ABSTRACT
Allergic diseases are reaching epidemic proportions in developed countries. In particular, food allergy is increasing in prevalence and severity, thus becoming an important socioeconomic burden. Numerous cell types and cell populations, which form an intricate and balanced network, are involved in an immune response. This balance is occasionally disturbed, leading to the onset of different diseases, such as allergic diseases. Antihistamines and corticosteroids provide some degree of relief from the symptoms of allergic conditions. However, the only treatment that can revert the disease is immunotherapy. Nevertheless, specific immunotherapy has at least 2 major drawbacks: it is time-consuming, and it can produce local and even systemic allergic side effects. Immunotherapy's potential goes beyond our current knowledge of the immune response; nevertheless, we can still design strategies to reach a safer immune modulation for treating allergies. This review deals with the use of adjuvants to reduce the undesirable side effects associated with specific allergen immunotherapy. For example, nanoparticles used as immunoadjuvants are offering promising results in preclinical assays.
Introduction, allergic diseases
Allergy represents the most prevalent disease in developed countries with a current rate of up to 30% in industrialized nations. There are robust epidemiological data demonstrating a steady increase in the prevalence of asthma, rhinitis and atopic dermatitis, Citation1 as well as an alarming increase in food allergy around the world. Citation2 These changes are reflected not only by a surge in allergic conditions, but also by an increase in the severity of allergic reactions Citation3-Citation5 with hen's eggs, cow's milk, and nuts being the foods most frequently associated with anaphylactic reactions. Allergic diseases have a serious impact on quality of life and represent a very significant socioeconomic burden Citation6,Citation7 due to healthcare costs, Citation8,Citation9 and decreased productivity, poor school performance, or absenteeism. Citation1 This is because allergic diseases have a high prevalence in children and young adults. Factors influencing this pattern of increasing risk of developing an allergy include early microbial and dietary exposure, even while within the uterus, that modifies gene expression. Citation10 Other factors affecting microbiota and which could orchestrate a Th2 pro-allergic immune type response are environmental changes, including climate, lifestyle, indoor life, habits and diet. However, the mechanisms underlying the current increase in the prevalence of allergic diseases has not been fully elucidated.
Allergy, from concepts to immune mechanisms
The overall biological function of our immune system is to defend the body by recognizing potential chemical or biological attacks. However, the overzealousness of the immune system occasionally causes an inappropriately excessive response, which we describe in terms such as allergy, atopy, hypersensitivity or anaphylaxis. The term allergy was introduced by von Pirquet in 1906 following his observations of early reactions noted on the skin 24 hours after cowpox vaccinations. In fact, he referred to it as “inadequate immunity” or “allergy” (from the Greek “allos,” other and “ergon,” work). However, he was dealing in sensu stricto with a “delayed hypersensitivity,” an immune process concept that was not understood at that time. Actually, it was in the 1960´s when Gell and Combs described the type I to IV hypersensitivity reactions, and only type I is now considered “allergic.” We currently classify any allergic symptoms (e.g., rhinitis, conjunctivitis, dermatitis, etc.) mediated by immunoglobulin E (IgE) as atopic (which means “out of place” in Greek). These symptoms come from a cascade of reactions involving mucus production, increase robustness of epithelial barriers, and other effects on the musculoskeletal and nervous systems that lead to mechanical, physical and chemical changes whose objectives are to prevent and remove these toxic environmental challenges. Citation11,Citation12 This immune response consequently manifests as lacrimation, vomiting, diarrhea, coughing, sneezing or itching. These reactions were historically called “anaphylactic” responses, literally, anti-protection (from the Greek “ana,” backward and “phylaxis,” protection), even though, as mentioned earlier, they are in fact natural responses designed to protect the host. Nowadays, an anaphylactic reaction is a clinical reference to the most severe type I hypersensitivity reaction.
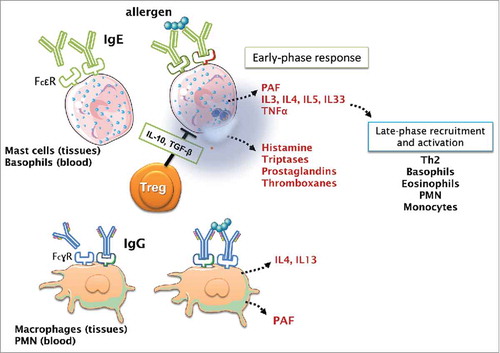
illustrates the IgE-mediated allergic immune response using the 2 classical common phases: sensitization and elicitation. Allergic sensitization occurs during initial exposure to a specific allergen. The allergen is taken up and processed by antigen-presenting cells (or dendritic cells, DCs, in the present case) which then present allergen peptides to T cells. T cells differentiate into Th2 cells which mainly release the cytokines IL-4, IL-5 and IL-13 resulting in allergen-specific IgE production by B cells, and the activation and recruitment of basophils and eosinophils. Secreted IgE binds to FcϵR receptors on the basophil and mast cells. The elicitation phase occurs after re-exposure to the same allergens when they cross-link IgE bound to mast cells and basophils, resulting in degranulation and release of inflammatory mediators, such as histamine, serotonin, tryptase and cytokines, which trigger different physical mechanisms (increased vascular permeability, muscle contraction, bronchoconstriction, with the concomitant mucous production) aimed at removing the “aggressor.” Alternatively, in the presence of a high dose of allergens, immunocomplexes with macrophage- or neutrophil-bound IgG will stimulate the release of PAF (platelet-activating factor). This is recognized as the “alternative” sensitization pathway and plays a major role when allergens enter the blood stream and form immune complexes with specific IgG. However, most anaphylactic reactions in humans are IgE-mediated because this pathway involves small quantities of antigen. Citation13
Figure 1. Sensitization to allergens and development of allergic responses. The sensitization pathway starts when an Antigen Presenting cell (APC) recognizes and processes an environmental protein as potentially dangerous, such as inhalants allergens (pollen, animal dander, house dust mites), or food proteins. This phase is known as sensitization. During allergen sensitization, naïve T cells are primed into CD4+ T helper type 2 T cells, producing Th2 cytokines as IL4 and IL-13, which are responsible of class switching to the IgE production by B cells. IgE will bind to the surface mast cells and basophils IgE receptor. Upon the next encounter with the allergen, the protein will crosslink 2 IgE molecules starting a signaling cascade that causing mast cells and basophils degranulation, releasing preformed mediators (histamine, proteases, prostaglandins, thromboxanes) constituting the early-phase response. In addition, mast cells and basophils also produce cytokines and chemokines that recruit inflammatory cells to the site (late-phase). This immune response type 2 (Th2) immunity is counterbalanced by Type 1 (Th1) immunity and T regulatory cells.
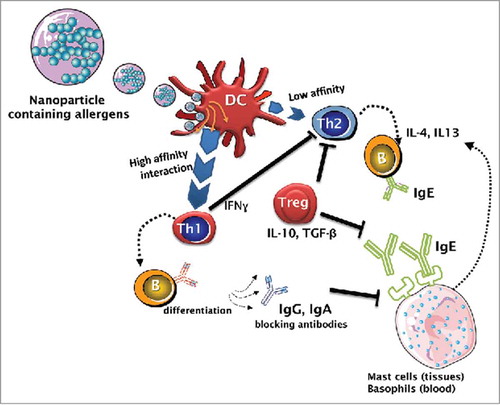
Allergic diseases are mediated by Th2 cytokine responses involving IL-3, IL-4, IL-5, IL-13 and GM-CSF (granulocyte-macrophage colony-stimulating factor), and a reduction in the number of Th1 IFN-γ-producing cells. It is well known that Th2 is counterbalanced by Th1 and regulatory T cells. Consequently, both IFN-γ (Th1) and IL-10 (Treg) cytokines have been shown to increase with allergen-specific immunotherapy (). Similar counterbalances have been described between Th17 and Th1, Th17 and Th2, or between Th17 and regulatory T subsets. Citation14 Thus, the immune response is likely to shift from the pro-allergic Th2 considering that cytokines secreted by Th1, Th17 and regulatory T subsets may suppress Th2 cell function. However, other cell types, like NK and Tc-cells, shall also be considered.
Figure 2. Immunological mechanism of immunotherapy based on nanoparticles carrying allergens. Dendritic cells (DC) are specialized cells able to shape the direction of the specific immune response from the allergic (Th2) to the inflammatory or to the tolerogenic ones. Currently, the allergen-specific IT is based on the gradual administration of increasing amounts of allergens. It is well documented that high-dose injections with short intervals induce T regulatory cells and suppress Th2 cells. However, it is also possible to reduce Th2 responses by increasing Th1 by the use of appropriate adjuvants. Thus, the uptake of allergens containing suitable nanoparticles increase of affinity of the interaction between DC and Th cells, rendering a Th1 response.
