ABSTRACT
Cytomegalovirus (CMV) is a herpesvirus that induces an extremely robust and sustained immune response. For this reason, CMV has been proposed as a vaccine vector to promote immunity to both pathogens and cancer. However, exploration of CMV as a vaccine vector is at an early stage and there are many questions. Using a mouse melanoma model, we recently found that a CMV-based vaccine induced large populations of melanoma-specific T cells, but was not effective at slowing tumor growth unless it was injected directly into the tumor. These surprising results have led us to hypothesize that CMV may be adept at modulating the tumor micro-environment through its infection of macrophages. Importantly, injection of CMV into the growing tumor synergized with blockade of the PD-1 checkpoint to clear well-established tumors. Here, we discuss our results in the context of CMV-based vaccines for pathogens and cancer.
Introduction
Effective therapeutic vaccines that elicit anti-tumor immunity are rare.Citation1 These failures are due to a lack of tumor associated antigen (TAA)-specific T cell infiltration into tumors, an immune suppressive environment, TAA-specific T cell dysfunction, previous immunity to viral vectors, and highly mutable tumors that may lose TAA or MHC expression.Citation1,2 It has been hypothesized that cytomegalovirus (CMV) could be a vaccine vector that could overcome these deficiencies. CMV is a β-herpesvirus that establishes a systemic, but asymptomatic infection. Like all herpesviruses, CMV becomes latent in infected cells and persists for life. However, latency must be controlled by continuous, life-long immune surveillance. In the case of CMV infection, this immune surveillance leads to exceptionally large cellular and humoral immune responses,Citation3 including gigantic populations of virus-specific CD8+ T cells, through a process known as “memory inflation”.Citation4-10 The use of CMV as a vaccine vector, particularly for cancers, is still in its infancy, but some pre-clinical work has shown the potential of CMV-based vaccines for cancers after a systemic vaccination. Surprisingly, when we tested a similar CMV-based vaccine in a mouse model of melanoma we found no benefit until we directly infected tumors. In this review, we discuss our unexpected results and their implications for the use of CMV to modulate the tumor environment and promote anti-tumor immunity.
Memory inflation and the use of cytomegalovirus as a viral vaccine vector
CMV has several features that may make it amenable to being a viral vaccine vector. CMV is a large virus and is able to accommodate large genetic insertions in its genome. The Mocarski laboratory showed that foreign DNA could be cloned into the viral immediate early 2 (IE2) locus without disrupting viral growth.Citation11 Therefore, recombinant antigens could be added to the CMV backbone to generate targeted immune responses. CMV can re-infect previously infected hosts, so natural CMV infections should not limit vaccination. Finally, CMV infections elicit robust immune responses.Citation3 The Reddehase laboratory described the maintenance of large anti-viral CD8+ T cell populations that were sustainedCitation12 or even enrichedCitation8 after the clearance of acute murine CMV (MCMV) infection. Subsequent work by the Klenerman laboratory demonstrated that persistent MCMV infection drove the slow accumulation of certain anti-viral CD8+ T cells, a process they termed “memory inflation”.Citation13 These “inflationary” CMV-specific T cells migrate to almost any tissue in the body, including tumors,Citation14-17 and are sustained by persistent antigen stimulation, which continuously promotes effector T cell differentiation.Citation15,18 Despite virus persistence and repeated antigen stimulation, CMV-specific T cells do not become exhausted, even over decades of time in humans.Citation17,19-21 The end result of this is that CMV-specific T cell populations can become the largest T cell populations in the circulation of healthy hosts, accounting for an average of 5% of all circulating T cells.Citation22 Thus, in theory, CMV-based vaccines might generate robust, sustained immunity that does not need to be recalled, but is always in an effector state.
The first CMV-based vaccines
CMV was first proposed as a vaccine vector to promote immune contraception in wild mouse populations in Australia.Citation23 Ultimately, this was tested with an MCMV encoding the zona pellucida 3 (ZP3) antigen, which sterilized female mice.Citation24 This effect was subsequently linked to antibodies targeting ZP3.Citation25 In addition, the Klenerman laboratory demonstrated that CD8+ T cell memory inflation could be elicited to recombinant antigens encoded in the CMV backbone and that these T cell responses could be protective against virus challenges.Citation5 Together, these studies provided the “proof of concept” that CMV-based vaccines could promote protective immunity.
Since this initial work, CMV-vectored vaccines have been used to protect against Ebola,Citation26 respiratory syncytial virus (RSV),Citation27 and Mycobacterium tuberculosis (MTb)Citation28 in mouse models, and some interesting outcomes of have been described. In the RSV vaccine study, the intranasal route of vaccination was critical to park protective T cells in the lung parenchyma.Citation27 In contrast, the MTb vaccine provided resistance primarily through an NK cell response.Citation28 Most dramatically, a rhesus macaque CMV (RhCMV) encoding simian immunodeficiency virus (SIV) antigens was profoundly protective against SIV challenge in rhesus macaques.Citation29-34 Strikingly, RhCMV vaccine vectors stimulated SIV-specific CD8+ T cells that were promiscuous and recognized non-canonical SIV viral epitopes restricted to MHC-II and HLA-E molecules, an effect that was traced to both evasion of class I MHC by the RhCMV vector as well as its altered tropism compared with wild-type vectors.Citation10,29,30 Although much work remains to elucidate how each vaccine is providing protection and how the vaccine design can be manipulated to promote the most desired immune responses, these successes have sustained and propelled the field.
