?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Adjuvants are essential for enhancing vaccine potency by improving the humoral and/or cell-mediated immune response to vaccine antigens. This study was performed to evaluate the immuno-enhancing characteristic of N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride (HTCC), the cationically modified chitosan, as an adjuvant for hepatitis E virus (HEV) recombinant polypeptide vaccine. Animal experiments showed that HTCC provides adjuvant activity when co-administered with HEV recombinant polypeptide vaccine by intramuscularly route. Vaccination using HTCC as an adjuvant was associated with increases of the serum HEV-specific IgG antibodies, splenocytes proliferation and the growths of CD4+CD8− T lymphocytes and IFN-γ-secreting T lymphocytes in peripheral blood. These findings suggested that HTCC had strong immuno-enhancing effect. Our findings are the first to demonstrate that HTCC is safe and effective in inducing a good antibody response and stimulating Th1-biased immune responses for HEV recombinant polypeptide vaccine.
Results
Characteristic of HTCC
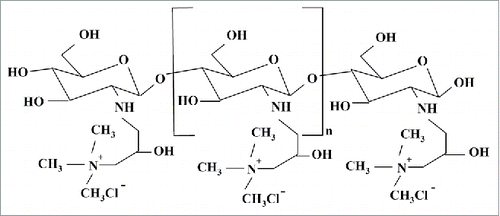
According to the 1H-NMR spectra of HTCC and the DQ formula, DQ of HTCC was 71.5%. The solubility of HTCC in distill water was more than 70.1 mg/ml and the chemical structure was showed in .
HEV-specific total IgG levels
We analyzed the sera HEV-specific total IgG levels on day 14, 35 and 56 after the primary immunized mice by an ELISA kit. According to the OD of negative control, the cutoff value was 0.22. Samples were considered positive if the OD > 0.22. On day 14, 35 and 56, Mock and HTCC groups were negative results, but the other 3 groups were all positive results. In particular, HTCC+HEV group generated stronger HEV-specific IgG antibodies as similar effect as Freund+HEV group ().
Table 1. HEV-specific total IgG levels and SI in immunized mice.
Splenocytes proliferation
SI value in HTCC+HEV group was significantly higher than Freund+HEV group, HEV group, HTCC group and mock group (P = 7.8 × 10−5, P = 7.2 × 10−6, P = 1.3 × 10−9 and P = 8.0 × 10−9, respectively). SI value of Freund+HEV group was also extremely significantly higher than the HEV group, HTCC group and mock group (P = 3.0 × 10−4, P = 4.0 × 10−6 and P = 3.0 × 10−7, respectively). The results suggested that HTCC and Freund's adjuvant could stimulated splenocytes proliferation when they respectively co administrated with HEV recombinant polypeptide vaccine, but HTCC had better effect ().
CD4+CD8− and CD4−CD8+ T lymphocytes subclasses
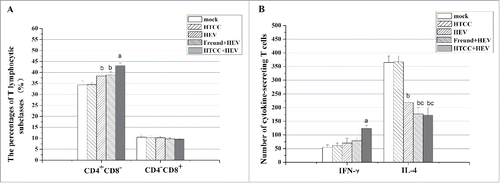
On day 56, the percentage of the cell population expressing CD4+ in the peripheral blood were significantly increased in HTCC+HEV group compared with Freund+HEV group, HEV group, HTCC group and mock group (P = 0.015, P = 0.0010, P = 0.0043 and P = 0.018, respectively). In Freund+HEV group and HEV group, the percentage of CD4+ T lymphocytes were also significantly higher than the mock group and HTCC group (P = 0.044 and P = 0.049; P = 0.014 and P = 0.030, respectively), but they had similar effects (P = 0.39) (). There were no significantly differences in the percentage of CD4−CD8+ () and the ratios of CD4+ and CD8+ T cell subclasses (data not shown) among the 5 groups (P > 0.05). These results indicated that HEV vaccine immunization with HTCC can promote stronger immune response in mice by improving CD4+CD8− helper T lymphocytes proliferation.
