ABSTRACT
Objective: To evaluate the long-term persistence of anti-hepatitis B surface (HBs) antibodies and the response to a HB challenge re-vaccination in children who had received a primary series of DTaP-IPV-HB-PRP∼T (Hexaxim™) or DTaP-IPV-HB/PRP∼T (Infanrix hexa™).
Methods: Two cohorts of participants who had previously received HB vaccine at birth followed by either DTaP-IPV-HB-PRP∼T or DTaP-IPV-HB/PRP∼T co-administered with PCV7 at 2, 4, 6 months of age in a randomized, Phase III, observer-blind study in Thailand, were followed up for anti-HBs antibodies (geometric mean concentrations [GMCs] and seroprotection [SP] rate [% of participants with a titer ≥10 mIU/mL]) at 12–18 months of age and 9–10 years of age. A monovalent HB challenge re-vaccination was administered at 9–10 years of age and the anamnestic response was evaluated.
Results: Anti-HBs GMCs and SP rates in the DTaP-IPV-HB-PRP∼T and DTaP-IPV-HB/PRP∼T groups were high and similar post-primary vaccination series (2477 mIU/mL and 99.5% and 2442 mIU/mL and 99.5%, respectively) and declined to a similar extent in each group at 12–18 months (154.5 mIU/mL and 90.8% and 162.3 mIU/mL and 96.5%, respectively). Antibody levels further declined at 9–10 years of age (13.3 mIU/mL and 49.3% and 8.0 mIU/mL and 42.9%) and a strong anamnestic response occurred in each group post-HB challenge re-vaccination (92.8% and 98.7%, respectively).
Conclusion: The kinetics of long-term anti-HBs antibody persistence were similar following a primary series of DTaP-IPV-HB-PRP∼T or DTaP-IPV-HB/PRP∼T. The response to a subsequent HB challenge re-vaccination was strong and similar in each group, demonstrating persisting immune memory.
Introduction
Multivalent pediatric vaccines are vital in achieving and maintaining high vaccine coverage against childhood diseases such as diphtheria (D), tetanus (T), pertussis, poliomyelitis, Haemophilus influenzae type b (Hib) invasive infections. The most modern of these vaccines are hexavalent including a hepatitis B antigen. Two such vaccines are currently widely available, a DTaP-IPV-HB/PRP∼T hexavalent vaccine (Infanrix hexa™) that is reconstituted prior to useCitation1-3 and a fully liquid DTaP-IPV-HB-PRP∼T hexavalent vaccine (Hexaxim™, Hexyon™, or Hexacima™, depending on the country of sale).Citation4-7 The fully liquid vaccine was first licensed in 2012. It is now licensed in 105 countries worldwide with more than 32 million doses having been distributed in 70 countries, and is pre-qualified by the World Health Organization.Citation8
Hexaxim is based on previous widely used and well-established tetravalent DTaP-IPV and pentavalent DTaP-IPV/PRP∼T vaccines (Tetraxim™/Tetravac™ and Pentaxim™/Pentavac™, respectively, depending on the country of sale)Citation9,Citation10 with the addition of 10 µg Hansenula polymorpha-derived HB surface antigen (HBsAg). The good safety and immunogenicity of this HBsAg have been well documented for both monovalent administration and as part of the hexavalent vaccine.Citation5,Citation11 The hexavalent vaccine has been shown to be safe and immunogenic in four continents when administered in a variety of pediatric vaccination schedules with or without coadministration of other common childhood vaccines; the HBsAg has been shown to be immunogenic in schedules with and without administration of a standalone HB vaccine prior to the first primary series dose of hexavalent vaccine according to national immunization schedules, including the challenging 6, 10, 14 week and 2, 3, 4 month schedules, and following a booster in the second year of life.Citation12-24
The persistence of immunity against HB virus (HBV) after vaccination in infancy is important as it drives the adult booster vaccination recommendations that should be in place for all infant cohorts routinely vaccinated against HBV and living in areas where the HBV endemicity and exposure justify the maintenance of continued immunity. Based on evidence over the last 3 decades, several national vaccination policy bodies have issued recommendations for HB vaccination where revaccination (boosters) is not justified in infants who have responded well to a primary HB vaccination seriesCitation25,Citation26 but some debate still persists.Citation27-29
A number of studies have shown that T-cell and B-cell memory against HBV can persist in individuals previously vaccinated with HBs-containing combination vaccines decades after the primary vaccination series with no regular booster vaccinationCitation30-33 and that this persistent memory can be observed even in the absence of detectable anti-HBs antibodies.Citation34,Citation35 This observation has even been reported for a previously licensed fully liquid DTaP-IPV-HB-PRP∼T vaccine (Hexavac™) that has been shown to induce lasting immune memoryCitation36-38 despite having initially presented with sub-optimal early anti-HBs responses.Citation39,Citation40
A previous study evaluated cohorts of participants who received either Hexaxim or Infanrix hexa at 2, 4, 6 months of age in Thailand, co-administered with a 7-valent pneumococcal vaccine (PCV7) following a birth dose of standalone HB vaccine,Citation15 with no difference in immunogenicity or safety between groups. Since no HB booster vaccination was given to these participants, according to the Thai national vaccination recommendations, their follow-up allowed the evaluation of short-term and long-term persistence of the HBsAg antibodies following an HB vaccination at birth followed by a pediatric 2, 4, 6 month primary vaccination series. In addition, the subsequent response to a HB challenge re-vaccination at 9–10 years of age evaluated the quality of the residual immune memory still present at that time.