Cytomegalovirus as a viral vaccine vector for cancer
The successes with CMV vectors in the infectious disease setting led to its use as an anti-tumor vaccine. The Jarvis laboratory was the first to publish a study demonstrating efficacy of a CMV vector promoting cancer-specific immunity. In their study, systemic infection (via the intraperitoneal route - IP) with a recombinant MCMV vector expressing an immunogenic peptide from prostate-specific antigen (PSA) was able to induce memory inflation of PSA-specific CD8+ T cells in mice that expressed PSA as a self-antigen, and a subsequent delay in growth of a prostate tumor model expressing human PSA.Citation35 Interestingly, encoding the full-length PSA in the MCMV backbone was not as effective as encoding only the PSA65–73 peptide, a result that correlated with increased PSA-specific CD8+ T cell responses after tumor challenge. In a second study, IP infection with MCMV expressing the melanoma protein Trp-2 induced prophylactic and therapeutic protection against B16 melanomas.Citation36 Surprisingly, the protective effect of this vaccine was antibody-dependent, and T-cell-independent. Importantly however, this vaccine worked when the vector was a “single-cycle,” spread-defective version of the vaccine that could only go through one round of infection. Thus, even a vaccine safe for immune compromised patients was effective. In another study, IP infection with MCMV expressing an altered form of the melanoma gp100 epitope (gp10025-33: E25K, S27P) induced memory inflation of gp100-specific CD8+ T cells and subsequent prophylactic and therapeutic protection in a B16 lung metastasis model.Citation37 Crucially, encoding the native form of gp100 in the viral backbone abrogated these effects presumably due to its failure to stimulate gp100-specific T cell responses.Citation37 Finally, a recent study has shown that MCMV encoding epitopes from the E6 and E7 proteins of human papillomavirus, delayed or prevented subsequent growth of the E6 and E7 expressing TC-1 tumor cells in a prophylactic setting.Citation38 Collectively, these experiments demonstrated that CMV-based cancer vaccines could be effective, but may work through diverse mechanisms depending on the antigen encoded in the genome. Thus, determining how to design vectors to promote the most desired anti-tumor immune responses is crucial to move the field forward, and this is an area of active investigation.Citation38-44
Intratumoral MCMV infection
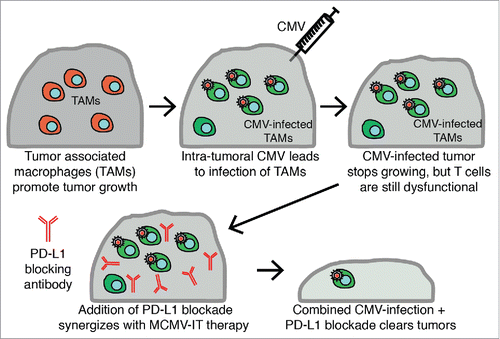
Keeping in line with the studies described above, we generated an MCMV-gp100 vaccine for melanoma using a modified gp100 peptide antigen (gp10025-33: S27P), which promoted robust gp100-specific CD8+ T cell memory inflation.Citation45 Given that a similar MCMV-gp100 vaccine delayed the growth of B16 lung nodulesCitation37 we expected to observe some impact on the growth of subcutaneously-implanted B16 melanomas. Surprisingly however, the IP route of vaccination did not delay the growth of subcutaneously-implanted B16 tumors, nor prolong mouse survival, despite promoting substantial numbers of tumor-localized gp100-specific T cells. Unsurprisingly, gp100-specific T cells recovered from the tumors were profoundly functionally impaired. We had previously shown that most “inflationary” T cells were confined to the blood and that many were stuck in the vasculature of the lungs,Citation14 where they might have access to tumor nodules delivered by intravenous (IV) injection, possibly explaining the different outcomes. Thus, we wondered whether vaccination directly into the tumor (intratumoral - IT) would better recruit T cells to the most needed site. While the IT route of infection did not increase T cell recruitment to the tumors, it caused a striking delay in tumor growth (45, summarized in ). We do not believe that the virus was directly killing tumor cells (i.e. acting as an oncolytic virus) as the only infected cells that we could find in the tumor were tumor-associated macrophages (TAMs).Citation45 Additionally, CD8+ T cells were required for the therapeutic effect. Thus, we currently hypothesize that IT delivery of MCMV alters TAMs in such a way as to boost CD8+ T cells responses. Interestingly, the function of gp100-specific T cells in the tumor was still impaired after IT vaccination.45 Thus, to improve the treatment, IT delivery of MCMV was combined with PD-1/PD-L1 checkpoint inhibition (, Citation45). These therapies robustly synergized leading to tumor clearance and long-term protection in ∼60% of the mice. Most surprisingly, the MCMV-gp100 vaccine was only marginally better than the wild-type MCMV at delaying tumor growth or synergizing with PD-L1 blockade. Thus, even an MCMV that did not specifically promote gp100-specific T cells was able to induce a nearly identical tumor growth delay.Citation45 We interpret these results to suggest that the major effect of MCMV was not to prime tumor-specific T cells, but rather to shift the tumor to a more pro-inflammatory, anti-tumor microenvironment.
Figure 1. Schematic of Intratumoral CMV therapy. Tumor-associated macrophages (TAM) in tumors help to promote and drive tumor growth. After injecting CMV intratumorally, CMV infected TAMs and this correlated with a delay in tumor growth. Combining this intratumoral CMV infection of TAM with anti-PD-L1 therapy synergized to induce clearance of more than 60% of tumors and long-term protection.