Figure 2. Number of T cells in immunized mice. Data are presented as mean ± SD. The error bars represent the SD with 10 mice per group per experiment. A: The percentage of CD4+ and CD8+ T lymphocytic subclasses. B: Number of cytokine-secreting T cells. a P < 0.05 in comparison with the other 4 groups. b P < 0.05 in comparison with the mock and HTCC groups. c P < 0.05 in comparison with the HEV group.
The number of producing IFN-γ or IL-4T lymphocytes
On day 56, a very significant increase in the number of producing IFN-γ T lymphocytes was observed from the samples in HTCC+HEV group compared with the other 4 groups (P = 1.8 × 10−3, P = 5.0 × 10−3, P = 3.4 × 10−3 and P = 4.0 × 10−3, respectively), while Freund+HEV group and HEV group had no difference compared with HTCC and mock group (P = 0.059 and P = 0.054; P = 0.12 and P = 0.087, respectively). Meanwhile the number of secreting IL-4 T lymphocytes in HTCC+HEV group, Freund+HEV group and HEV group were all extremely significantly lower than HTCC group and mock group (P = 1.7 × 10−5 and P = 2.5 × 10−4; P = 5.9 × 10−4 and P = 1.7 × 10−3; P = 7.9 × 10−4 and P = 1.2 × 10−3, respectively), while HTCC+HEV and Freund+HEV group were also significantly lower than HEV group (P = 0.0050 and P = 0.020), but they had no significant difference (P = 0.20) (). That meant the balanced Th1/Th2 cells response based on the cytokine production.Take together these results suggested that HTCC promoted cell-mediated immune response in immunized mice.
Discussion
Adjuvants improve vaccine effectiveness by modulating the immunogenicity of antigen delivered in a vaccine product. In addition to increasing antibody titer, immunologic adjuvants are now being engineered to alter the adaptive immune response to an antigen for shortened time to immunity, altered immune polarization and increased potency and duration.Citation1 Now alum remains the sole adjuvant approved for human use, but it has little capacity to stimulate cell-mediated immune responses.Citation2
HTCC is a cationically modified chitosan (a polymer derived from chitin through partial deacetylation). Its biological effects are similar to non-modified polymer. However, HTCC shows some unique beneficial properties.Citation3 For example, HTCC is already well-known for its better antibacterial properties overall a wide range of pH.Citation4,5 HTCC shows good solubility,Citation6 nontoxic, biocompatible and cell growth at physiological pH for tissue engineeringCitation7 and gene engineering applications (such as gene delivery vector).Citation8 HTCC has the ability to bind heparin at physiological PH.Citation9 HTCC is easily absorbed within 1h after oral administration. HTCC is distributed to lung, heart, and kidneys.Citation10 HTCC stimulates and enhances blood platelet aggregation and decreases erythrocyte deformability.Citation3 Moreover, HTCC seems to decrease both plasma total cholesterol level and LDL-cholesterol level.Citation11 At present, there are more and more researches about the immune-stimulatory effect of HTCC, such as Zaire Ebola virus glycoprotein antigenCitation12 and H5N1 influenza split antigen vaccine.Citation13,14 Lymphocyte proliferation is essential to activate both humoral and cell-mediated immune responses.Citation15 As we know, splenocytes induced by specific antigen may evaluate splenocytes immune activity.Citation15 Our results showed that HTCC, co-administrated with HEV recombinant polypeptide vaccine enhanced the splenocytes proliferation of immunized mice. This suggested that HTCC could induce a definite and clear immuno-enhancing effect on splenocytes immune activity. The results were consistent with the research provided by Fu et al.Citation16
We also examined the CD4+CD8− and CD4−CD8+ T lymphocytes subclasses in peripheral blood to investigate the immune activity of immunized mice. The CD4+CD8− T lymphocytes can enhance the ability of B lymphocytes to produce antibodies and regulate immune activity and cytokine production,Citation17 while CD4−CD8+ T lymphocytes can cause direct cytolysis of infected target cells.Citation18,19 In our study, the number of CD4+CD8− T lymphocytes in the peripheral blood in HTCC+HEV group increased, while CD4−CD8+ T cells decreased. Growths in CD4+CD8− T lymphocytes counts have been associated with increased resistance to subsequent infections.Citation20 Our results meant that HTCC was a useful tool to assist vaccine by directing the immune response toward the desired CD4+CD8− T lymphocytes response to combat pathogen.Citation21