Allergy, from strategies to therapy
The appropriate drugs can, to some extent, help alleviate the symptoms of allergic conditions and may improve patients' quality of life. However, currently, the only treatment that can reverse the disease course is immunotherapy. Nevertheless, in spite of its efficacy, the underlying mechanisms of immunotherapy are not completely understood. Immunotherapy means to “act specifically” (allergen-specific immunotherapy, IT) to desensitize the host to a specific allergen; in other words, the host develops a “tolerance” to that allergen. Thus, successful desensitization will mean that a greater amount of allergen is required to induce an allergic reaction or that the allergen no longer induces a reaction. Citation15 IT was traditionally based on a system with an empirical basis. Thus, a century ago, Leonard Noon started to use subcutaneous injections of a grass pollen extract to treat hay fever. Citation16 However, IT is time-consuming, and subcutaneous injections are associated with local and systemic allergic side effects. New forms of immunotherapy with allergens were later introduced to improve the immunotherapy safety profile. The administration routes and number of injections were modified in some cases. In other cases, modifications were introduced in the allergenic extracts. Finally, adjuvants have been added in attempts to modulate the immune response to a tolerogenic one, to increase the time interval between injections and/or to enable a particular administration route ( and ).
Table 1. Types of Allergen Immunotherapy (routes, main groups of allergens and adjuvants) currently in use in clinical practice.
Table 2. Future developments of Allergen Immunotherapy. New routes, allergens and adjuvants that are nowadays at different development stages for future use in clinical practice.
Administration routes
With regard to administration routes, subcutaneous immunotherapy (SCIT) is the best established method. What we currently consider to be classical IT consists of repeated subcutaneous inoculation of doses of allergen over a long period of time. However, in spite of its efficacy, there are downfalls to the use of SCIT. These include local allergic reactions, anaphylaxis (occasionally) and, near-fatal reactions (rarely). Citation17,Citation18 Considering these risks, subcutaneous immunotherapy should only be administered by trained specialists. On the other hand, guidelines recommend at least 3 to 5 y of treatment to obtain a long-term immunological response, which requires good patient compliance. SCIT is performed in 2 phases: (i) starting phase (build-up), a weekly dose is administered with increasing amounts of allergen until the maximum dose is reached; and (ii) maintenance phase, the maximum dose of allergen is administered every 4 weeks. Other disadvantages related to the use of classical subcutaneous immunotherapy derive from some patients' fear of needles and the risks associated with their use. Citation19 A plausible alternative is the practice of cluster regimens, in an attempt to reduce the number of hospital visits during the build-up phase. Cluster regimens consist of administering multiple injections each day on non-consecutive days, and represent an alternative to conventional up-dosing schedules as they reach the maintenance dose quickly, shortening the build-up phase. These regimens have not been associated with an increased risk of systemic reactions compared with conventional schedules for subcutaneous allergen administration. Moreover, they shorten the time required to reach maintenance therapy, as seen with dust mite and grass-pollen immunotherapy. Citation20
The use of sublingual immunotherapy (SLIT) has become more extensive over the last 20 y. Citation22 In general, the risk of anaphylactic reaction is smaller in SLIT compared with SCIT and so patients can self-administer at home; but it appears to be less effective than SCIT. Citation23,Citation24 The need to administer SLIT daily or several times a week also results in poor treatment adherence. Citation25 SLIT with tablets should solve this problem. Citation26,Citation27 However, in the particular case of IT for food allergies, where subcutaneous immunotherapy has been ruled out due to the prevalence of severe adverse reactions, SLIT has shown only limited efficacy.
The decision to use either SCIT or SLIT currently depends on the allergist's preference, and not on scientific criteria. The main arguments in favor of the subcutaneous route are because it achieves higher patient compliance and there is indirect evidence that it is more effective than the sublingual route. Nevertheless, the sublingual route could be the selection of choice because it is administered at home and presents less risk of provoking a severe reaction. Studies with appropriate methodologies are required to make a comparison of both administration routes. Citation28
Oral immunotherapy
(OIT) has been introduced with good results in terms of efficacy, but with frequent adverse reactions. OIT is being used to treat food-allergic patients, especially for milk, egg or peanut allergies. It comprises a dose-escalation phase, in which an increasing oral dose is administered weekly at the hospital, followed by a maintenance phase, in which the maximum dose (if it was well-tolerated) is administered on a daily basis at home. OIT is now used under research protocols, and can induce desensitization (the food is tolerated while receiving treatment) or, in some patients, a sustained unresponsiveness after discontinuation. Citation29
Epicutaneous and intralymphatic routes are currently being evaluated. Epicutaneous immunotherapy (EPIT) has been shown to be effective in the treatment of grass pollen allergic rhinoconjunctivitis and is being studied for the treatment of food allergies, such as milk or peanut, where its efficacy has also already been proven. Citation30 The intralymphatic route consists of administering 3 injections of allergenic extract into the inguinal lymph nodes over the course of a year; the technique has proven to be effective and safe in the treatment of cat dander or grass pollen-induced allergic rhinoconjunctivitis. Citation31
Modified allergens
Attempts to develop safer, more convenient and more effective strategies include the use of hypoallergenic glycol-conjugates or recombinant allergens, also known as modified allergens, which do not induce IgE-mediated activation. Citation33-Citation34 Another possibility being explored is fusing allergens with proteins containing allergen specific T epitopes (35, which have the advantage that they do not stimulate the allergen-specific T cells). However, one major disadvantage is that they can cause delayed reactions. Citation36 These B peptides must be linked to a carrier protein. Citation37 When modified allergens are used, the build-up phase can be significantly shortened due to the reduced risk of an adverse reaction. However, there are no studies available comparing the efficacy of using modified allergens compared with native ones. A further benefit of these novel forms is that they can improve the standardization, reproducibility and quality of the extracts. Citation36,Citation38
Adjuvants in allergen-specific immunotherapy: Safety and efficacy
Adjuvants have been used extensively in conventional prophylactic vaccines for preventing infectious diseases. An adjuvant is any “substance” with the capacity to increase the immunogenicity of the accompanying antigen and/or modulate the quality of the elicited immune response. In this regard, adjuvants can be selected to favor the development of any of the specific T cell branches. Consequently, over the years, adjuvants have been widely used in allergy vaccines to enhance and simplify immunization regimens, besides improving the safety profile of allergen-specific immunotherapy treatments. Citation39 However, they can also cause adverse events, Citation40 raising clinical concerns about their use. Recent advances in developing new forms of adjuvants for immunotherapy have focused on reducing their potential risks while modulating the immunogenicity of the allergen-specific immunotherapy. Citation41 Among others, novel and promising allergen delivery systems with immunomodulatory properties appear to fulfil the ideal requirements for allergy immunotherapy.