Intratumoral cancer therapies
Intratumoral administration of cancer therapeutics has been around for more than a century. Dr. William Coley first documented the use of IT therapy in the late 1800s when he isolated the bacteria that caused erysipelas and used it to treat solid tumors.Citation46 In 1975, successful treatment of a man with advanced melanoma using IT Bacille Calmette-Guérin (BCG) reignited the field.Citation47,48 Chemotherapy, gene therapy, oncolytic viruses and immunotherapy have now been tested with varying success as IT therapies.Citation46,47,49-53 IT chemotherapy and gene therapy can lessen their side effects such as non-specific cytotoxicity and severe systemic inflammation respectively. Although usually limited to easily accessible tumors, minimally invasive surgery has been used to administer IT therapies to unresectable tumors (e.g. Citation54).
Recent work has highlighted the potential for IT delivered oncolytic viruses. IT delivery of Herpes Simplex Virus T-VEC, which encodes GM-CSF, to metastatic melanoma became the first FDA approved oncolytic virus in the US in 2015.Citation51 Other oncolytic HSVs that encode microRNA targets, tissue-specific promoters, or targeted attachment proteins are currently in development. In addition to T-VEC, the oncolytic adenovirus H101 is approved in China for IT administration in combination with chemotherapy for head and neck cancer.Citation51 Additionally, the GM-CSF encoding vaccinia virus Pexavec and the coxsackievirus A21 drug Cavatek are both in clinical trials as oncolytic IT therapies.Citation51,52 T-VEC, H101, Pexavec, and Cavatek all work primarily by directly infecting and lysing tumor cells when delivered IT. Our data suggest that MCMV did not work in this way and in fact, it may not have infected tumor cells much at all.
MCMV infection of tumor associated macrophages
Unlike oncolytic viruses, MCMV delivered IT seemed to primarily infect tumor associated macrophages (TAMs), which are crucial for tumor immune evasion and outgrowth and are now being targeted in several clinical trials.Citation55 Thus, we speculate that MCMV infection of TAMs may have altered TAMs to enable improved tumor-specific immune responses (). There is a clear correlation between tumor infiltration by macrophages and poor clinical prognosis in several cancersCitation55-57 and depletion of macrophages with clodronate in mouse models has demonstrated the importance of macrophages in tumor progression and development.Citation58 Typically, TAMs are divided into pro-inflammatory (‘M1-like') and anti-inflammatory (‘M2-like'). Pro-inflammatory TAMs are thought to promote tumor-specific immunity that can eliminate tumor cells or hold tumors in equilibrium (). Aside from producing pro-inflammatory cytokines, and other inflammatory mediators like reactive oxygen and nitrogen species, pro-inflammatory TAMs also have high expression of MHC-I and II, enabling potent antigen presentation and activation of T cells.Citation55,59 However, tumor progression and late stages of tumor growth are often associated with elevated numbers of anti-inflammatory, ‘M2-like' macrophages.Citation55,59 Anti-inflammatory, ‘M2-like' TAMs produce high levels of IL-10 and TGF-β, lose the expression of pro-inflammatory TH1 cytokines and MHC-II, express high levels of PD-L1, and contribute to poor CD8+ T cell responses.Citation55,57,59 Furthermore, anti-inflammatory, ‘M2-like' TAMs can release CCL22, causing TREG infiltration, as well as CCL2, which causes an accumulation of TAMs, and are able to help induce and seed metastasis.Citation55,57,59 Thus, TAMs are an important part of the tumor microenvironment ().
Figure 2. Tumor associate macrophage and changes induced by CMV infection. A) Pro-inflammatory tumor associated macrophages (TAM) express high levels of MHC-I, MHC-II and release pro-inflammatory cytokines. These attributes can contribute to T cell activation and proliferation, and subsequent anti-tumor effects. B) Tumor progression is associated with an accumulation of anti-inflammatory TAMs, which express lower levels of MHC-I, MHC-II and proinflammatory cytokines and increase expression of anti inflammatory cytokines, leading to diminished T cell responses. Additionally, anti-inflammatory TAMS release VEGF, which induces angiogenesis, CCL2, which can induce the accumulation of anti-inflammatory macrophages, and CCL22, which can induce TREG infiltration. Together, these attributes contribute to tumor progression. C) After IT infection with MCMV, TAM became infected. CMV infection of macrophages is known to promote a shift toward a more pro-inflammatory phenotype. Additionally, CMV encodes its own virally-encoded chemokines, which attract inflammatory macrophages. Thus, although more work is needed to define the changes in TAM, we hypothesize that MCMV infection of TAMs leads to increased T cell activation and proliferation and increased recruitment of pro-inflammatory macrophages to the tumor. Together, these attributes of TAM MCMV infection may shift the tumor microenvironment from anti-inflammatory to pro-inflammatory.