Cytokine production of T lymphocytes reflects the subset of CD4+ T helper (Th) cells polarized in immune responses leading to the different mechanisms of host protection process.Citation22 Th1 cells secrete IFN-γ, IL-2 and IFN-β, while Th2 cells secrete IL-4 and IL-5, IL-10 and IL-13. Th1 cells protect against intracellular pathogens, activate phagocytes and promote delayed-type hypersensitivity response, whereas Th2 cells protect against extracellular pathogens, and promote humoral responses. The growths of IFN-γ-secreting T lymphocytes confirmed that HTCC tended to induce a Th1-biased immune response or cell-mediated immunity, while the reductions of T lymphocytes producing IL-4 meant the balance of humoral/cell-mediated (Th1/Th2) immune response. The similar results were provided by Wu et al.Citation12
In addition, there were no cell culture systems and susceptible HEV animal models,Citation23,24 so that we couldn't present the biological significance of these findings. Although we found Z:ZCLA Mongolian gerbils can be infected by human HEV, there were no replicated virus shedding in stools.Citation25 So now the development of HEV vaccine focuses on the recombinant polypeptide. Otherwise, we also measured the HEV-specific IgA antibodies of serum and intestine by ELISA since the mucosal surfaces of the gastrointestinal, respiratory and genitourinary tracts were the first lines of defense against the entry of the virus.Citation26 But there were all negative results (data not shown). That may be associated with a variety factors, such as the immunization route, dosage, detection methods, and need to further study.
Materials and methods
Materials
Chitosan (Lot: 20061201, deacetylation degree 94%, weight-average molecular weight 288kDa) was purchased from Qingdao Lizhong Chitin Company (Qingdao, Liaoning, CHN). 3-chloro-2-hydroxypropyltrimethylammonium chloride (CTA) was purchased from Shanxi Dasheng Company (Xian, Shanxi, CHN). HEV recombinant polypeptide vaccine that included HEV partial open reading frame 2 (ORF2) gene (394–660aa) (GenBank ID BAG09239.1) and complete ORF3 gene (GenBank ID BAG09238.1)Citation27 was prepared and kept by our laboratory.Citation28 Specific-pathogen-free female BALB/c mice 6–8 weeks old (Sino-British SIPPR/BK Lab., Animal Ltd., Shanhai, CHN) were kept at a temperature-controlled room under a 12/12 hour light/dark cycle with free access to food and water. All animal procedures were approved and performed in accordance with the Institutional Guidelines for Animal Experiments of the Animal Care and Use Committee at the Zhejiang Academy of Medical Sciences.
HTCC synthesizing
HTCC was synthesized as previous paper.Citation8 18ml 40%NaOH and 180 ml isopropanol were mixed with 12 g chitosan, and then stirred at 55°C for 5 h. After heating up to 60°C, the reactor was added 96 ml 50% CTA drop by drop and was stirred for 10 h. Then, the obtained transparent yellowish solution was precipitated in acetone and was kept at room temperature overnight. The material was dried by infrared fast dryer to constant weight. After confirming the successful HTCC synthesizing by FTIR and 1H-NMR, the DQ of HTCC was calculated by the formula .Citation29
Mice immunization experiments
50 female mice were randomly divided into 5 groups and immunized intramuscularly in hind legs on day 0. Animals were treated 50μl per hind leg of mice with HTCC (1.5 mg/ml) (HTCC group) alone or with HEV recombinant polypeptide vaccine (0.2 mg/ml) (HEV group) alone or with incomplete Freund's adjuvant (Bio Basic Inc., Toronto, CA) and HEV recombinant polypeptide vaccine (0.2 mg/ml) (Freund+HEV group) or with HTCC (1.5 mg/ml) and HEV recombinant polypeptide vaccine (0.2 mg/ml) (HTCC+HEV group) in phosphate buffered saline (PBS) or with PBS alone in the control group (mock group). On day 21, all mice were vaccinated again.