Two different ‘generations’ of adjuvants for allergy vaccines have been described: the first generation refers to those molecules that constitute vehicles for binding the allergen. These molecules have the ability to co-stimulate innate cells, including antigen-presenting cells. Second-generation adjuvants are considered specific immunomodulatory molecules capable of inducing specific immunopotentiation of either immune cells or pathways. Citation42
First-generation adjuvants
The efficacy of aluminum hydroxide (alum) and calcium phosphate for subcutaneous allergy vaccines has been demonstrated in humans as they can elicit proinflammatory responses after inflammasome activation. Citation43,Citation44 Alum has been used as an adjuvant in vaccines against infectious diseases since 1926, Citation45 and for treating allergic conditions since 1937. Citation46-Citation49 Today, most subcutaneous allergen-specific immunotherapy is performed with Al(OH)3 as the adjuvant, Citation39 and can generally be regarded as safe in terms of acute local or systemic side effects. However, several reports of chronic aluminum toxicity are accumulating and are being inspected by national authorities. Citation46
Microcrystalline tyrosine (MCT) constitutes a relatively new depot adjuvant formulation that can co-precipitate the allergens, thus decelerating allergen bioavailabilty, reducing the rate of dissemination from the injection site and the prevalence of systemic reactions. Citation50 It is frequently used in combination with monophosphoryl lipid A (see below), leading to a reduction in the up-dosing phase. Its safety has been demonstrated in different studies in both animal models and in humans. Citation51-Citation54
Second-generation adjuvants
Second-generation adjuvants contain immune modulators that stimulate a Th1 and/or T regulatory response. They include microbial products (Toll-like receptor agonists) and vector-delivery systems (microparticles, nanoparticles, liposomes, virosomes, or immunostimulating complexes, among others). Citation55
Microbial products (TLR agonists)
Microbial products contain inflammatory “danger” signals, such as those known as “pathogen-associated molecular patterns” (PAMP). The host senses PAMPs as danger molecules, eliciting the release of cytokines and chemokines that initiate an inflammatory defensive response. Immune system cells have special receptors for PAMPs known as PRRs (pattern recognition receptors), including Toll-like receptors (TLRs), Nod-like receptors (NLRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs). Citation56-Citation59 One of the main PAMPs is lipopolysaccharide (LPS) found in Gram negative bacteria, a TLR4 agonist. Both natural and synthetic derivatives of LPS with reduced toxicity are currently being developed. Monophosphoryl lipid A (MPL) extracted from Salmonella enterica serovar Minnesota is included as an adjuvant, alone or in combination with alum, in some current IT applications. Citation60 MPL has shown efficacy in murine asthma models Citation39 and in patients with asthma after receiving the first cycle of allergen-specific SCIT for grass pollen combined with MPL. Citation61 The efficacy of this adjuvant relies on the strong induction of allergen-specific IgG antibodies which may have protective effects by inhibiting immediate-type reactions, as confirmed through the decrease in nasal reactivity after nasal allergen challenge in the case of an MPL-adjuvanted grass pollen allergy vaccine administered using SLIT. Citation62 The LPS extracted from Brucella ovis has also been studied due to its low toxicity but maintained capacity to induce Th1 responses to the carried antigens. Citation63 Flagellin, the monomeric protein that forms the bacterial flagellum, is a potent TLR5 agonist and induces the maturation of intestinal DC, activates CD4+ T cells in vivo and promotes the development of effector Th1 cell responses. Other PRR agonists that induce strong Th1 responses include some unmethylated CpG oligodeoxynucleotides (CpG ODN) which interact with TLR9, or the bacterial second messenger 3´,5´-cyclic diguanylic acid, recognized by 2 distinct PRRs, STING and DDX41. Citation59,Citation64 Moreover, the CpG dinucleotides have been proposed via different allergen immunotherapy administration routes (sublingual, intradermal, nasal, subcutaneous). Citation41 However, the subcutaneous use of a type B CpG ODN with ragweed pollen did not yield reproducible clinical data in a large phase III study. Citation65 Some authors have recently suggested that immunotherapy could be effective even in the absence of allergens, as reported with the use of virus-like particles and CpG-motifs, either as standalone products, or as adjuvants for allergen-specific immunotherapy. Citation42,Citation66 However, this strategy is a double-edged sword due to the potential side effects associated with inflammation, such as pain, swelling, and cell death.
Probiotics
Symbiotic bacteria, such as Lactococcus lactis, Lactobacillus plantarum and Bifidobacterium bifidum, have been studied for use in mucosally administered immunotherapy (e.g., intranasal or sublingual), showing efficacy in animal models through the induction of Th1 responses, regulatory T cells and even reductions in asthma symptoms. Citation39 In a double-blind randomized clinical trial, Tang and coworkers Citation67 evaluated the effects of a combined probiotic (Lactobacillus rhamnosus strain CGMCC, NCC4007) and peanut oral immunotherapy administered to children with peanut allergy. They reported that nearly 90% of the subjects receiving the combined oral immunotherapy were desensitized to peanut allergy, including induction of Treg and Th1 cytokine responses. The efficacy relies on the probiotics' capacity to induce beneficial gut immune modulation, which could promote the patient's sustained unresponsiveness after discontinuing the OIT. Further studies are needed before considering probiotics efficient adjuvants for allergen immunotherapy, especially in children, where the mucosal route, in our experience, appears to be more acceptable for the patient and their parents. Citation68,Citation69
Small molecule compounds
The active metabolite of vitamin D3, 1α,25-dihydroxyvitamin D3 (VD3), has been described as a potent inducer of T regulatory cells, and thus, a good adjuvant in allergen immunotherapy. A recent study showed that its addition to a conventional immunotherapy vaccine could reduce the allergoid dose needed and, therefore, the cost. Furthermore, this would also help patients adhere to the treatment, hopefully increasing the effectiveness of the immunotherapy. Citation70 Baris and co. Citation71 evaluated the efficacy, safety and T regulatory cell response of vitamin D as adjuvant for allergen-specific IT; 50 children with bronchial asthma and sensitization to dust mite were randomized into 3 groups: one receiving SCIT with vitamin D supplementation, and the other 2 with SCIT alone and pharmacotherapy alone. They reported similar results for the 2 SCIT groups, although some more favorable outcomes were observed in those treated with vitamin D supplementation, and both were better than those for pharmacotherapy alone. Citation71
In a double-blind placebo-controlled study in 50 children, Jerzynsak et al. reported a better response and reduced symptoms of allergic rhinoconjunctivitis after 5 months of treatment with SLIT grass tablets plus 1000 units of vitamin D compared with those who received SLIT alone. Citation72 It has previously been suggested that vitamin D regulates immune function by inhibiting the differentiation and maturation of human dendritic cells, enhancing IL-10 production by CD4+ T cells and upregulation of Foxp3+ CD4+ T cells. Citation73-Citation75
Other synthetic molecules involved in adjuvant immunotherapy are the calcineurin inhibitor cyclosporine A, rapamycin, aspirin and mycophenolate mofetil which seem to enhance IL-10 production. Citation39
Particle delivery systems
Particle delivery systems are adjuvants whose aim is to facilitate the work of antigen-presenting cells by increasing the length of contact between the allergen and the patient's mucosa. They extend contact duration via different mechanisms, such as the use of mucoadhesive polymers in the oral mucosa or by increasing the influx of professional APCs to the injection site in the case of SCIT. Particle delivery systems have also shown efficacy in animal models by successfully targeting immunotherapy via parenteral routes. Citation76,Citation77 Among the systems described in the literature, the use of virus-like particles (VLP) as adjuvants in allergen-specific immunotherapy seems to increase the safety in patients with a high risk of systemic reactions as they appear to limit the bioavailability of active substances that may cause massive mast cell degranulation and, therefore, systemic reactions during treatment. Citation42,Citation66 The VLP technology platform has been explored for different administration routes, such as subcutaneous, intralymphatic and intramuscular. Citation42
Nanoparticles
The use of micro- and nanoparticles based on polymers and macromolecules has received considerable attention in recent years because of their potential to enhance the antigen delivery to the immune system. In fact, these compounds constitute a group of delivery systems with interesting abilities as adjuvants; since they are easily captured and internalized by different immune cells, such as DCs and macrophages, then they can enhance the delivery of the loaded antigen/allergen to the immune system (). Citation70,Citation76,Citation77 The terms microparticle and nanoparticle refer here to particles with dimensions best described in micrometers and nanometers, respectively. Size brings real differences at many levels, including formulation techniques, in vivo administration or cellular uptake and intracellular fate. Citation55 Furthermore, nanoparticles (NPs) can be used to load biologically active material (i.e., allergens) and thereby offer the following advantages: (i) protection from hydrolysis and/or enzymatic degradation, (ii) controlled and sustained load release properties, and (iii) co-encapsulation of the allergen and immunopotentiators (i.e., CpG motifs or LPS). Most of these nanoparticles can easily be tailored to improve their targeting abilities. A present disadvantage is that only a few materials are currently available for preparing nanoparticles with an acceptable safety profile. For parenteral delivery purposes, some polyester-based nanomedicines [e.g., poly(lactic) acid (PLA) and poly(D,L-lactic-co-glycolic) (PLGA)] or human serum albumin nanoparticles are now available. For oral delivery purposes, nanoparticles based on either chitosan Citation78 or Gantrez ANm Citation79 have been demonstrated to be safe, including in subchronic toxicity studies.