Results
Participants studied
A total of 412 participants were randomized to receive a primary series of either DTaP-IPV-HB-PRP∼T and PCV7 (N = 206) or DTaP-IPV-HB/PRP∼T and PCV7 (N = 206).Citation15 Of these, a blood sample was drawn from 65 and 57 participants, respectively, for the assessment of anti-HBs antibody persistence at 12–18 months of age. At 9–10 years of age 71 and 79 participants, respectively, received the HB challenge re-vaccination and provided blood samples pre- and post-vaccination (all of whom had provided a blood sample post-primary vaccination series). Overall, 27 and 28 participants provided a blood sample at each of the three timepoints (post-primary vaccination, 12–18 months and 9–10 years of age). Participant disposition is presented in . The sex ratio (male:female) of the two groups of subjects who contributed to the follow-up evaluations was similar across the two groups post-primary series vaccination and at both 12–18 months and 9–10 years of age (overall 1.7 for the DTaP-IPV-HB-PRP∼T and PCV7 group and 1.8 for the DTaP-IPV-HB/PRP∼T and PCV7).
Immunogenicity
Anti-HBsAg GMCs and SP rates were high and similar in each group at 1 month after the primary series vaccination ( and ).Citation15 Anti-HBs GMCs decreased 6–12 months later (12-18 months of age) to a similar extent in both groups (from 2477 mIU/mL to 154.5 mIU/mL in the DTaP-IPV-HB-PRP∼T and PCV7 group and from 2442 to 162.3 mIU/mL in the DTaP-IPV-HB/PRP∼T and PCV7 group) and SP rates remained similar in each group ( and ).
Figure 2. Anti-HBs antibody GMC profile post-primary vaccination, persistence at 12–18 months and 9–10 years of age, and response to HB challenge re-vaccination at 9–10 years of age.
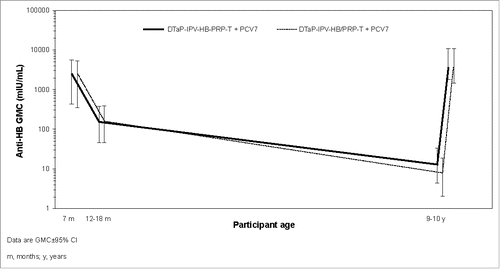
Table 1. Anti-HBs antibody response post-primary vaccination, persistence at 12–18 months and 9–10 years of age, and response to HB challenge re-vaccination at 9–10 years of age.
Anti-HBs antibody levels continued to decrease at 9–10 years after the primary series to a similar extent in each group: anti-HBs GMCs prior to the HB challenge re-vaccination were 13.3 mIU/mL and 8.0 mIU/mL, respectively. In terms of SP rate, the persistence of anti-HBs antibody concentrations ≥10 mIU/mL was similar in each group (49.3% and 42.9%, respectively) ().
An anamnestic response was observed that was similar in each group (92.8% and 98.7%) after the HB challenge re-vaccination at 9–10 years of age with GMCs increasing to 3692 mIU/mL and 4241 mIU/mL, respectively, and SP rates of 92.8% and 98.7% (≥10 mIU/mL) and 89.9% and 97.4% (≥100 mIU/mL) ( and ).
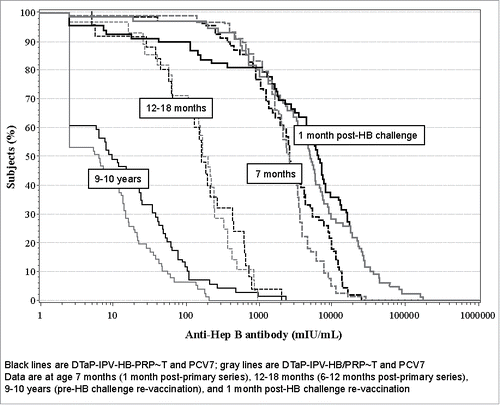
The reverse cumulative distribution curves () show the strong anti-HBs responses post-primary vaccination series and post-HB challenge re-vaccination, as well as the declining antibody levels at 12–18 months and 9–10 years of age.