Sample collection
Blood samples were collected from the tail of mice on day 14, 28 and 56 after primary immunization, centrifuged at 800 × g, 5 min, and sera were stored at −20°C for future assays. A single-cell suspension of splenocytes was prepared as following description. A single-cell suspension of splenocytes was prepared in RMPI1640 containing 10% FBS and antibiotics (GIBCO, Invitrogen Corporation, Grand Island, NY, USA).
HEV-specific total IgG determination
Serum HEV-specific antibodies were detected by HEV-specific total IgG ELISA kit (Beijing Wantai Biological pharmaceutical co., Ltd, Beijing, CHN). Briefly, 20-fold serum samples were added in the 96-well plate and incubated at 37°C for 30min. Six washes with PBST were followed by incubation with HRP goat anti-mouse IgG (1:12000) for 30min at 37°C. After washing with PBST, 100 μl 3, 3′, 3, 5′-tetramethylbenzidine substrate was added, and the reaction was terminated with 2M sulfuric acid. The absorbance was measured at 450 nm using a Multiskan MK3 Microplate Reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples were considered positive if the OD (optical degree) sample≥ 2.1 × ODnegative.
Splenocytes proliferation analysis
Splenocytes were covered in 96-well microtiter plate (Corning Inc., Corning, NY, USA) at a density of 5 × 105 cell per well, and were stimulated with HEV recombinant polypeptide vaccine (40 μg/ml) or 1640 medium (Corning Inc., Corning, NY, USA) (negative control), respectively, then incubated for 68 h at 37°C, then added 40 μl MTS (CellTiter 96®AQueous One Soultion Cell Proliferation Assay, Promega, Madison, WI, USA) per well and incubated for another 4 h at 37°C. And the absorbance of light at 490 nm wavelength was measured using a Multiskan MK3 Microplate Reader. Stimulation index (SI) was calculated as described by Guo et al.Citation30 .
Identification of T lymphocyte subpolulations
T lymphocyte subpopulations in peripheral blood were analyzed for expression of phenotypic markers by using fluorescent antibodies specific for the PerCP-CD4, APC-CD8a, FITC-IFN-γ and PE-IL-4. The Fixation/Permeabilization kit (with BD GolgiStop™ protein transport inhibitor), phorbol myristate acetate ionomycin and 4 fluorescent antibodies were all purchased from BD PharMingen™, BD Biosciences, San Diego, CA, USA. Flow cytometry data from 10,000 cells/sample were acquired on a FACSCalibur (BD Biosciences, San Diego, CA, USA) and analyzed using the computer program CELLQuest™software (BD Biosciences, San Diego, CA, USA).
Statistical analysis
Data were summarized as mean ± SD. The IBM SPSS Statistics 20 software was used for statistical analysis. P < 0.05 was considered to be statistically different; P < 0.01 was considered to be extremely statistically different.
Abbreviations
| CTA | = | 3-chloro-2-hydroxypropyltrimethylammonium chloride |
| DQ | = | degree of quaternization |
| HTCC | = | N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride |
| 1SI10 | = | stimulation index |
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by Traditional Chinese Medicine Science and Technology Planning Project of Zhejiang Province, China (Grant Nos. 2017ZB023); Public Technology Research and Social Development Project of Zhejiang Province, China (Grant Nos. 2014C33276).