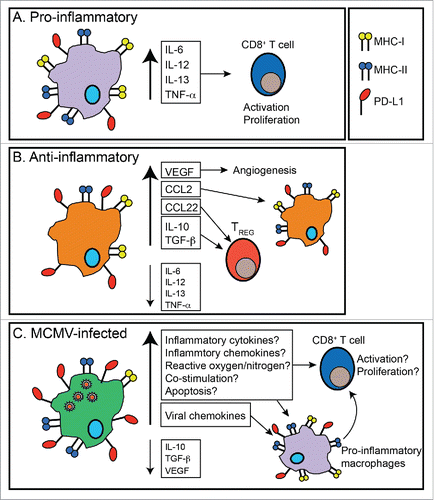
Macrophages are also important targets for CMV biology during normal viral infection (). Both MCMV and HCMV establish latency in macrophages, monocytes and progenitors of monocytes and dendritic cells.Citation60-72 Moreover, CMVs express several chemokines, which can directly or indirectly recruit inflammatory monocytes to the site of infection.Citation73-79 In addition, HCMV promotes monocyte differentiation into immune-stimulatory, ‘M1-like' macrophagesCitation80,81 and preferentially infects immune-suppressive ‘M2-like' macrophages over ‘M1-like' macrophages.Citation82 After HCMV infection, macrophages release pro-inflammatory cytokines, have increased expression of toll-like receptors (TLRs) and improved MyD88 signaling,Citation80-84 leading to increased T cell proliferation and function. Thus, MCMV infection of TAMs and recruitment of new pro-inflammatory TAMs, may shift the tumor microenvironment from immune suppressive to pro-inflammatory. MCMV infection of TAMs also may induce their apoptosis, leading to the delayed tumor growth. This, however, is unlikely in our model as there were large percentages of TAMs present in MCMV IT infected tumors 5 d after the initial infection.Citation45 Additionally, MCMV infection may decrease the ability of TAMs to induce pro-angiogenic factors such as VEGF, leading to less vascularization of the tumor and slowed tumor growth. However, it must be noted that MCMV infection of macrophages may also increase VEGF production, leading to increased vascularization,Citation85 an effect that could promote, rather than inhibit, tumor growth. Thus, the specific interactions between CMV and the tumor environment must be dissected so that the desired outcomes can be promoted ().
The interaction between MCMV and TAMs may also suggest additional avenues of synergy. For example, if MCMV IT therapy skews TAMs toward pro-inflammatory responses or depletes them, then synergy may be achieved by combining MCMV IT therapy with a drug like Paclitaxel, a common chemotherapy that enhances the infiltration of TAMs in breast cancer.Citation86 Likewise, since Cox-2 expression in the tumor microenvironment correlates with anti-inflammatory TAMs, the combination of IT-MCMV and Cox2 inhibitors may be synergistic by extending the therapeutic effect beyond the directly infected cells, to further decrease anti-inflammatory TAMs.Citation87-89
It is also possible that the PD-L1 blocking antibody was synergistic because it could alter TAMs. Indeed, PD-L1 expression was increased on both haematopoietic and non-haematopoietic cells after IT-MCMV infection.Citation45 Thus, it is possible that the anti-PD-L1 antibody may be depleting PD-L1hi TAMs, as seen with other checkpoint inhibitors,Citation90-92 or altering TAMs by disrupting the PD-1/PD-L1 signaling, which has been linked to an increase in immune suppressive TAMs.Citation87,88
Finally, improving CMV's ability to kill macrophages may be ideal for improving the therapy. CMV delays apoptosis and programmed necrosis of infected macrophages in vitro through the expression of several genes.Citation93 Using viruses deficient in one or more of these genes might kill TAMs more quickly and have a larger effect if loss of TAMs is part of the therapeutic effect. In addition, CMVs lacking the genes that prevent cell death are much less pathogenic and therefore would be expected to be safer. Together, these approaches may offer ways to improve the efficacy and/or safety of MCMV IT therapy and may also offer a better understanding of the impact of MCMV on the tumor environment.
MCMV IT therapy's effect on CD8+ T cells
As CD8+ T cells were crucial for prolonged survival after MCMV IT therapy, and the therapy synergized with PD-L1 blockade, the effect of the on CD8+ T cells must be determined. It was surprising that IT delivery of both wild-type MCMV and MCMV-gp100 had nearly equivalent impacts on tumor growth and equivalent abilities to synergize with PD-L1 blockade. These data imply that IT delivery of MCMV improves the function of pre-existing tumor-specific T cells, or enables priming of new tumor-specific T cell responses. We favor the former hypothesis because IT delivery of wild-type MCMV did not promote expansion of Pmel-I transgenic (gp100-specific) T cells except in one animal.Citation45
Stimulation of the innate immune system by CMV
Although our data suggest that IT delivery of MCMV worked through the adaptive immune system, there is substantial data arguing for an effect of MCMV and HCMV infection on the innate immune system. Both MCMV and HCMV can promote robust NK cell activation, although our data suggest that NK cells were not crucial in the efficacy of IT MCMV infection in the B16 melanoma model.Citation45 Likewise, CMV has been shown to promote γδ-T cells that are protective against CMV infection and can also cross-react with cancer cells, presumably due to similar expression of stress-induced ligands by infected cells and cancer cells.Citation94 Interestingly, early work from the Reddehase laboratory in a model of liver-adapted lymphoma showed that MCMV infection could induce apoptosis of the tumor cells in a manner that still remains unclear, but is not due to viral infection of the tumor cells, or the induction of T cells, B cells or NK cells.Citation95-97 Thus, other innate immune activation and alternative mechanisms to trigger tumor cell death cannot be ruled out at this time.