Parenteral administration
Intramuscularly, subcutaneously or intradermally administered micro- and nanoparticles are expected to essentially remain at the injection site and then control the release of the loaded antigen. Therefore, slowly degradable polymers such as polyesters [PLA, PLGA, or poly(epsilon-caprolactone) (PEC)] are suitable for this application as they provide an adequately prolonged release of the loaded allergen. Schöll and coworkers Citation80 recently demonstrated the capacity of PLGA nanoparticles in immunotherapy to treat birch pollen (Bet-v1) allergy in BALB/c mice. PLGA nanoparticles loaded with Bet-v1, the major birch pollen allergen, enhanced the immunogenicity of the allergen and did not lead to novel sensitization in naïve animals. The authors assumed that vaccination with PLGA nanoparticles can counterbalance an ongoing Th2 response to Bet-v1, readdressing it to a Th1 protective response. In the same way, carbohydrate-modified ultrafine hydroxyapatite-based nanoparticles (aquasomes) also induced a shift in mouse immune response against ovalbumin (used as a model allergen) when administered intradermally. In sensitized mice, these nanoparticles elicited lower serum IgE and histamine levels and a higher survival rate in comparison with alum-adsorbed ovalbumin. Citation81 Another interesting nanoplatform for allergen-specific immunotherapy is based on the use of protamines, which are arginine-rich proteins that can spontaneously assemble into nanoparticles with DNA or RNA via electrostatic interactions. Citation82,Citation83 These protamine-based nanoparticles, combined with CpG sequences, were evaluated as an allergen delivery system using Ara h 2 as a model allergen. When the protamine nanoparticles were subcutaneously injected into naïve BALB/c mice, a favorable increase in Ara h 2-specific IgG2a antibodies was observed, whereas no Arah 2-specific IgE was detected. Citation84 Nanoparticles based on tetrafunctional block copolymer 704 have also been proposed for gene delivery. Citation85 When these nanoparticles, which contain a pDNA encoding for the allergen Dermatophagoides farinae 1 (Der f 1), were intramuscularly administered to healthy mice they induced strong humoral and cellular responses with a Th-1 profile. However, in asthmatic mice, the same nanoparticles led to a reduction of symptoms and a significant decrease in the level of inflammatory cytokines in bronchoalveolar lavage fluids. Citation85
Mucosal administration
Important advances in nanotechnology have enabled the development of new nanoparticles for use as allergen delivery systems. These have been investigated in recent years as they offer an alternative administration route, as well as reducing the dose and diminishing allergen exposure to IgE bound to mast cells or basophils. Citation86 The advantages of polymer nanoparticles include their use for either subcutaneous or mucosal routes and the fact they are biodegradable, in contrast to traditional alum mineral salts. Citation45 For allergen delivery, nanoparticles based on poly(DL-lactide-co-glycolide) (PLGA) or its derivatives, chitosan or poly(anhydrides), among other materials, have given encouraging results. Most of these studies involved the use of allergen-loaded nanoparticles administered via a mucosal route.
PLGA microparticles were the first devices proposed to elicit oral tolerance to an allergen. Citation87 In this work, Pecquet et al. demonstrated that the oral administration of β-lactoglobulin encapsulated in PLGA particles produced a greater tolerance than the free protein (10,000 times higher). Citation88 In another interesting study, the possibility of increasing the capacity of PLGA nanoparticles to promote Th1 responses was evaluated through their combination with CpG sequences. Citation89 CpG motifs provide steric hindrance that reduces IgE binding and enhances Th1 responses by stimulating TLR9, APCs and NK cells to secrete gamma-interferon and IL-12. Citation89 The oral administration of PLGA nanoparticles loaded with a peanut extract and CpG over a 4 week period provided long-lasting protection from anaphylaxis to repeated challenges in animals. During this time, animals treated with the nanoparticles displayed lower levels of specific IgE, IgG1 and pro-Th2 cytokines (IL-4, IL-5 and IL-13) than the controls. Citation89
Nanoparticles based on the methyl vinyl ether-maleic anhydride copolymer (Gantrez AN®) respresent another interesting class of potential nanocarriers for oral allergen delivery. These poly(anhydride) nanoparticles have demonstrated their efficacy to induce Th1 immune responses, Citation90,Citation91 which is mediated by their capacity to activate TLR-2 and TLR-4 and induce DC maturation with a significant upregulation of the CD40, CD80, and CD86 receptors. Citation92,Citation93 Using ovalbumin (OVA) as an allergen model, a single oral dose of these poly(anhydride) nanoparticles given to mice induced high serum levels of OVA-specific IgG2a, IgA and IL-10. Citation94 These nanoparticles were also able to decrease the anaphylactic symptoms of sensitized animals and protect them from death after an intraperitoneal challenge with the native protein, in contrast to the control group. Citation94 Interestingly, the co-encapsulation of LPS in these OVA-loaded poly(anhydride) nanoparticles, but not its binding to their surface, also protected sensitized mice during the challenge. Citation94 In another study using a peanut protein extract, similar poly(anhydride) nanoparticles were prepared and orally administered to mice. After a single dose, the nanoparticles induced a balanced Th1 and Th2 antibody response and low levels of specific IgE. Moreover, animals treated with peanut protein-loaded nanoparticles displayed lower levels of pro-Th2 cytokines (IL-4, IL-5, IL-6) and higher levels of IFN-γ and IL-10 than control animals. Citation95
Other interesting materials for the preparation of nanoparticle adjuvants are chitosan and its derivatives. Chitosans, particularly those with a deacetylation of 75–90%, can drive potent cell-mediated immunity, promoting DC maturation by inducing type I interferons and enhancing antigen-specific Th1 responses. Citation96 In addition, chitosan-based nanoparticles possess mucoadhesive properties that increase their contact time at mucosal surfaces. Citation97 Last but not least, the cationic character of chitosan makes it ideal for designing devices used to deliver polyanions, such as DNA or RNA. Citation98 In this context chitosan nanoparticles were proposed for the oral delivery of a pDNA encoding for Ara h 2, a dominant peanut allergen. Compared with the controls (non-immunized mice or mice treated with ‘naked’ DNA), animals immunized with nanoparticles showed a substantial reduction in allergen-induced anaphylaxis associated with reduced levels of IgE and plasma histamine. Citation98 More recently, chitosan nanoparticles containing a plasmid DNA encoding for Der p1 (a house dust mite allergen), primed a Th1-skewed immune response against this allergenic protein. Citation99 In another interesting publication, in which chitosan nanoparticles were used to deliver a pDNA encoding for Dermatophagoides pteronyssinus 2 allergen (Der p 2), Li and coworkers demonstrated the expression of Der p 2 in the epithelial cells of both the stomach and small intestine and the induction of a Th1-type immune response. Citation100
Adjuvant limitations
Empirically developed vaccines have been in use for decades. The field is now applying increased use of rational design based on our knowledge of the mechanisms of the protective immune response against allergies and the adjuvants' mechanisms of action. Concerning adjuvants, the use of alum compounds illustrates the need for new safer adjuvants with immunological functions oriented toward an effective therapy. As stated above, alum is still the most commonly used adjuvant in allergy immunotherapy. Its strength is acknowledged despite several important disadvantages, for example it stimulates high and persistent levels of IgE antibodies, limiting its use in long-term therapies. Citation101 Alum's intrinsic physicochemical properties can also limit its use. The adsorption capacity of aluminum is based on a combination of reversible interactions, including electrostatic and van der Waals forces. Therefore, if dealing with simple antigens (e.g., tetanus toxoid) we can predict the adsorption; however, the avidity and kinetics of such interactions are unknown in the case of the antigenic complexes that are currently used in immunotherapy. Furthermore, there is no data available regarding the dose of alum required to achieve adequate adsorption to the antigens, limiting our predictions on toxicity, stability, and the effect of the final product. Citation102,Citation103
On the other hand, the mechanism of action of alum remains elusive, raising safety and efficacy concerns over its application. Citation104 Our newly acquired knowledge of the role of antigen-presenting cell receptors for danger signals, which dictates the fate of the immune response, has opened the door to novel, better-characterized adjuvants. Thus, MPL was the first well-characterized TLR agonist used as an immunoadjuvant in allergy immunotherapy (see above). Its immunological function is to orientate the antibody response from IgE to IgG1 and IgG4. Citation105 However, MPL does not have a unique, well-defined structure; on the contrary, there are several MPL products available on the market, from synthetic MPL derivatives from Escherichia coli, to both natural and synthetic lipid A derivatives from Salmonella enterica serovar Minnesota R595. These macromolecules consist of a mixture of lipid A species with different fatty acid chains. Variables such as the number of fatty acids, chain length, origin and the ratio of molecules can affect the physicochemical and adjuvant properties. Citation106 Consequently, further studies into the safety and efficacy of MPL derivatives are required before drawing conclusions regarding the benefits and risks during their application in vaccines and immunotherapies. Citation107
Few studies have explored the influence of administration route on the immune response elicited during immunotherapy, Citation105 e.g., MPL studies only focused on parenteral applications. However, rational designs for alternative therapies must consider the neglected natural routes, the mucosal routes. Citation108 Several delivery systems such as polymeric nanoparticles have been developed for mucosal routes with very promising results. Citation109 Yet these systems are still a long way from being authorized. Particularly, in the case of polymeric delivery systems for allergy IT, more investigation into safety during prolonged therapy is required.