Figure 3. Reverse cumulative distribution curves for anti-HB antibody post-primary vaccination, persistence at 12–18 months and 9–10 years of age, and response to HB challenge re-vaccination at 9–10 years of age.
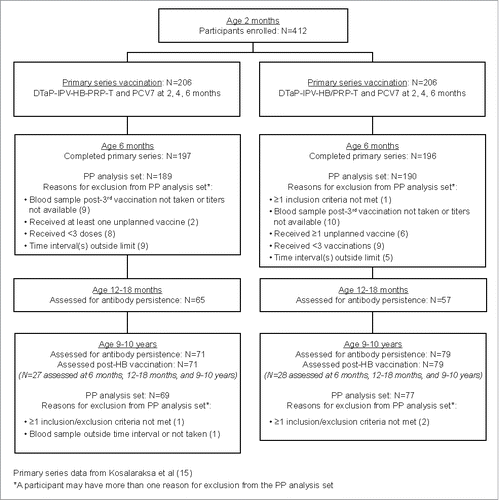
Individual anti-HBs results for the participants who provided samples post-primary vaccination series and 9–10 years of age, irrespective of whether a sample was provided at 12–18 months of age (N = 71 and N = 79 for the DTaP-IPV-HB-PRP∼T and DTaP-IPV-HB/PRP∼T groups, respectively) are presented in . These data support the overall anti-HBs profiles described for each group. However, five participants who had received DTaP-IPV-HB-PRP∼T and one participant who had received DTaP-IPV-HB/PRP∼T did not develop anti-HBs antibody levels above the 10 mIU/mL threshold after the HB challenge re-vaccination. Of these, one of the five participants who had received DTaP-IPV-HB-PRP∼T and the participant who had received DTaP-IPV-HB/PRP∼T had not responded to the initial primary series and were considered to be non-responders. No assessment of anti-HBc antibody or HBs antigen was performed for these participants.
Safety
The primary series vaccinations were well tolerated in each group with no vaccine-related SAEsCitation15 and there were no immediate AEs nor any SAEs following the HB challenge re-vaccination.
Discussion
Pediatric vaccination against HBV can be performed using a range of schedules depending on national specificities.Citation41 The HB antigen is generally delivered using combination vaccines with the exception of HB vaccination at birth, which is administered using a standalone monovalent HB vaccine if required by national recommendations to protect against maternal HB virus transmission in areas of high endemicity.Citation26 Although the exact duration of protection is not defined for all age groups, primary vaccination series, and types of vaccine used for immune priming, both long-term persistence of anti-HBs antibodies and HB immune memory up to 20–30 years after the primary vaccination series have been demonstrated.Citation30-32,Citation34,Citation42-50 Long-term protection against HB is perhaps more dependent on persistence of immune memory than the persistence of detectable antibodies per se and according to WHO ‘the substantial body of evidence does not provide a compelling basis for recommending a booster dose of hepatitis B vaccine after completion of the primary vaccination series for persons with normal immune status’.Citation26 As such, although booster vaccination against HB is often performed in the second year of life for operational simplicity, e.g. if a HB antigen is contained in the booster combination vaccine, this is not considered to be essential.
The present studies represent the longest follow-up (9-10 years) of anti-HBs antibody persistence for the DTaP-IPV-HB-PRP∼T vaccine. Good persistence was shown in the second year of life following a schedule combining HB vaccination at birth with a 2, 4, 6 month HBsAg-containing hexavalent primary series and no further booster vaccination; there was no difference between participants who received Hexaxim or Infanrix hexa. Although antibody levels were lower at 9–10 years of age, a strong anamnestic response was demonstrated for the vast majority of participants following a challenge re-vaccination with a standalone HB vaccine. These results demonstrate that persisting immune memory resulting from good priming is more important than actual levels of anti-HBs antibodies in terms of a strong resultant anti-HBs response to subsequent challenge vaccination, and further support the WHO statement that the evidence for routine booster HB vaccination is not compelling. Studies in areas of high endemicity have shown that although breakthrough HB virus infections can occur, fully vaccinated individuals do not develop clinical symptoms.Citation51,Citation52 Since fully vaccinated individuals will inevitably include a small percentage of non-responders, as observed in the present studies, even individuals who do not respond to a secondary HB challenge re-vaccination would therefore not be expected to develop clinical manifestations if exposed to HB virus. However, with less than 50% of participants in each group having anti-HBs ≥10 mIU/mL at 9–10 years of age a monovalent HB vaccine booster, with low reactogenicity and a good anamnestic response, could be considered at that time in areas of high HB endemicity. Importantly, long-term follow up of cohorts who had received Hexavac, which was withdrawn by the EMA as described earlier due to concerns over long-term HB protection,Citation39,Citation40 has shown no breakthrough HB infection.Citation38 Moreover, 92% of Hexavac-vaccinated infants showed an anamnestic response to a HB booster at 4–7 years of age.Citation37 These and other studies further support the importance of the role played by immune memory rather than simply anti-HBs antibodiesCitation30,Citation32,Citation34,Citation35,Citation42,Citation44–47,Citation53,Citation54 in long-term protection against HB.