References
- Powell BS, Andrianov AK, Fusco PC. Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. Clin Exp Vaccine Res 2015; 4(1):23-45; PMID:25648619; https://doi.org/10.7774/cevr.2015.4.1.23
- Ghimire TR, Benson RA, Garside P, Brewer JM. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol Lett 2012; 147(1-2):55-62; PMID:22732235; https://doi.org/10.1016/j.imlet.2012.06.002
- Ye LL, Zeng RY, Bai Y, Roopenian DC, Zhu XP. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol 2011; 29(2):158-63; PMID:21240266; https://doi.org/10.1038/nbt.1742
- Lim SH, Hudson SM. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res 2004; 339(2):313-9; PMID:14698889; https://doi.org/10.1016/j.carres.2003.10.024
- Wen Y, Yao F, Sun F, Tan Z, Tian L, Xie L, Song Q. Antibacterial action mode of quaternized carboxymethyl chitosan/poly (amidoamine) dendrimer core-shell nanoparticles against Escherichia coli correlated with molecular chain conformation. Mater Sci Eng C Mater Biol Appl 2015; 48:220-7; PMID:25579917; https://doi.org/10.1016/j.msec.2014.11.066
- Huang J, Cheng ZH, Xie HH, Gong JY, Lou J, Ge Q, Wang YJ, Wu YF, Liu SW, Sun PL, et al. Effect of quaternization degree on physiochemical and biological activities of chitosan from squid pens. Int J Biol Macromol 2014; 70:545-50; PMID:25077838; https://doi.org/10.1016/j.ijbiomac.2014.07.017
- Aliabadi M, Dastjerdi R, Kabiri K. HTCC-modified nanoclay for tissue engineering applications: a synergistic cell growth and antibacterial efficiency. Biomed Res Int 2013; 2013:749240; PMID:23998128; https://doi.org/10.1155/2013/749240
- Zhong J, He Z, Tao W, Hong Y, Chen Y. Quaternary ammonium salt of chitosan as a gene delivery vector. J Clin Rehabilitative Tissue Engineering Res 2009; 13(12):2373-7; https://doi.org/10.3969/j.issn.1673-8225.2009.12.040
- Kaminski K, Szczubialka K, Zazakowny K, Lach R, Nowakowska M. Chitosan derivatives as novel potential heparin reversal agents. J Med Chem 2010; 53(10):4141-7; PMID:20423087; https://doi.org/10.1021/jm1001666
- Zeng L, Qin C, Wang W, Chi W, Li W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr Polym 2009; 71(3):435-40; https://doi.org/10.1016/j.carbpol.2007.06.016
- Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol 2004;55(3):503-17; PMID:15381823
- Wu Y, Wu S, Hou L, Wei W, Zhou M, Su Z, Wu J, Chen W, Ma G. Novel thermal-sensitivehydrogel enhances both humoral and cell-mediated immune responses by intranasal vaccine delivery. Eur J Pharm Biopharm 2012; 81(3):486-97; PMID:22507968; https://doi.org/10.1016/j.ejpb.2012.03.021
- Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel basedon quaternized chitosan and poly (ethylene glycol) for nasal drug deliverysystem. Biomaterials 2007; 28(13):2220-32; PMID:17291582; https://doi.org/10.1016/j.jconrel.2015.05.083
- Wu Y, Wei W, Zhou M, Wang Y, Wu J, Ma G, Su Z. Thermal-sensitive hydrogelas adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials 2012; 33(7):2351-60; PMID:22192540; https://doi.org/10.1016/j.biomaterials.2011.11.068
- Chen WX, Zhang WY, Shen WB, Wang KC. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell Immunol 2010; 262(1):69-74; PMID:20138259; https://doi.org/10.1016/j.cellimm.2010.01.001
- Fu T, Hong Y, Chen Y, He Z, Tao W. Preliminary research of 3-chlorine-2-hydroxypropyl trimethyl chitosan (HTCC)-pgD nanoparticles on immune responses to HSV-2 DNA vaccine in mice. Chinese J Health Laboratory Technol 2012; 22(2):205-10.