The Future of CMV IT therapy
MCMV IT therapy preferentially infected TAMs and induced CD8+ T cell- dependent improved survival of tumor bearing animals. The therapy synergized with anti-PD-L1 therapy, inducing tumor clearance and protection in over half the animals. Thus, MCMV IT therapy represents a potentially powerful anti-tumor treatment. However, there are many unknowns. The importance and effect of TAMs infection on the tumor microenvironment and the subsequent induction of CD8+ T cell dependent responses must be investigated to be able to improve the therapy and define the mechanisms of action. Additionally, we need to know whether the IT CMV therapy will remain effective with killed, replication-defective or spread-defective versions of CMV to develop a safe clinical reagent. It will be important to determine whether altering the immune response at one tumor site will have effects on distant tumor sites and metastases. Otherwise, the therapy would be restricted to tumors that are available for injection. Lastly, it is crucial to determine whether IT CMV therapy is effective in a multitude of different cancers, besides melanoma. Since macrophages may play distinct roles in different cancers, this type of therapy is unlikely to be equally effective in all tumor types. In sum, MCMV IT therapy represents a potentially potent therapy by acting directly on tumor-associated macrophages, but it must be better understood for future improvement.
Disclosure of potential conflicts of interest
C.M.S. has a financial interest in UbiVac CMV, for the development of spread-defective, CMV-based therapeutics.
References
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: Are we there yet? Immunol Rev 2011; 239(1):27-44; PMID:21198663; https://doi.org/10.1111/j.1600-065X.2010.00979.x
- Saxena M, Van TT, Baird FJ, Coloe PJ, Smooker PM. Pre-existing immunity against vaccine vectors–friend or foe? Microbiology 2013; 159(Pt 1):1-11; PMID:23175507; https://doi.org/10.1099/mic.0.049601-0
- Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16(6):367-77; PMID:27108521; https://doi.org/10.1038/nri.2016.38
- Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse cd8 t cell response to murine cytomegalovirus. J Immunol 2006; 176:3760-6; PMID:16517745; https://doi.org/10.4049/jimmunol.176.6.3760
- Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol 2004; 78(5):2255-64; PMID:14963122; https://doi.org/10.1128/JVI.78.5.2255-2264.2004
- Komatsu H, Sierro S, V Cuero A, Klenerman P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin Exp Immunol 2003; 134(1):9-12; PMID:12974748; https://doi.org/10.1046/j.1365-2249.2003.02266.x
- Holtappels R, Grzimek NK, Simon CO, Thomas D, Dreis D, Reddehase MJ. Processing and presentation of murine cytomegalovirus pORFm164-derived peptide in fibroblasts in the face of all viral immunosubversive early gene functions. J Virol 2002; 76(12):6044-53; PMID:12021337; https://doi.org/10.1128/JVI.76.12.6044-6053.2002
- Holtappels R, et al. Enrichment of immediate-early 1 (m123/pp89) peptide-specific cd8 t cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 2000; 74(24):11495-503; https://doi.org/10.1128/JVI.74.24.11495-11503.2000
- Simon CO, Holtappels R, Tervo HM, Böhm V, Däubner T, Oehrlein-Karpi SA, Kühnapfel B, Renzaho A, Strand D, Podlech J, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol 2006; 80(21):10436-56; PMID:16928768; https://doi.org/10.1128/JVI.01248-06
- Quinn M, Erkes DA, Snyder CM. Cytomegalovirus and immunotherapy: Opportunistic pathogen, novel target for cancer and a promising vaccine vector. Immunotherapy 2016; 8(2):211-21; PMID:26786895; https://doi.org/10.2217/imt.15.110
- Cardin RD, Abenes GB, Stoddart CA, Mocarski ES. Murine cytomegalovirus IE2, an activator of gene expression, is dispensable for growth and latency in mice. Virology 1995; 209(1):236-41; PMID:7747475; https://doi.org/10.1006/viro.1995.1249
- Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: Persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 2000; 74(16):7496-507; PMID:10906203; https://doi.org/10.1128/JVI.74.16.7496-7507.2000
- Karrer R, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory Inflation: Continuous accumulation of antiviral CD8 + T cells over time. J Immunol 2003; 170:2022-9; PMID:12574372; https://doi.org/10.4049/jimmunol.170.4.2022
- Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog 2014; 10(7):e1004233; PMID:24992722; https://doi.org/10.1371/journal.ppat.1004233
- Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep 2015; 13(6):1137-48; PMID:26526996; https://doi.org/10.1016/j.celrep.2015.09.076
- Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol 2005; 35(4):1113-23; PMID:15756645; https://doi.org/10.1002/eji.200425534
- Erkes DA, Smith CJ, Wilski NA, Caldeira-Dantas S, Mohgbeli T, Snyder CM. Virus-specific CD8+ T cells infiltrate melanoma lesions and retain function independently of PD-1 expression. J Immunol 2017; 198(7):2979-88; PMID:28202614; https://doi.org/10.4049/jimmunol.1601064