The use of probiotics as tolerance-inducing immunomodulators is specially promising. Nevertheless, variations among individuals may reduce the potential applications of probiotics, and further new clinical trials are required to demonstrate the long-term sustained reduction of specific IgE response.
Finally, despite recent advances in adjuvants, the costs of developing new products mean alum is still the primary adjuvant for human vaccines worldwide. This is really discouraging because if alum hadn't been in use for all these years then it would be refused registration by today's standards on the basis of safety concerns. Citation110 Many adjuvants have been proposed but costs are, so far, a major limitation. Consequently, there is an urgent need for innovations that streamline manufacturing processes and reduce costs in accordance with the needs and priorities of immunotherapy and vaccines. On the other hand, there is an increasing cost-benefit value from the use of adjuvants as if the use of adjuvants would improve efficacy or result in the use of fewer doses and physician visits, cost would not be an issue limiting their use.
A greater knowledge of the mechanisms of action of allergen-specific immunotherapy techniques has fostered the inclusion of novel adjuvants that will improve treatment safety and efficacy. Citation22 Moreover, the contemplation of wider indications besides what we know already, such as the preventive effect on the development of bronchial asthma Citation111,Citation112 or identification of the optimal maintenance dose and dose-ranging for common allergens from grass pollen, dust mite allergens or ragweed pollen in large trials studies have constituted important advances in the field of specific allergen immunotherapy. Citation27,Citation113,Citation114 However, in our opinion, there is only limited data available in terms of efficacy of the different adjuvants and there are still unmet requirements, such as the performance of ad hoc clinical trials to evaluate the different types of adjuvants (traditional and novel ones) in order improve the safety of the immunotherapy and to individualize each patient's indication according to their allergies.
Conclusions
As in the case of vaccines against infectious disease, adjuvants in immunotherapy for allergic conditions are expected to enhance and modulate immune responses to allergens, enabling fewer doses and/or lower dosage levels of allergen. The adjuvant should provide better immunomodulation that would ideally balance the overactivation of Th2 cells and, consequently, the overproduction of IgE, thus achieving a quicker and longer-lasting efficacy (see for on-going clinical trials; www.clinicaltrials.gov).
Table 3. Currently open ongoing clinical trials recruiting (R) or not yet recruiting (NR) subjects [www.clinicaltrials.gov]
On the one hand, allergen immunotherapy has proven its long-term efficacy, with a protection rate of over 97% in the case of hymenoptera. Allergen immunotherapy has the potential to balance the immune dysregulation causing the allergic inflammation and it has demonstrated its ability to prevent future sensitizations. It is well accepted by patients because it is not a synthetic drug. However, allergen immunotherapy also has several disadvantages: it requires time to build up the immune response, and in the case of subcutaneous immunotherapy it must be administered by trained personal at a healthcare facility, with monthly doses for an average of at least 3 y. Although allergen immunotherapy may be effective, it also presents a risk since patients can have an allergic reaction at any given dose. Recent developments in allergy research have focused on allergen components, molecular diagnosis and targets for biological drugs, rather than developing new strategies to design safer, more precise, standardized, and faster immunotherapy.
Some important innovations in immunotherapy are currently at different developmental stages as researchers try to achieve: greater extract efficacy, more reproducible production at lower manufacturing costs, safer immunotherapy requiring fewer doses, potential application to food allergies, a lack of sensitizing potential, and the use of IT in a prophylactic manner. Citation36 The influence of probiotics on the immune system is an exciting area of research and, specifically, the role of probiotics as adjuvants in vaccinations or immunotherapy is very promising. Citation115
Nanoparticles are being studied as the next generation of mucosal adjuvants since they can enhance the delivery of the loaded antigen to gut lymphoid cells thanks to their special bioadhesive properties. NPs may stimulate “protective” Th1 and/or T regulatory responses while avoiding the systemic side effects associated with IgE cross-linking on mast cells and basophils, which is the risk associated with conventional whole allergen extracts. These properties open new possibilities for treating not only infectious diseases but also allergic diseases.
Important advances in developing new forms of adjuvants have been made in the past few years. However, further research is needed to develop an ‘ideal’ adjuvant that shows promising efficacy in modulating immune response, facilitating shorter up-dosing phases (which would increase adherence), reducing adverse reactions during treatment and finally inducing sustained unresponsiveness in allergic patients.
Disclosure of potential conflicts of interest
The authors report no potential conflicts of interest.
Authors' contributions
CG, JMI, CD, GG, and MF all contributed to the conceptual framework, writing, and revision of the manuscript.
References
- Mukherjee M , Stoddart A , Gupta RP , Nwaru BI , Farr A , Heaven M , Fitzsimmons D , Bandyopadhyay A , Aftab C , Simpson CR , et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med 2016; 14(113):13; PMID:26817443
- Nwaru BI , Hickstein L , Panesar SS , Muraro A , Werfel T , Cardona V , Dubois AE , Halken S , Hoffmann-Sommergruber K , Poulsen LK , et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 2014; 69(1):62-75; PMID:24205824; https://doi.org/10.1111/all.12305
- Turner PJ , Gowland MH , Sharma V , Ierodiakonou D , Harper N , Garcez T , Pumphrey R , Boyle RJ. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol 2015; 135(4):956-63; PMID:25468198; https://doi.org/10.1016/j.jaci.2014.10.021
- Grabenhenrich LB , Dolle S , Moneret-Vautrin A , Kohli A , Lange L , Spindler T , Ruëff F , Nemat K , Maris I , Roumpedaki E , et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol 2016; 137(4):1128-37; PMID:26806049; https://doi.org/10.1016/j.jaci.2015.11.015
- Tejedor-Alonso MA , Moro-Moro M , Mosquera Gonzalez M , Rodriguez-Alvarez M , Perez Fernandez E , Latasa Zamalloa P , Farias Aquino E , Gil Prieto R , Gil de Miguel A . Increased incidence of admissions for anaphylaxis in Spain 1998–2011. Allergy 2015; 70(7):880-3; PMID:25808198; https://doi.org/10.1111/all.12613
- Nunes C , Pereira AM , Morais-Almeida M . Asthma costs and social impact. Asthma Res Pract 2017; 3:1; PMID:28078100; https://doi.org/10.1186/s40733-016-0029-3
- Linneberg AA-O , Dam Petersen K , Hahn-Pedersen J , Hammerby E , Serup-Hansen N , Boxall N . Burden of allergic respiratory disease: a systematic review. Clinical and molecular allergy: CMA 2016; 14:12; PMID:27708552; https://doi.org/10.1186/s12948-016-0049-9
- Tavakoli H , FitzGerald JM , Chen W , Lynd L , Kendzerska T , Aaron S , Gershon A , Marra C , Sadatsafavi, M , Canadian Respiratory Research Network . Ten-year trends in direct costs of asthma: a population-based study. Allergy 2017; 72(2):291-9; PMID:27455382; https://doi.org/10.1111/all.12993
- Caulley L , Thavorn K , Rudmik L , Cameron C , Kilty SJ . Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol 2015; 136(6):1517-22; PMID:26483176; https://doi.org/10.1016/j.jaci.2015.08.037
- Martino DJ , Prescott SL . Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy 2010; 65(1):7-15; PMID:19796189; https://doi.org/10.1111/j.1398-9995.2009.02186.x
- Gause WC , Wynn TA , Allen JE . Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 2013; 13(8):607-614; PMID:23827958; https://doi.org/10.1038/nri3476
- Palm NW , Rosenstein RK , Medzhitov R . Allergic host defences. Nature 2012; 484(7395):465-472; PMID:22538607; https://doi.org/10.1038/nature11047
- Finkelman J. Anaphylaxis . Lessons from mouse models allergy. Clin Immunol; 115: 449-57 (2005).
- Peck A , Mellins ED . Plasticity of T‐cell phenotype and function: the T helper type 17 example. Immunology 2010; 129(2):147-153; PMID:19922424; https://doi.org/10.1111/j.1365-2567.2009.03189.x
- Akdis CA , and Akdis M . Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 2009; 123 4:735-746; https://doi.org/10.1016/j.jaci.2009.02.030
- Noon L . Prophylactic inoculations against hay fever. Lancet; 1911 1:1572-1573; https://doi.org/10.1016/S0140-6736(00)78276-6
- Ragusa, VF and Massolo A . Non-fatal systemic reactions to subcutaneous immunotherapy: a 20-year experience comparison of two 10-year periods. Eur Ann Allergy Clin Immunol 2004; 36(2):52-55; PMID:15061395
- Amin, HS , Liss, GM , and Bernstein, DI . Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clini Immunol 2006; 117(1):169-175; PMID:16387602; https://doi.org/10.1016/j.jaci.2005.10.010
- National Center for Immunization and Respiratory Diseases . General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1-64.