A limitation of the present studies is that it was not possible to collect follow-up data for all participants included in the primary series study. Due to this reduced sample size it is not possible to accurately define the distribution of non-responders between the Hexaxim and Infanrix hexa groups, however, as an observational follow up study, the sample size is considered to be sufficient to support a descriptive assessment of anti-HBs antibody persistence and the effect of an HB challenge re-vaccination 9–10 years after a three dose primary vaccination series. Additionally, as this was an observational study and the sample size was strictly controlled the study was not powered to perform any statistical analyses, such as non-inferiority, and the group comparison is descriptive. We also accept the possibility of a natural booster effect, but this was not evaluated and also represents a study limitation. A last limitation is that anti-HBc and HBsAg data were not collected.
In conclusion, the results described are consistent with previous observations following the administration of similar vaccines in similar cohorts that were followed for similar periods of time and support the long-term persistence of clinical protection against HB virus infection afforded by multivalent HB-containing vaccines. The importance of immune memory is underlined, with strong anamnestic responses observed even in individuals with low anti-HBs antibody levels.
Materials and methods
Study design and participants
A Phase III randomized, observer-blind, controlled, trial was conducted in 4 study sites to investigate the safety and immunogenicity of a primary series of the DTaP-IPV-HB-PRP∼T vaccine versus the DTaP-IPV-HB/PRP∼T vaccine given at 2, 4, 6 months of age and co-administered with PCV7 in Thailand between October 2006 and November 2007Citation15 (ClinicalTrials.gov identifier: NCT00401531). All study participants had received a standalone HB vaccine at birth. Blood samples were subsequently collected at 12–18 months of age within the scope of a separate evaluation of the immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine (NCT02610348)Citation55 and at 9–10 years of age (NCT02697474) to evaluate long-term persistence of anti-HBs antibodies. At 9–10 years of age all participants received a standalone HB vaccine and their anti-HBs response 1 month later was also assessed.
All study protocols and amendments were approved by independent ethics committees at each study site and the studies were performed according to Thai regulations, Good Clinical Practice, applicable International Conference on Harmonization guidelines, and the ethical principles of the Declaration of Helsinki (Edinburgh revision, October 2000). Signed informed consent was provided by at least one parent or legally acceptable representative of each participant for each part of the study, and signed assent was obtained from each participant at 9–10 years of age.
For the primary series study, healthy infants aged 2 months who had received a HB vaccine at birth, born at full-term (≥37 weeks), with birth weight ≥2.5 kg were eligible for inclusion in the study. Standard exclusion criteria were used which are described in full elsewhereCitation15 and included known personal or maternal history of hepatitis B (HBsAg carrier), human immunodeficiency virus (HIV), or hepatitis C seropositivity so that all participants were born to mothers uninfected with HB, HIV, and hepatitis C. Participants were randomized in a 1:1 ratio to receive either the investigational DTaP-IPV-HB-PRP∼T vaccine or the control DTaP-IPV-HB/PRP∼T vaccine at 2, 4, 6 months of age coadministered with PCV7. At 12–18 months of age blood samples were taken from participants who had received the primary vaccination seriesCitation55; as this was a non-interventional study no extra inclusion or exclusion criteria applied, although samples from participants who had received any vaccine containing D, T, aP, IPV, HB, or PRP∼T since the primary series were not analyzed. At 9–10 years of age, participants who had received the primary series vaccines were again re-contacted and received a standalone HB (challenge) re-vaccination. For participants with a low anti-HBs response (<10 mIU/mL) after the HB challenge re-vaccination, two additional standalone HB vaccinations were provided outside the scope of the study. Participants were excluded at 9–10 years of age for any of the following reasons: participation in another clinical trial in the previous 4 weeks, history of HB infection, receipt of HB vaccine since the primary series, receipt of any vaccine in the previous 4 weeks (except for Bacille Calmette Guerin vaccine or oral poliovirus vaccine [OPV] given in the context of an OPV national immunization day), receipt of blood products or immunosuppressant drugs in the previous 3 months, acquired immunodeficiency or hepatitis C infection since the primary series study, chronic illness following the primary series (e.g. leukemia, lymphoma, Crohn's disease), and any acute illness or febrile illness.