- Quinn M, Turula H, Tandon M, Deslouches B, Moghbeli T, Snyder CM. Memory T cells specific for murine cytomegalovirus re-emerge after multiple challenges and recapitulate immunity in various adoptive transfer scenarios. J Immunol 2015; 194(4):1726-36; PMID:25595792; https://doi.org/10.4049/jimmunol.1402757
- Hertoghs KM, Moerland PD, van Stijn A, Remmerswaal EB, Yong SL, van de Berg PJ, van Ham SM, Baas F, ten Berge IJ, van Lier RA. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J Clin Invest 2010; 120(11):4077-90; PMID:20921622; https://doi.org/10.1172/JCI42758
- Snyder CM, Loewendorf A, Bonnett EL, Croft M, Benedict CA, Hill AB. CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol 2009; 183(6):3932-41; PMID:19692644; https://doi.org/10.4049/jimmunol.0900227
- Walton S, Mandaric S, Oxenius A. CD4 T cell responses in latent and chronic viral infections. Front Immunol 2013; 4:105; PMID:23717308; https://doi.org/10.3389/fimmu.2013.00105
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202(5):673-85; PMID:16147978; https://doi.org/10.1084/jem.20050882
- Shellam GR. The potential of murine cytomegalovirus as a viral vector for immunocontraception. Reprod Fertil Dev 1994; 6(3):401-9; PMID:7831489; https://doi.org/10.1071/RD9940401
- Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol Reprod 2003; 68(6):2024-32; PMID:12606395; https://doi.org/10.1095/biolreprod.102.012880
- Lloyd ML, Papadimitriou JM, O'Leary S, Robertson SA, Shellam GR. Immunoglobulin to zona pellucida 3 mediates ovarian damage and infertility after contraceptive vaccination in mice. J Autoimmun 2010; 35(1):77-85; PMID:20382503; https://doi.org/10.1016/j.jaut.2010.03.002
- Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine 2015; 33(19):2261-6; PMID:25820063; https://doi.org/10.1016/j.vaccine.2015.03.029
- Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol 2017; 10(2):545-54; PMID:27220815; https://doi.org/10.1038/mi.2016.48
- Beverley PC, Ruzsics Z, Hey A, Hutchings C, Boos S, Bolinger B, Marchi E, O'Hara G, Klenerman P, Koszinowski UH, et al. A novel murine cytomegalovirus vaccine vector protects against mycobacterium tuberculosis. J Immunol 2014; 193(5):2306-16; PMID:25070842; https://doi.org/10.4049/jimmunol.1302523
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 2016; 351(6274):714-20; PMID:26797147; https://doi.org/10.1126/science.aac9475
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 2013; 340(6135):1237874; PMID:23704576; https://doi.org/10.1126/science.1237874
- Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502(7469):100-4; PMID:24025770; https://doi.org/10.1038/nature12519
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473(7348):523-7; PMID:21562493; https://doi.org/10.1038/nature10003
- Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 2010; 328(5974):102-6; PMID:20360110; https://doi.org/10.1126/science.1185350
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15(3):293-9; PMID:19219024; https://doi.org/10.1038/nm.1935
- Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother 2012; 35(5):390-9; PMID:22576344; https://doi.org/10.1097/CJI.0b013e3182585d50
- Xu G, Smith T, Grey F, Hill AB. Cytomegalovirus-based cancer vaccines expressing TRP2 induce rejection of melanoma in mice. Biochem Biophys Res Commun 2013; 437(2):287-91; PMID:23811402; https://doi.org/10.1016/j.bbrc.2013.06.068
- Qiu Z, Huang H, Grenier JM, Perez OA, Smilowitz HM, Adler B, Khanna KM. Cytomegalovirus based vaccine expressing a modified tumor antigen induces potent tumor-specific CD8+ T cell response and protects mice from melanoma. Cancer Immunol Res 2015; 3(5):1-11; PMID:25568067; https://doi.org/10.1158/2326-6066.CIR-14-0044
- Dekhtiarenko I, Ratts RB, Blatnik R, Lee LN, Fischer S, Borkner L, Oduro JD, Marandu TF, Hoppe S, Ruzsics Z, et al. Peptide processing is critical for T-cell memory inflation and may be optimized to improve immune protection by CMV-based vaccine vectors. PLoS Pathog 2016; 12(12):e1006072; PMID:27977791; https://doi.org/10.1371/journal.ppat.1006072
- Colston JM, Bolinger B, Cottingham MG, Gilbert S, Klenerman P. Modification of antigen impacts on memory quality after adenovirus vaccination. J Immunol 2016; 196(8):3354-63; PMID:26944930; https://doi.org/10.4049/jimmunol.1502687
- Turula H, Smith CJ, Grey F, Zurbach KA, Snyder CM. Competition between T cells maintains clonal dominance during memory inflation induced by MCMV. Eur J Immunol 2013; 43(5):1252-63; PMID:23404526; https://doi.org/10.1002/eji.201242940
- Farrington LA, Smith TA, Grey F, Hill AB, Snyder CM. Competition for antigen at the level of the APC is a major determinant of immunodominance during memory inflation in murine cytomegalovirus infection. J Immunol 2013; 190(7):3410-6; PMID:23455500; https://doi.org/10.4049/jimmunol.1203151
- Dekhtiarenko I, Jarvis MA, Ruzsics Z, Čičin-Šain L. The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J Immunol 2013; 190(7):3399-409; PMID:23460738; https://doi.org/10.4049/jimmunol.1203173
- Hutchinson S, Sims S, O'Hara G, Silk J, Gileadi U, Cerundolo V, Klenerman P. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PLoS One 2011; 6(2):e14646; PMID:21304910; https://doi.org/10.1371/journal.pone.0014646
- Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kühnapfel B, Däubner T, Emde SF, Podlech J, Grzimek NK, et al. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol 2008; 82(12):5781-96; PMID:18367531; https://doi.org/10.1128/JVI.00155-08
- Erkes DA, Xu G, Daskalakis C, Zurbach KA, Wilski NA, Moghbeli T, Hill AB, Snyder CM. Intratumoral infection with murine cytomegalovirus synergizes with PD-L1 blockade to clear melanoma lesions and induce long-term immunity. Mol Ther 2016; 24(8):1444-55; In Press; PMID:27434584; https://doi.org/10.1038/mt.2016.121
- Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: A new paradigm for cancer therapy. Clin Cancer Res 2014; 20(7):1747-56; PMID:24691639; https://doi.org/10.1158/1078-0432.CCR-13-2116
- Agarwala SS. Intralesional therapy for advanced melanoma: Promise and limitation. Curr Opin Oncol 2015; 27(2):151-6; PMID:25629369; https://doi.org/10.1097/CCO.0000000000000158
- Mastrangelo MJ, Bellet RE, Berkelhammer J, Clark WH Jr. Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer 1975; 36(4):1305-8; PMID:1175129; https://doi.org/10.1002/1097-0142(197510)36:4%3c1305::AID-CNCR2820360417%3e3.0.CO;2-
- Cheok CF. Protecting normal cells from the cytotoxicity of chemotherapy. Cell Cycle 2012; 11(12):2227-32; PMID:22684296; https://doi.org/10.4161/cc.20961
- Gottesman MM. Cancer gene therapy: An awkward adolescence. Cancer Gene Ther 2003; 10(7):501-8; PMID:12833130; https://doi.org/10.1038/sj.cgt.7700602
- Russell SJ, Peng KW. Oncolytic virotherapy: A contest between apples and oranges. Mol Ther 2017; 25(5):1107-16; PMID:28392162; https://doi.org/10.1016/j.ymthe.2017.03.026
- Bommareddy PK, Silk AW, Kaufman HL. Intratumoral approached for the treatment of melanoma. Cancer K 2017; 23(1):40-7; PMID:28114253; https://doi.org/10.1097/PPO.0000000000000234
- Weide B, Martens A, Wistuba-Hamprecht K, Zelba H, Maier L, Lipp HP, Klumpp BD, Soffel D, Eigentler TK, Garbe C. Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma-safety and efficacy in a phase II study. Cancer Immunol Immunother 2017; 66(4):441-9; PMID:28008452; https://doi.org/10.1007/s00262-016-1944-0
- Bruno MJ. Interventional endoscopic ultrasonography: Where are we headed? Dig Endosc 2017; 29(4):503-11; PMID:28181708; https://doi.org/10.1111/den.12842
- Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; [epub ahead of print]; PMID:28117416; https://doi.org/10.1038/nrclinonc.2016.217
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18(5):349-55; PMID:18467122; https://doi.org/10.1016/j.semcancer.2008.03.004
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12(4):253-68; PMID:22437938; https://doi.org/10.1038/nri3175
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: A new and highly effective antiangiogenic therapy approach. Br J Cancer 2006; 95(3):272-81; PMID:16832418; https://doi.org/10.1038/sj.bjc.6603240
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014; 6(3):1670-90; PMID:25125485; https://doi.org/10.3390/cancers6031670
- Koffron AJ, Hummel M, Patterson BK, Yan S, Kaufman DB, Fryer JP, Stuart FP, Abecassis MI. Cellular localization of laten murine cytomegalovirus. J Virol 1998; 72(1):95-103; PMID:9420204
- Hahn G, Jores R, Mocarkski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. PNAS 1998; 95:3937-42; PMID:9520471; https://doi.org/10.1073/pnas.95.7.3937
- Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol 1996; 77:3099-102; PMID:9000102; https://doi.org/10.1099/0022-1317-77-12-3099
- Kondo K, Xu J, Mocarkski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. PNAS 1996; 93:11137-42; PMID:8855322; https://doi.org/10.1073/pnas.93.20.11137
- Kondo K, Kaneshima H, Mocarkski ES. Human cytomegalovirus latent infection of granulovyte-macrophage progenitors. PNAS 1994; 91:11879-83; PMID:7991550; https://doi.org/10.1073/pnas.91.25.11879
- Sindre H, Tjøonnfjord GE, Rollag H, Ranneberg-Nilsen T, Veiby OP, Beck S, Degré M, Hestdal K. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood 1996; 88(12):4526-33; PMID:8977244
- Reevers MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A 2005; 102(11):4140-5; PMID:15738399; https://doi.org/10.1073/pnas.0408994102
- Pollock JL, Presti RM, Paetzold S. Virgin HW 4th., Latent Murine Cytomegalovirus Infection in Macrophages. Virology 1997; 227:168-79; PMID:9007070; https://doi.org/10.1006/viro.1996.8303
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of huma cytomegalovirus in peripharl blood mononuclear cells. J Gen Virol 1991; 72:2059-64; PMID:1654370; https://doi.org/10.1099/0022-1317-72-9-2059
- Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate disseminiation of murine cytomegalovirus. J Virol 1994; 68(10):6243-53; PMID:8083964
- Slobedman B, Mocarkski ES. Quantitative analysis of latent human cytomegalovirus. J Virol 1999; 73(6):4806-12; PMID:10233941
- Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997; 91:119-26; PMID:9335340; https://doi.org/10.1016/S0092-8674(01)80014-3
- Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol 2001; 75(16):7543-54; PMID:11462026; https://doi.org/10.1128/JVI.75.16.7543-7554.2001
- Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe 2014; 15(3):351-62; PMID:24629341; https://doi.org/10.1016/j.chom.2014.02.002
- Daley-Bauer LP, Wynn GM, Mocarski ES. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity 2012; 37(1):122-33; PMID:22840843; https://doi.org/10.1016/j.immuni.2012.04.014
- Noda S, Aguirre SA, Bitmansour A, Brown JM, Sparer TE, Huang J, Mocarski ES. Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood 2006; 107(1):30-8; PMID:16046529; https://doi.org/10.1182/blood-2005-05-1833
- Zheng Q, Tao R, Gao H, Xu J, Shang S, Zhao N. HCMV-encoded UL128 enhances TNF-alpha and IL-6 expression and promotes PBMC proliferation through the MAPK/ERK pathway in vitro. Viral Immunol 2012; 25(2):98-105; PMID:22486303; https://doi.org/10.1089/vim.2011.0064
- Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol 2006; 80(23):11539-55; PMID:16987970; https://doi.org/10.1128/JVI.01016-06
- Hong SS, Choi JH, Lee SY, Park YH, Park KY, Lee JY, Kim J, Gajulapati V, Goo JI, Singh S, et al. A novel small-molecule inhibitor targeting the IL-6 receptor beta subunit, glycoprotein 130. J Immunol 2015; 195(1):237-45; PMID:26026064; https://doi.org/10.4049/jimmunol.1402908
- Gao H, Tao R, Zheng Q, Xu J, Shang S. Recombinant HCMV UL128 expression and functional identification of PBMC-attracting activity in vitro. Arch Virol 2013; 158(1):173-7; PMID:22851009; https://doi.org/10.1007/s00705-012-1558-6
- Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol 2008; 181:698-711; PMID:18566437; https://doi.org/10.4049/jimmunol.181.1.698
- Chan G, Nogalski MT, Yurochko AD. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J Virol 2012; 86(19):10714-23; PMID:22837201; https://doi.org/10.1128/JVI.07129-11
- Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Söderberg-Naucler C, Mertens T, Frascaroli G. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. J Virol 2013; 87(1):67-79; PMID:23055571; https://doi.org/10.1128/JVI.01585-12
- Yamaguchi T, Shinagawa Y, Pollard RB. Relationship between the produciton of murine cytomegalovirus and inferferon in macrophages. J Gen Virol 1988; 69:2961-71; PMID:2462012; https://doi.org/10.1099/0022-1317-69-12-2961
- Smith PD, Shimamura M, Musgrove LC, Dennis EA, Bimczok D, Novak L, Ballestas M, Fenton A, Dandekar S, Britt WJ, et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J Immunol 2014; 193(11):5604-12; PMID:25355920; https://doi.org/10.4049/jimmunol.1302608
- Cousins SW, Espinosa-Heidmann DG, Miller DM, Pereira-Simon S, Hernandez EP, Chien H, Meier-Jewett C, Dix RD. Macrophage activation associated with chronic murine cytomegalovirus infection results in more severe experimental choroidal neovascularization. PLoS Pathog 2012; 8(4):e1002671; PMID:22570607; https://doi.org/10.1371/journal.ppat.1002671
- Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 2015; 212(4):435-45; PMID:25753580; https://doi.org/10.1084/jem.20150295
- Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, Li W, Wu G, Ren J, Wang Z, et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016; 5(2):e1074374; PMID:27057439; https://doi.org/10.1080/2162402X.2015.1074374
- Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 2015; 162(6):1257-70; PMID:26343581; https://doi.org/10.1016/j.cell.2015.08.015
- Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A 2017; 114(5):1117-22; PMID:28096371; https://doi.org/10.1073/pnas.1612920114
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124(2):687-95; PMID:24382348; https://doi.org/10.1172/JCI67313
- Schalper K, et al. Clinical significance of PD-L1 protein expression on tumor-associated macrophages in lung cancer. J Immuno Ther Cancer 2015; 3(Suppl 2):P415; https://doi.org/10.1186/2051-1426-3-S2-P415
- Zippelius A, Schreiner J, Herzig P, Müller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res 2015; 3(3):236-44; PMID:25623164; https://doi.org/10.1158/2326-6066.CIR-14-0226
- Handke W, Krause E, Brune W. Live or let die: Manipulation of cellular suicide programs by murine cytomegalovirus. Med Microbiol Immunol 2012; 201(4):475-86; PMID:22965170; https://doi.org/10.1007/s00430-012-0264-z
- Khairallah C, Dechanet-Merville J, Capone M. γδ T cell-mediated immunity to cytomegalovirus infection. Front Immunol 2017; 8(105):1-10; PMID:28232834; https://doi.org/10.3389/fimmu.2017.00105
- Erlach KC, Böhm V, Knabe M, Deegen P, Reddehase MJ, Podlech J. Activation of hepatic natural killer cells and control of liver-adapted lymphoma in the murine model of cytomegalovirus infection. Med Microbiol Immunol 2008; 197(2):167-78; PMID:18309517; https://doi.org/10.1007/s00430-008-0084-3
- Erlach KC, Böhm V, Seckert CK, Reddehase MJ, Podlech J. Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection. J Virol 2006; 80(10):4801-19; PMID:16641273; https://doi.org/10.1128/JVI.80.10.4801-4819.2006
- Erlach KC, Podlech J, Rojan A, Reddehase MJ. Tumor control in a model of bone marrow transplantation and acute liver-infiltrating B-cell lymphoma: An unpredicted novel function of cytomegalovirus. J Virol 2002; 76(6):2857-70; PMID:11861853; https://doi.org/10.1128/JVI.76.6.2857-2870.2002