- Jutel M . International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015;136: 556-568; PMID:26162571; https://doi.org/10.1016/j.jaci.2015.04.047
- Klimek L , Uhlig J , Mösges R , Rettig K . O Pfaar A high polymerized grass pollen extract is efficacious and safe in randomized double-blind, placebo-controlled study using a novel up-dosing cluster-protocol. Allergy 2014; 69(12): 1629-38. Published online 2014 Oct 6. https://doi.org/10.1111/all.12513
- Passalacqua G , Canonica GW . Allergen Immunotherapy: History and Future Developments. Immunol Allergy Clin North Am 2016; 36:1-12; PMID:26617223; https://doi.org/10.1016/j.iac.2015.08.001
- Di Bona D , Plaia A , Leto-Barone MS , La Piana S , Di Lorenzo G . Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol 2012; 130:1097-107; PMID:23021885; https://doi.org/10.1016/j.jaci.2012.08.012
- Nelson HS , Makatsori M , Calderon MA . Subcutaneous Immunotherapy and Sublingual Immunotherapy: Comparative Efficacy, Current and Potential Indications, and Warnings–United States Versus Europe. Immunol Allergy Clin North Am 2016; 36:13-24; PMID:26617224; https://doi.org/10.1016/j.iac.2015.08.005
- Kiel M , Röder E , Gerth van Wijk R , Al M , Hop W , Rutten-van Mölken M . Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol 2013; 132:353-60; PMID:23651609; https://doi.org/10.1016/j.jaci.2013.03.013
- Dahl R , Kapp A , Colombo G , de Monchy JG , Rak S , Emminger W , Rivas MF , Ribel M , Durham SR . Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006; 118:434-40; PMID:16890769; https://doi.org/10.1016/j.jaci.2006.05.003
- Didier A , Malling HJ , Worm M , Horak F , Jäger S , Montagut A , André C , de Beaumont O , Melac M . Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5 grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol 2007; 120:1338-45; PMID:17935764; https://doi.org/10.1016/j.jaci.2007.07.046
- Durham SR , Penagos M . Sublingual or subcutaneous immunotherapy for allergic rhinitis?. J Allergy Clin Immunol 2016; 137:339-349; PMID:26853126; https://doi.org/10.1016/j.jaci.2015.12.1298
- Nurmatov U , Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, Roberts G, Akdis C, Alvaro-Lozano M, Beyer K, et al. Allergen immunotherapy for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy 72(8):1133-1147; https://doi.org/10.1111/all.13124.
- Jones SM , Sicherer SH , Burks AW , Leung DY , Lindblad RW , Dawson P , Henning AK , Berin MC , Chiang D , Vickery BP , et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 2017; 139(4): 1242-1252.e9. https://doi.org/10.1016/j.jaci.2016.08.017
- Hylander T , Larsson O , Petersson-Westin U , Eriksson M , Kumlien Georén S , Winqvist O , Cardell LO . Intralymphatic immunotherapy of pollen-induced rhinoconjunctivitis: a double-blind placebo-controlled trial. Respir Res 2016; 17:10; https://doi.org/10.1186/s12931-016-0324-9
- Niederberger V , Horak F , Vrtala S , Spitzauer S , Krauth MT , Valent P , Valenta R . Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci 2004; 101:14677-14682; https://doi.org/10.1073/pnas.0404735101
- Weiss R , Scheiblhofer S , Machado Y , Thalhamer J . New approaches to transcutaneous immunotherapy: targeting dendritic cells with novel allergen conjugates. Curr Opin Allergy Clin Immunol 2013; 13(6):669-76; PMID:24169433; https://doi.org/10.1097/ACI.0b013e328364f4df
- Weinberger EE , Himly M , Myschik J , Hauser M , Altmann F , Isakovic A , Weiss R . Generation of hypoallergenic neoglycoconjugates for dendritic cell targeted vaccination: A novel tool for specific immunotherapy. J Control Release 2013; 165(2):101-109; PMID:23147517; https://doi.org/10.1016/j.jconrel.2012.11.002
- King TP , Jim SY , Monsalve RI , Kagey-Sobotka A , Lichtenstein LM , Spangfort MD . Recombinant allergens with reduced allergenicity but retaining immunogenicity of the natural allergens: hybrids of yellow jacket and paper wasp venom allergen antigen 5s. J Immunol 2001; 166:6057-65; PMID:11342623; https://doi.org/10.4049/jimmunol.166.10.6057
- Valenta R , Campana R , Focke-Tejkl M , Niederberger V . Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol 2016; 137:351-7; PMID:26853127; https://doi.org/10.1016/j.jaci.2015.12.1299
- Cornelius C , Schöneweis K , Georgi F , Weber M , Niederberger V , Zieglmayer P , Niespodziana K , Trauner M , Hofer H , Urban S , Valenta R . Immunotherapy with the PreS-based Grass Pollen Allergy Vaccine BM32 Induces Antibody Responses Protecting Against Hepatitis B Infection. EBioMedicine 2016; 11:58-67; PMID:27568223; https://doi.org/10.1016/j.ebiom.2016.07.023
- Meyer W , Narkus A , Salapatek AM , Häfner D . Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy 2013; 68:724-31; PMID:23621350; https://doi.org/10.1111/all.12148
- Moingeon P . Adjuvants for allergy vaccines. Hum Vaccin Immunother 2012; 8(10):1492-8; PMID:23095872; https://doi.org/10.4161/hv.21688
- Chesné J , S.-Schmidt-Weber CB , Esse von-Bieren J . The use of adjuvants for enhancing allergen immunotherapy efficacy. Immunol Allergy Clin North Am 2016; 36:125-45.
- Brehler R . [Adjuvants]. Der Hautarzt. 2017; 68(4):292-296.
- Klimek L , Schmidt-Weber CB , Kramer MF , Skinner MA , Heath MD . Clinical use of adjuvants in allergen-immunotherapy. Expert Rev Clin Immunol 2017; 13(6):599-610; PMID:28162007; https://doi.org/10.1080/1744666X.2017.1292133
- Leynadier F , Banoun L , Dollois B , Terrier P , Epstein M , Guinnepain MT , Firon D , Traube C , Fadel R , André C . Immunotherapy with a calcium phosphate-adsorbed five-grass-pollen extract in seasonal rhinoconjunctivitis: a double-blind, placebo-controlled study. Clin Exp Allergy 2001; 31(7):988-96; PMID:11467988; https://doi.org/10.1046/j.1365-2222.2001.01145.x
- Wilcock, LK , Francis JN , and Durham SR . Aluminium hydroxide down-regulates T helper 2 responses by allergen-stimulated human peripheral blood mononuclear cells. Clin Exp Allergy 2004; 34(9):1373-8; PMID:15347369; https://doi.org/10.1111/j.1365-2222.2004.02052.x
- Kramer MF , Heath, MD . Aluminium in allergen-specific subcutaneous immunotherapy - a german perspective. Vaccine 2014; 32:4140-8; PMID:24892252; https://doi.org/10.1016/j.vaccine.2014.05.063
- Jensen-Jarolim E . Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ J 2015; 8(1):7; PMID:25780491; https://doi.org/10.1186/s40413-015-0060-5
- Lauren CTBD , Morel KD , LaRussa P . Case Report of Subcutaneous Nodules and Sterile Abscesses Due to Delayed Type Hypersensitivity to Aluminum-Containing Vaccines. Pediatrics 2016; 138: pii: e20141690.
- Perricone C , Colafrancesco S , Mazor RD , Soriano A , Agmon-Levin N , Shoenfeld Y . Corrigendum to “Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects” [J. Autoimmun. 47C (2013) 1–16]. J Autoimmun 2016; 72:126; PMID:27269045
- Soriano A , Nesher G , Shoenfeld Y . Predicting post-vaccination autoimmunity: who might be at risk? Pharmacol Res 2015; 92:18-22.
- Baldrick P , Richardson D , Wheeler AW . Review of L -Tyrosine Confirming its Safe Human Use as an Adjuvant. Journal of Applied Toxicology 2002; 22:333-44; PMID:12355563; https://doi.org/10.1002/jat.869
- Wheeler AW , Moran DM , Robins BE , Driscoll A . L-Tyrosine as an immunological adjuvant. Int Arch Allergy Appl Immun 1982; 69:113-9; https://doi.org/10.1159/000233157
- Wheeler AW , Woroniecki SR . Immunological adjuvants in allergy vaccines: past, present and future. Allergol Int 2001; 50(4):295-301; https://doi.org/10.1046/j.1440-1592.2001.00230.x
- Baldrick P , Richardson, D , Wheeler AW . Review of l-tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol 2002; 22:333-44.