The hexavalent and PCV7 primary series vaccines were administered intramuscularly into the anterolateral area of the right and left thigh, respectively; the HB challenge re-vaccination was given by intramuscular administration into the deltoid muscle.
Vaccines
The investigational DTaP-IPV-HB-PRP∼T vaccine (Hexaxim: batch number S4106) was manufactured by Sanofi Pasteur, France and supplied as a preservative-free, fully liquid suspension for injection in single dose pre-filled syringes. Each 0.5 mL dose contained ≥20 IU (30 limit of flocculation [Lf]) D-toxoid, ≥40 IU (10 Lf) T-toxoid, 25 µg PT, 25 µg FHA, 40, 8 and 32 D antigen units of IPV type 1, 2 and 3, respectively, 10 µg HBsAg, 12 µg Hib polysaccharide conjugated to 22–36 µg tetanus toxoid protein (PRP∼T), and 0.6 mg aluminum hydroxide.
The control DTaP-IPV-HB/PRP∼T vaccine (Infanrix hexa™: batch number A21CA130A) was manufactured by GlaxoSmithKline (GSK) Biologicals and supplied as two separate components (a DTaP-HB-IPV vaccine suspension in a pre-filled syringe and a PRP∼T vaccine as a white lyophilized pellet in a glass vial) that were to be reconstituted as a 0.5 mL dose prior to injection. Each dose contained ≥30 IU of D-toxoid, ≥40 IU T-toxoid, 25 µg PT, 25 µg FHA, 8 µg pertactin, 40, 8 and 32 antigen units of IPV type 1, 2 and 3, respectively, 10 µg HBsAg, and a total aluminum content of 0.82 mg (0.5 mg hydrated aluminum hydroxide and 0.32 mg aluminum phosphate).
The HB vaccine administered at birth was in accordance with the national immunization calendar in Thailand and outside the scope of this study. The recombinant standalone HB vaccine administered at 9–10 years of age (Euvax-B™: batch number UFA14010) was manufactured by LG Life Sciences and presented as a thimerosal-preserved liquid suspension in multi-dose glass vials. Each 0.5 mL dose contained 10 µg purified HBsAg and 0.25 mg aluminium hydroxide gel.
The PCV7 vaccine (Prevnar™: batch number 20882) was manufactured by Wyeth (now Pfizer Incorporated) and supplied in single-dose pre-filled syringes.
Serology
Anti-HBs antibodies were analyzed using blood samples taken at 1 month after the primary series (7 months of age), at 12–18 months of age, prior to the HB challenge re-vaccination at 9–10 years of age, and 1 month after the HB challenge re-vaccination.
All serological analysis of anti-HBsAg antibodies was performed in a blinded manner at the Sanofi Pasteur Global Clinical Immunology laboratory (Swiftwater, PA, USA) using the commercially available VITROS ECi/ECiQ Immunodiagnostic System assay based on a chemiluminescence detection technology. The consistency of assay performance over time was demonstrated by trending of control serum samples that extended over the range of low, mid, and high titer sera.
Safety
Primary series reactogenicity and safety are reported elsewhere.Citation15 No safety data were collected at 12–18 months of age since no vaccine was administered. For the HB challenge re-vaccination at 9–10 years of age, participants were kept under observation for 30 minutes per usual practice following a vaccination, but due to the well-established good safety profile of the HB vaccine only SAEs were to be collected to 1 month post-vaccination. No other assessment of safety or reactogenicity was performed.
Statistical analyses
The objective of these three studies together was to describe the anti-HBsAg antibody profile at 1 month after a 2, 4, 6 months of age primary series vaccination of the investigational and control vaccines, co-administered with PCV7, at 12–18 months of age, and before and after a HB challenge re-vaccination at 9–10 years of age. With the exception of the primary series, for which a non-inferiority analysis is described elsewhere,Citation15 all analyses were descriptive.
The confidence interval (CI) for single proportions were calculated using the exact binomial method (Clopper-Pearson method).Citation56 For the anti-HBs concentration data, assuming that the log10 transformation followed a normal distribution, at first, the mean and the 95% CI were calculated on log10 using the usual calculation for normal distribution (using Student's t distribution with n-1 degree of freedom), and then antilog transformations were applied to the results to provide geometric mean concentrations (GMCs) and their 95% CIs.
As well as GMCs, seroprotection (SP) rates were calculated based on thresholds of 10 mIU/mL and 100 mIU/mL, and the percentage of participants with an anamnestic response post-HB challenge re-vaccination was calculated for each group (see for definition). Reverse cumulative distribution curves of anti-HBs antibody concentrations for the four time points evaluated (i.e. 7 months of age, 12–18 months of age, and pre- and post-HB challenge re-vaccination at 9–10 years of age) were also prepared.