- Cantor H, Boyse EA. Regulation of Cellular and Humoral Immune Responses by T-cell Subclasses. Cold Spring Harbor Symposia on Quantitative Biology 1997; 41 Pt 1:23-32; PMID:302190; https://doi.org/10.1101/SQB.1977.041.01.006
- Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol 2007; 179(1):53-63; PMID:17579021; https://doi.org/10.4049/jimmunol.179.1.53
- Smith KA. The molecular mechanisms of regulatory T cell immunosuppression. Front Immunol 2012; 3:379; PMID:23248628; https://doi.org/10.3389/fimmu.2012.00379
- Pike R, Filby A, Ploquin MJ, Eksmond U, Marques R, Antunes I, Hasenkrug K, Kassiotis G. Race between Retroviral Spread and CD4+ T-Cell Response Determines the Outcome of Acute Friend Virus Infection. J Virol 2009; 83(21):11211-22; PMID:19692462; https://doi.org/10.1128/JVI.01225-09
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci USA 2009; 106(10):3877-82; PMID:19237568; https://doi.org/10.1073/pnas.0813390106
- Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol 2000; 18:709-37; PMID:10837073; https://doi.org/10.1146/annurev.immunol.18.1.709
- Wei S, Walsh P, Huang R, To SS. 93G, a novel sporadic strain of hepatitis E virus in South China isolated by cell culture. J Med Virol 2000; 61(3):311-8; PMID:10861638; https://doi.org/10.1002/1096-9071(200007)61:3<311::AID-JMV5>3.0.CO;2-H
- Zhang F, Qi Y, Harrison TJ, Luo B, Zhou Y, Li X, Song A, Huang W, Wang Y. Hepatitis E genotype 4 virus from feces of monkeys infected experimentally can be cultured in PLC/PRF/5 cells and upregulate host interferon-inducible genes. J Med Virol 2014; 86(10):1736-44; PMID:25042677; https://doi.org/10.1002/jmv.24014
- Hong Y, He ZJ, Tao W, Fu T, Wang YK, Chen Y. Experimental infection of Z:ZCLA Mongolian gerbils with human hepatitis E virus. World J Gastroenterol 2015; 21(3):862-7; PMID:25624719; https://doi.org/10.3748/wjg.v21.i3.862
- Harandi AM, Medaglini D. Mucosal adjuvants. Curr HIV Res 2010; 8(4):330-5; PMID:20353395; https://doi.org/10.2174/157016210791208695
- Aye TT, Uchida T, Ma XZ, Iida F, Shikata T, Zhuang H, Win KM. Complete nucleotide sequence of a hepatitis E virus isolated from the Xinjiang epidemic (1986-1988) of China. Nucleic Acids Res 1992; 20(13):3512; PMID:1630924; https://doi.org/10.1093/nar/20.13.3512
- Hong Y, Chen Y, Yang L, Wang Y, Jing L, Hu H. Primary research on immunogenicity of hepatitis E virus recombinant chimeric protein. Chinses J Health Laboratory Technol 2005; 15(9):1037-9; https://doi.org/10.3969/j.issn.1004-8685.2005.09.005
- Hamman JH, Kotzé AF. Effect of the type of base and number of reaction steps on the degree of quaternization and molecular weight of N-trimethyl chitosan chloride. Drug Dev Ind Pharm 2001; 27(5):373-80: PMID:11448044; https://doi.org/10.1081/DDC-100104312
- Guo XY, Jia H, Yuan WF, Zhang Q, Hou S, Sun Y, Zhu H, Sánchez-Vizcaíno JM. Construction of Swine-Specific CpG Motif Enriched Plasmid and the Study of Its Immunostimulatory Effects Both In Vitro and In Vivo. J Vet Med Sci 2012; 74(12):1647-50; PMID:22814086; https://doi.org/10.1292/jvms.11-0543