- DuBuske LM , Frew, AJ , Horak, F , Keith PK , Corrigan CJ , Aberer W , Holdich T , von Weikersthal-Drachenberg KJ . Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc 2011; 32:239-47; PMID:21535913; https://doi.org/10.2500/aap.2011.32.3453
- Irache JM , Esparza I , Gamazo C , Agüeros M , Espuelas, S . Nanomedicine: novel approaches in human and veterinary therapeutics. Vet Parasitol 2011;180(1–2):47-71; PMID:21680101; https://doi.org/10.1016/j.vetpar.2011.05.028
- O'Neill LAJ , Golenbock D and Bowie AG . The history of Toll-like receptors, redefining innate immunity. Nature Reviews Immunology 2013; 13:453-60; PMID:23681101; https://doi.org/10.1038/nri3446
- Elinav E , Strowig T , Henao-Mejia J , Flavell RA . Regulation of the antimicrobial response by NLR proteins. Immunity 2011; 34:665-79; PMID:21616436; https://doi.org/10.1016/j.immuni.2011.05.007
- Loo Y-M , Gale M . Immune signaling by RIG-I-like receptors. Immunity 2011;34:680-92; PMID:21616437; https://doi.org/10.1016/j.immuni.2011.05.003
- Taro K and Akira S . The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology 2010; 11,373-84; PMID:20404851; https://doi.org/10.1038/ni.1863
- O'Hagan DT. , De Gregorio E . The path to a successful vaccine adjuvant. The long and winding road. Drug Discovery Today 2009; 14:541-51; https://doi.org/10.1016/j.drudis.2009.02.009
- Rosewich MGK , Zielen S , Schubert R , Schulze J . Induction of Bronchial Tolerance After 1 Cycle of Monophosphoryl-A-Adjuvanted SpecificImmunotherapy in Children With Grass Pollen Allergies. Allergy Asthma Immunol Res 2016; 8:253-67.
- Mothes N1HM , Drachenberg KJ , Sperr WR , Krauth MT , Majlesi Y , Semper H , Valent P , Niederberger V , Kraft D , Valenta R . Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy 2003; 33:1198-208… PMID:12956739
- Gómez S , Gamazo C , San Román B , Ferrer M , Sanz ML , Espuelas S , Irache JM . Allergen immunotherapy with nanoparticles containing lipopolysaccharide from Brucella ovis. Eur. J. Pharm. Biopharm 2008; 70:711-717; PMID:18582571; https://doi.org/10.1016/j.ejpb.2008.05.016
- Burdette DL , and Vance RE . STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 2013; 14: 19-26; PMID:23238760; https://doi.org/10.1038/ni.2491
- Creticos PS , Schroeder, JT , Hamilton RG , Balcer-Whaley SL , Khattignavong AP , Lindblad R , Li H , Coffman R , Seyfert V , Eiden JJ , et al. Immunotherapy with a ragweed–toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med 2006; 355:1445-55.
- Klimek L , Bachmann MF , Senti G , Kündig TM . Immunotherapy of type-1 allergies with virus-like particles and CpG-motifs. Expert Rev Clin Immunol 2014;. 10(8):1059-67.
- Tang ML , Ponsonby, AL , Orsini F , Tey D , Robinson M , Su EL , Licciardi P , Burks W , Donath S . Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol 2015; 135:737–44…; PMID:25592987; https://doi.org/10.1016/j.jaci.2014.11.034
- Ghadimi DF-HR , de Vrese M , Winkler P , Heller KJ , Schrezenmeir J . Effects of probiotic bacteria and their genomic DNA on Th1/Th2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology 2008; 213:677-92; PMID:18950596; https://doi.org/10.1016/j.imbio.2008.02.001
- Zahra Aryan, EC , Giorgio Walter Canonica , Nima Rezaei . Allergen-specific immunotherapy in asthmatic children: from the basis to clinical applications. Clin Exp Vaccines 2013; 12:639-59; https://doi.org/10.1586/erv.13.45
- Petrarca C , Clemente E , Amato V , Gatta A , Cortese S , Lamolinara A , Rossi C , Zanotta S , Mistrello G , Paganelli R , et al. Vitamin D3 improves the effects of low dose Der p 2 allergoid treatment in Der p 2 sensitized BALB/c mice. Clin Mol Allergy 2016; 14:7.
- Baris S. , Kiykim A. , Ozen A. , Tulunay A. , Karakoc-Aydiner E. , Barlan, IB . -Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014; 69(2):246-53; PMID:24180595; https://doi.org/10.1111/all.12278
- Jerzynsak J , Stelmach W , Rychlik B , Lechańska J , Podlecka D , Stelmach I . The clinical effect of vitamin D Supplementation combined with grass-specific sublingual immunotherapy in children with allergic rhinitis. Allergy Asthma Proc 2016; 37:105-14; PMID:26932169; https://doi.org/10.2500/aap.2016.37.3921
- Penna G , Adorini L . 1 Alpha, 25-di-hydroxyvitamin D3 inhibits differentiation,maturation, activation, and survival of den-dritic cells leading to impaired alloreactive Tcell activation. J Immunol 2000; 164:2405-11; PMID:10679076; https://doi.org/10.4049/jimmunol.164.5.2405
- Cantorna MT , Snyder L , Lin YD , Yang L . Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015; 7(4):3011-21; https://doi.org/10.3390/nu7043011
- van Hemelen D . Adjuvants for immunotherapy: lost in translation? Clin Exp Allergy 2009; 39(12):1783-5; https://doi.org/10.1111/j.1365-2222.2009.03396.x
- Schöll I , Weissenböck, A , Förster-Waldl, E , Untersmayr E , Walter F , Willheim M , Boltz-Nitulescu G , Scheiner O , Gabor F , Jensen-Jarolim E . Allergen-loaded biodegradable poly(D,L-lactic-coglycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy 2004; 34:315-21; PMID:14987314; https://doi.org/10.1111/j.1365-2222.2004.01884.x
- Sanders MT , Brown, LE , Deliyannis, G , Pearse MJ . ISCOM-based vaccines: the second decade. Immunol Cell Biol 2005; 83:119-28; PMID:15748208; https://doi.org/10.1111/j.1440-1711.2005.01319.x
- Yoksan R , Chirachanchai S . Amphiphilic chitosan nanosphere: studies on formation, toxicity, and guest molecule incorporation. Bioorg Med Chem 2008; 16(5):2687-96; PMID:18061462; https://doi.org/10.1016/j.bmc.2007.11.037
- Ojer P , de Cerain AL , Areses P , Peñuelas I , Irache JM . Toxicity studies of poly(anhydride) nanoparticles as carriers for oral drug delivery. Pharm Res 2012; 29(9):2615-27; PMID:22638871; https://doi.org/10.1007/s11095-012-0791-8
- Schöll I , Kopp T , Bohle B , Jensen-Jarolim E . Biodegradable PLGA particles for improved systemic and mucosal treatment of Type I allergy. Immunol Allergy Clin N Am 2006; 26:349-64; https://doi.org/10.1016/j.iac.2006.02.007
- Pandey RS , Sahu S , Sudheesh MS , Madan J , Kumar M , Dixit VK . Carbohydrate modified ultrafine ceramic nanoparticles for allergen immunotherapy. Int Immunopharmacol 2011; 11:925-31; PMID:21333772; https://doi.org/10.1016/j.intimp.2011.02.004
- Wang CQ , Wu JL , Zhuo RX , Cheng SX . Protamine sulfate-calcium carbonate-plasmid DNA ternary nanoparticles for efficient gene delivery. Mol Biosyst 2014; 10(3):672-8; PMID:24442276; https://doi.org/10.1039/c3mb70502a
- Nouri HR , Varasteh A , Jaafari MR , Davies JM , Sankian M . Induction of a Th1 immune response and suppression of IgE via immunotherapy with a recombinant hybrid molecule encapsulated in liposome-protamine-DNA nanoparticles in a model of experimental allergy. Immunol Res 2015; 62(3):280-91; https://doi.org/10.1007/s12026-015-8659-8
- Pali-Schöll I , Szöllösi H , Starkl P , Scheicher B , Stremnitzer C , Hofmeister A , Roth-Walter F , Lukschal A , Diesner SC , Zimmer A , Jensen-Jarolim E . Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur J Pharm Biopharm 2013; 85 656-64; PMID:23523543; https://doi.org/10.1016/j.ejpb.2013.03.003