The sample size calculation for the primary series study is described elsewhere.Citation15 As many participants who had completed the primary series as possible were re-contacted at 12–18 months and 9–10 years.
The primary vaccination and HB challenge re-vaccination data are presented for the per protocol (PP) analysis set (all participants who received all planned vaccinations and had no protocol deviations). For the assessment at 12–18 months, all participants who provided a blood sample are included (no analysis sets were defined).
All statistical analyses were performed under the responsibility of the Sponsor's Biostatistics Platform using SAS® software, Version 9.2 or 9.4 (SAS Institute, Cary, North Carolina, USA).
Disclosure of potential conflicts of interest
No clinical investigator involved in this study received any direct payment from Sanofi Pasteur with regard to their contribution to this manuscript, but could receive expenses for conference attendance for the presentation of data from this study. SC, SB'C, XDC, and EV are employees of Sanofi Pasteur
Authors contributions
PK, KC, and SW contributed to the conception, design, and clinical conduct of the studies.
All authors contributed to the analysis and interpretation of study data and all approved the final version of this article.
Acknowledgments
The authors acknowledge the study personnel for the conduct of the studies and also all enrolled infants and parents/legal guardian(s) for their participation.
This manuscript was prepared with the assistance of a professional medical writer, Dr Andrew Lane (Lane Medical Writing) funded by Sanofi Pasteur, in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
All studies were funded by Sanofi Pasteur.
Additional information
Funding
References
- Dhillon S. DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa): A review of its use as primary and booster vaccination. Drugs. 2010;70(8):1021–58. doi:10.2165/11204830-000000000-00000. PMID:20481658.
- Zepp F, Schmitt HJ, Cleerbout J, Verstraeten T, Schuerman L, Jacquet JM. Review of 8 years of experience with Infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines. 2009;8(6):663–78. doi:10.1586/erv.09.32. PMID:19485747.
- Baldo V, Bonanni P, Castro M, Gabutti G, Franco E, Marchetti F, et al. Combined hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type B vaccine; Infanrix hexa: twelve years of experience in Italy. Human Vaccines Immunotherapeutics. 2014;10(1):129–37. doi:10.4161/hv.26269.
- Lyseng-Williams KA, McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexyon®/Haxacima®): a guide to its use in the primary and booster vaccination of infants and toddlers in Europe. Drugs Therapy Perspectives. 2013;29(11):329–35. doi:10.1007/s40267-013-0078-0.
- McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexaxim®): a review of its use in primary and booster vaccination. Paediatric Drugs. 2013;15(1):59–70. doi:10.1007/s40272-013-0007-7. PMID:23338932.
- Nunes MC, Madhi SA. Review of a new fully liquid, hexavalent vaccine: Hexaxim. Expert Opinion Biol Therapy. 2013;13(4):575–93. doi:10.1517/14712598.2013.774368. PMID:23441818.
- Santos-Lima E, B'Chir S, Lane A. Combined immunogenicity data for a new DTaP-IPV-Hep B-PRP-T vaccine (Hexaxim) following primary series administration at 2, 4, 6 months of age in Latin America. Vaccine. 2013;31(9):1255–8. doi:10.1016/j.vaccine.2012.11.087. PMID:23246307.
- World Health Organization. WHO prequalified vaccines 2017. Available from: https://extranet.who.int/gavi/PQ_Web/ ( accessed 15 November 2017).
- Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP-IPV//PRP∼T vaccine (Pentaxim): a review of 16 years' clinical experience. Expert Rev Vaccines. 2011;10(7):981–1005. doi:10.1586/erv.11.72. PMID:21749196.
- Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccines. 2008;4(5):328–40. doi:10.4161/hv.4.5.6008. PMID:18398308.
- Tregnaghi MW, Voelker R, Santos-Lima E, Zambrano B. Immunogenicity and safety of a novel yeast Hansenula polymorpha-derived recombinant Hepatitis B candidate vaccine in healthy adolescents and adults aged 10–45 years. Vaccine. 2010;28(20):3595–601. doi:10.1016/j.vaccine.2010.02.049. PMID:20189492.
- Aquino AG, Brito MG, Doniz CE, Herrera JF, Macias M, Zambrano B, Plennevaux E, Santos-Lima E. A fully liquid DTaP-IPV-Hep B-PRP-T hexavalent vaccine for primary and booster vaccination of healthy Mexican children. Vaccine. 2012;30(45):6492–500. doi:10.1016/j.vaccine.2012.07.040. PMID:22863658.
- Ceyhan M, Yildirim I, Tezer H, Devrim I, Feroldi E. A fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine for primary and booster vaccination of healthy Turkish infants and toddlers. Turkish J Medical Sci. 2017;47:1247–56. doi:10.3906/sag-1609-62. PMID:29156870.
