AA, Pilon-Thomas S. Immune checkpoint blockade to improve tumor infiltrating lymphocytes for adoptive cell therapy. PloS One 2016; 11:e0153053; PMID:27050669; http://dx.doi.org/10.1371/journal.pone.0153053
- Shin JH, Park HB, Oh YM, Lim DP, Lee JE, Seo HH, Lee SJ, Eom HS, Kim IH, Lee SH, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 2012; 119:5678-87; PMID:22538857; http://dx.doi.org/10.1182/blood-2011-09-380519
- Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res Lett 2011; 6:283; PMID:21711861; http://dx.doi.org/10.1186/1556-276X-6-283
- Steinfeld U, Pauli C, Kaltz N, Bergemann C, Lee HH. T lymphocytes as potential therapeutic drug carrier for cancer treatment. Int J Pharmaceutics 2006; 311:229-36; PMID:16460895; http://dx.doi.org/10.1016/j.ijpharm.2005.12.040
- Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials 2005; 26:5898-906; PMID:15949555; http://dx.doi.org/10.1016/j.biomaterials.2005.02.038
- Wu XY. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Exp Opin Drug Delivery 2016; 13:609-12; PMID:26978527; http://dx.doi.org/10.1517/17425247.2016.1165662
- Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - Strategies and perspectives. J Controlled Release 2016; 240:489-503
- Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Materials 2012; 11:895-905; PMID:22797827; http://dx.doi.org/10.1038/nmat3355
- Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, et al. A critical role for Stat3 signaling in immune tolerance. Immunity 2003; 19:425-36; PMID:14499117; http://dx.doi.org/10.1016/S1074-7613(03)00232-2
- Kim JH, Noh YW, Heo MB, Cho MY, Lim YT. Multifunctional hybrid nanoconjugates for efficient in vivo delivery of immunomodulating oligonucleotides and enhanced antitumor immunity. Angewandte Chemie 2012; 51:9670-3; PMID:22915476; http://dx.doi.org/10.1002/anie.201204989
- Heo MB, Lim YT. Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells. Biomaterials 2014; 35:590-600; PMID:24125775; http://dx.doi.org/10.1016/j.biomaterials.2013.10.009
- Chu TW, Yang J, Zhang R, Sima M, Kopecek J. Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis. ACS Nano 2014; 8:719-30; PMID:24308267; http://dx.doi.org/10.1021/nn4053827
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Eng J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- First immunotherapy combo approved for cancer. Cancer Discov 2015; 5:1228; PMID:26487304
- Qiao J, Liu Z, Fu YX. Adapting conventional cancer treatment for immunotherapy. J Mol Med 2016; 94:489-95; PMID:26910191; http://dx.doi.org/10.1007/s00109-016-1393-4
- Heo MB, Kim SY, Yun WS, Lim YT. Sequential delivery of an anticancer drug and combined immunomodulatory nanoparticles for efficient chemoimmunotherapy. Int J Nanomed 2015; 10:5981-92; PMID:26451105
- Wyluda EJ, Cheng J, Schell TD, Haley JS, Mallon C, Neves RI, Robertson G, Sivik J, Mackley H, Talamo G, et al. Durable complete responses off all treatment in patients with metastatic malignant melanoma after sequential immunotherapy followed by a finite course of BRAF inhibitor therapy. Cancer Biol Therapy 2015; 16:662-70; PMID:25806780; http://dx.doi.org/10.1080/15384047.2015.1026507
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237-51; PMID:22437869; http://dx.doi.org/10.1038/nrc3237
- Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res 2000; 20:2665-76; PMID:10953341
- McDermott D, Lebbe C, Hodi FS, Maio M, Weber JS, Wolchok JD, Thompson JA, Balch CM. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treatment Rev 2014; 40:1056-64; PMID:25060490; http://dx.doi.org/10.1016/j.ctrv.2014.06.012
- Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev 2012; 3: 1–11; PMID:23240070; http://dx.doi.org/10.3402/nano.v3i0.18496
- Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, George AJ, Hedrick JL, Yang YY, Ghaem-Maghami S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Adv Health Care Materials 2015; 4:1180-9; PMID:25866054; http://dx.doi.org/10.1002/adhm.201500089
- Haq K, Jia Y, Krishnan L. Archaeal lipid vaccine adjuvants for induction of cell-mediated immunity. Exp Rev Vaccines 2016; 8:1-10; PMID:27276183; http://dx.doi.org/10.1080/14760584.2016.1195265
- Krishnan L, Deschatelets L, Stark FC, Gurnani K, Sprott GD. Archaeosome adjuvant overcomes tolerance to tumor-associated melanoma antigens inducing protective CD8 T cell responses. Clin Dev Immunol 2010; 2010:1–13; PMID:21318177; http://dx.doi.org/10.1155/2010/578432
- Zhao M, Sun Y, Zhu X, Chen D, Feng S, Guo S, Li H. Smart drug delivery system: chapter 6. Biomedical Engineering 2016
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007; 2:751-60; PMID:18654426; http://dx.doi.org/10.1038/nnano.2007.387