- Beilvert F , Tissot A , Langelot M , Mevel M , Chatin B , Lair D , Magnan A , Pitard B . DNA/amphiphilic block copolymer nanospheres reduce asthmatic response in a mouse model of allergic asthma. Hum Gene Ther 2012; 23:597-608; PMID:22429072; https://doi.org/10.1089/hum.2012.024
- Gamazo CGG , Ferrer M , Sanz ML , Irache JM . Nanoparticle based-immunotherapy against allergy. Immunotherapy 2014(6):885-97; PMID:25290419; https://doi.org/10.2217/imt.14.63
- Pecquet S , Leo E , Fritsche R , Pfeifer A , Couvreur P , Fattal E . Oral tolerance elicited in mice by β-lactoglobulin entrapped in biodegradable microspheres. Vaccine 2000; 18:1196-202; PMID:10649620; https://doi.org/10.1016/S0264-410X(99)00384-9
- Srivastava KD , Siefert A , Fahmy TM , Caplan MJ , Li XM , Sampson HA . Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol 2016; https://doi.org/10.1016/j.jaci.2016.01.047
- Bohle B , Jahn-Schmid B , Maurer D , Kraft D , Ebner C . Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-g production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur J Immunol 1999; 29:2344-53; PMID:10427997; https://doi.org/10.1002/(SICI)1521-4141(199907)29:07%3c2344::AID-IMMU2344%3e3.0.CO;2-R
- Ochoa J , Irache JM , Tamayo I , Walz A , DelVecchio VG , Gamazo C . Protective immunity of biodegradable nanoparticle-based vaccine against an experimental challenge with Salmonella Enteritidis in mice. Vaccine 2007; 25:4410-19; PMID:17434651; https://doi.org/10.1016/j.vaccine.2007.03.025
- Gómez S , Gamazo C , San Roman B , Vauthier C , Ferrer M , Irache JM . Development of a novel vaccine delivery system based on Gantrez nanoparticles. J Nanosci Nanotechnol 2006; 6:3283-3289; https://doi.org/10.1166/jnn.2006.471
- Camacho AI , Da Costa Martins R , Tamayo I , de Souza J , Lasarte JJ , Mansilla C , Esparza I , Irache JM , Gamazo C . Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine 2011; 29:7130-5; PMID:21651945; https://doi.org/10.1016/j.vaccine.2011.05.072
- Denzel A , Maus UA , Rodriguez Gomez M , Moll C , Niedermeier M , Winter C , Maus R , Hollingshead S , Briles DE , Kunz-Schughart LA , et al. Basophils enhance immunological memory responses. Nat Immunol 2008; 9:733-42; PMID:18516038; https://doi.org/10.1038/ni.1621
- Gomez S , Gamazo C , San Roman B , Ferrer M , Sanz ML , Irache JM . Gantrez AN nanoparticles as an adjuvant for oral immunotherapy with allergens. Vaccine 2007; 25:5263-71; PMID:17576025; https://doi.org/10.1016/j.vaccine.2007.05.020
- De Souza Reboucas J , Irache JM , Camacho AI , Gastaminza G , Sanz ML , Ferrer M , Gamazo C . Immunogenicity of peanut proteins containing poly(anhydride) nanoparticles. Clin Vaccine Immunol 2014; 21:1106-12; PMID:24899075; https://doi.org/10.1128/CVI.00359-14
- Carroll EC , Jin L , Mori A , Munoz-Wolf N , Oleszycka E , Moran HB , Mansouri S , McEntee CP , Lambe E , Agger EM , et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 2016; 44:597-608; PMID:26944200; https://doi.org/10.1016/j.immuni.2016.02.004
- Sogias IA , Williams AC , Khutoryanskiy VV . Why is chitosan mucoadhesive? Biomacromolecules 2008; 9(7):1837-42.
- Roy K , Mao HQ , Huang SK , Leong KW . Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med 1999; 5:387-91; PMID:10202926; https://doi.org/10.1038/7385
- Chew JL , Wolfowicz CB , Mao HQ , Leong KW , Chua KY . Chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in mice. Vaccine 2003; 21:2720-29; PMID:12798609; https://doi.org/10.1016/S0264-410X(03)00228-7
- Li GP , Liu ZG , Liao B , Zhong NS . Induction of Th1-type immune response by chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen Der p 2 for oral vaccination in mice. Cell Mol. Immunol 2009; 6:45-50; PMID:19254479; https://doi.org/10.1038/cmi.2009.6
- Chesné J , Schmidt-Weber CB , Esser von-Bieren J . The use of adjuvants for enhancing allergen immunotherapy efficacy. Immunol Allergy Clin North Am 2016; 36(1):125-45; PMID:26617231; https://doi.org/10.1016/j.iac.2015.08.009
- Mold M , Shardlow E , Exley C . Insight into the cellular fate and toxicity of aluminium adjuvants used in clinically approved human vaccinations. Sci Rep 2016; 6:31578; PMID:27515230; https://doi.org/10.1038/srep31578
- Committee World Health Organization Vaccine Safety Advisory . Macrophagic myofasciitis and aluminum-containing vaccines. Wkly Epidemiol Rec 1999; 74:338-40.
- Gherardi RK , Aouizerate J , Cadusseau J , Yara S , Authier FJ . Morphologie. Aluminum adjuvants of vaccines injected into the muscle: Normal fate, pathology and associated disease. Morphologie 2016; 100(329):85-94; https://doi.org/10.1016/j.morpho.2016.01.002
- De Souza Rebouças J , Esparza I , Ferrer M , Sanz ML , Irache JM , Gamazo C . Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J Biomed Biotechnol 2012; 2012:474605; PMID:22496608; https://doi.org/10.1155/2012/474605
- Anderson RC , Fox CB , Dutill TS , Shaverdian N , Evers TL , Poshusta GR , Chesko J , Coler RN , Friede M , Reed SG , et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids and Surfaces B: Biointerfaces 2010; 75:123-32; https://doi.org/10.1016/j.colsurfb.2009.08.022
- Schülke S , Vogel L , Junker AC , Hanschmann KM , Flaczyk A , Vieths S , Scheurer S . A fusion protein consisting of the vaccine adjuvant monophosphoryl lipid A and the allergen ovalbumin boosts allergen-specific Th1, Th2, and Th17 responses in vitro. J Immunol Res 2016; 2016:415-426.
- Johansen P , von Moos S , Mohanan D , Kündig TM , Senti G . New routes for allergen immunotherapy. Hum Vaccin Immunother 2012; 8(10):1525-33; PMID:23095873; https://doi.org/10.4161/hv.21948
- Irache JM , Salman HH , Gomez S , Espuelas S , Gamazo C . Poly(anhydride) nanoparticles as adjuvants for mucosal vaccination. Frontiers in Bioscience 2010; 2:876-90; https://doi.org/10.2741/s108
- Petrovsky, N and Aguilar JC . Vaccine adjuvants: Current state and future trends. Immunology and Cell Biology 2004; 82:488-96; PMID:15479434; https://doi.org/10.1111/j.0818-9641.2004.01272.x
- Moller C , Dreborg S , Ferdousi HA , Halken S , Høst A , Jacobsen L , Koivikko A , Koller DY , Niggemann B , Norberg LA , et al. Pollen immunotherapy reduces thedevelopment of asthma in children with seasonal rhinoconjunctivitis (the PATstudy). J Allergy Clin Immunol 2002; 109:251-6.
- Marogna M , Tomassetti D , Bernasconi A , Colombo F , Massolo A , Businco AD , Canonica GW , Passalacqua G , Tripodi S . Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol 2008; 101:206-11; PMID:18727478; https://doi.org/10.1016/S1081-1206(10)60211-6
- Durham SR , Yang WH , Pedersen MR , Johansen N , Rak S . Sublingual immunotherapy with once daily grass-allergen tablets: a randomised controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006; 117:802; PMID:16630937; https://doi.org/10.1016/j.jaci.2005.12.1358
- Creticos PS , Maloney J , Bernstein DI , Casale T , Kaur A , Fisher R , Liu N , Murphy K , Nékám K , Nolte H . Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol 2013; 131:1342-9; PMID:23622121; https://doi.org/10.1016/j.jaci.2013.03.019
- Fiocchi A , Burks, W , Bahna, SL , Bielory L , Boyle RJ , Cocco R , Dreborg S , Goodman R , Kuitunen M , Haahtela T , et al. Clinical use of probiotics in pediatric allergy (CUPPA): a World Allergy Organization position paper. World Allergy Organ J 2012; 5:148-67.