- Chhatwal J, Lalwani S, Vidor E. Immunogenicity and safety of a liquid hexavalent vaccine in Indian infants. Indian Pediatrics. 2017;54:15–20. doi:10.1007/s13312-017-0989-2. PMID:27889711.
- Kosalaraksa P, Thisyakorn U, Benjaponpitak S, Chokephaibulkit K, Santos-Lima E. Immunogenicity and safety study of a new DTaP-IPV-Hep B-PRP-T combined vaccine compared to a licensed DTaP-IPV-Hep B//PRP-T comparator, both concomitantly administered with a 7-valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. Int J Infect Dis. 2011;15(4):e249–56. doi:10.1016/j.ijid.2010.12.004. PMID:21334243.
- Lanata C, Zambrano B, Ecker L, Amemiya I, Gil A, Santos-Lima E. Immunogenicity and safety of a fully liquid DTaP-IPV-Hep B-PRP-T vaccine at 2-4-6 months of age in Peru. Vaccines Vaccination. 2012;3:128. doi:10.4172/2157-7560.1000128.
- López P, Mohs A, Vásquez A, Consuelo-Miranda M, Feroldi E, Noriega F, Jordanov E, B Chir S, Zambrano B. A randomized, controlled study of a fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine for primary and booster vaccinations of healthy infants and toddlers in Latin America. Pediatric Infect Dis J. 2017;36(11):e272–e282. doi:10.1097/INF.0000000000001682.
- Macias M, Lanata CF, Zambrano B, Gil AI, Amemiya I, Mispireta M, Ecker L, Santos-Lima E. Safety and immunogenicity of an investigational fully liquid hexavalent DTaP-IPV-Hep B-PRP-T vaccine at two, four and six months of age compared with licensed vaccines in Latin America. Pediatric Infect Dis J. 2012;31(8):e126–32. doi:10.1097/INF.0b013e318258400d.
- Madhi SA, Koen A, Cutland C, Groome M, Santos-Lima E. Antibody persistence and booster vaccination of a fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15–18 months of age in healthy South African infants. Pediatric Infect Dis J. 2013;32(8):889–97.
- Madhi SA, Mitha I, Cutland C, Groome M, Santos-Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatric Infect Dis J. 2011;30(4):e68–74. doi:10.1097/INF.0b013e31820b93d2.
- Tregnaghi M, Zambrano B, Santos-Lima E. Antibody persistence after a primary series of a new DTaP-IPV-Hep B-PRP-T combined vaccine or separate DTaP-IPV//PRP-T and hepatitis B vaccines at 2, 4, and 6 months of age and the effect of a subsequent DTaP-IPV//PRP-T booster vaccination at 18 months of age in healthy Argentinean infants. Pediatric Infect Dis J. 2012;31(1):e24–30. doi:10.1097/INF.0b013e318242460a.
- Tregnaghi MW, Zambrano B, Santos-Lima E. Immunogenicity and safety of an investigational hexavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B-Haemophilus influenzae B conjugate combined vaccine in healthy 2-, 4-, and 6-month-old Argentinean infants. Pediatric Infect Dis J. 2011;30(6):e88–96. doi:10.1097/INF.0b013e318212eb80. PMID:21372751.
- Vesikari T, Borrow R, Da Costa X, Richard P, Eymin C, Boisnard F, Lockhart S. Concomitant administration of a fully liquid, ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal serogroup C conjugate vaccine in infants. Vaccine. 2017;35(3):452–8. doi:10.1016/j.vaccine.2016.11.053. PMID:27939054.
- Vesikari TS, Silfverdal SA, Jordanov E, Feroldi E. A randomized, controlled study of DTaP-IPV-HB-PRP-T, a fully liquid hexavalent vaccine, administered in a 3, 5 and 11–12 month schedule. Pediatric Infect Dis J. 2017;36(1):87–93. doi:10.1097/INF.0000000000001358. PMID:27753797.
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355(9203):561–5. doi:10.1016/S0140-6736(99)07239-6. PMID:10683019.
- WHO. Hepatitis B vaccines: WHO position paper – July 2017. Weekly Epidemiological Record. 2017;92(27):369–92. PMID:28685564.
- Lao TT. Immune persistence after hepatitis B vaccination in infancy – fact or fancy? Hum Vaccines Immunotherapeutics. 2016;12(5):1172–6. doi:10.1080/21645515.2015.1130195.
- Lao TT. Long-term persistence of immunity after hepatitis B vaccination: is this substantiated by the literature? Hum Vaccines Immunotherapeutics. 2017;13(4):918–20. doi:10.1080/21645515.2016.1267084.
- Trevisan A. Long-term persistence of immunity after hepatitis B vaccination: a fact, not a fancy. Hum Vaccines Immunotherapeutics. 2017;13(4):916–7. doi:10.1080/21645515.2016.1257451.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28(3):730–6. doi:10.1016/j.vaccine.2009.10.074. PMID:19892043.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccines Immunotherapeutics. 2013;9(8):1679–84. doi:10.4161/hv.24844.
- Van Der Meeren O, Bleckmann G, Crasta PD. Immune memory to hepatitis B persists in children aged 7–8 years, who were vaccinated in infancy with 4 doses of hexavalent DTPa-HBV-IPV/Hib (Infanrix hexa) vaccine. Hum Vaccines Immunotherapeutics. 2014;10(6):1682–7. doi:10.4161/hv.28480.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Human Vaccines Immunotherapeutics. 2012;8(7):896–904. doi:10.4161/hv.19989.
- Carollo M, Palazzo R, Bianco M, Pandolfi E, Chionne P, Fedele G, Tozzi AE, Carsetti R, Romanò L, Ausiello CM, et al. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine. 2013;31(3):506–13. doi:10.1016/j.vaccine.2012.11.029. PMID:23174200.
- Rosado MM, Scarsella M, Pandolfi E, Cascioli S, Giorda E, Chionne P, et al. Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example. European J Immunol. 2011;41(6):1800–8. doi:10.1002/eji.201041187.
- Zanetti A, Desole MG, Romano L, d'Alessandro A, Conversano M, Ferrera G, et al. Safety and immune response to a challenge dose of hepatitis B vaccine in healthy children primed 10 years earlier with hexavalent vaccines in a 3, 5, 11-month schedule: an open-label, controlled, multicentre trial in Italy. Vaccine. 2017;35(32):4034–40. doi:10.1016/j.vaccine.2017.05.047. PMID:28624307.
- Zanetti A, Parlato A, Romano L, Desole MG, Ferrera G, Giurdanella F, Zuliani M, Richard P, Thomas S, Fiquet A. Challenge with a hepatitis B vaccine in two cohorts of 4-7-year-old children primed with hexavalent vaccines: an open-label, randomised trial in Italy. Vaccine. 2012;30(39):5770–5. doi:10.1016/j.vaccine.2012.06.078. PMID:22789511.
- Zanetti AR, Romano L, Giambi C, Pavan A, Carnelli V, Baitelli G, Malchiodi G, Valerio E, Barale A, Marchisio MA, et al. Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis. 2010;10(11):755–61. doi:10.1016/S1473-3099(10)70195-X. PMID:20884297.
- European Medicines Agency. European Medicines Agency recommends suspension of Hexavac 2005. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/12/WC500017695.pdf ( accessed 15 November 2017).
- European Medicines Agency. Scientific conclusions and grounds for the suspension of the marketing authorisation of Hexavac presented by the EMEA. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Conclusion/human/000298/WC500074684.pdf ( accessed 15 November 2017).
- Thisyakorn U, Montellano, M., Lane A. Routine newborn hepatitis B immunization: a review of schedules. Infect Dis Clin Practice. 2011;19(5):326. doi:10.1097/IPC.0b013e31822b7dda.
- Anderson CL, Remschmidt C, Drobnitzky FP, Falkenhorst G, Zimmermann R, Wichmann O, Harder T. Hepatitis B immune status in adolescents vaccinated during infancy: a retrospective cohort study from a pediatric practice in Germany. Hum Vaccines Immunotherapeutics. 2016;12(3):779–84. doi:10.1080/21645515.2015.1105414.
- Avdicova M, Prikazsky V, Hudeckova H, Schuerman L, Willems P. Immunogenicity and reactogenicity of a novel hexavalent DTPa-HBV-IPV/Hib vaccine compared to separate concomitant injections of DTPa-IPV/Hib and HBV vaccines, when administered according to a 3, 5 and 11 month vaccination schedule. Eur J Pediatrics. 2002;161(11):581–7. doi:10.1007/s00431-002-1079-5.
- Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi:10.1093/infdis/jiv748. PMID:26802139.
- Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P, Ouakki M, Trudeau G, Massé R, Dionne M. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31(3):448–51. doi:10.1016/j.vaccine.2012.11.037. PMID:23206974.
- Middleman AB, Baker CJ, Kozinetz CA, Kamili S, Nguyen C, Hu DJ, Spradling PR. Duration of protection after infant hepatitis B vaccination series. Pediatrics. 2014;133(6):e1500–7. doi:10.1542/peds.2013-2940. PMID:24843060.
- Van Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in adolescents 15–16 years of age vaccinated in infancy with three doses of hepatitis B vaccine. Vaccine. 2016;34(24):2745–9. doi:10.1016/j.vaccine.2016.04.013. PMID:27095043.
- Braeckman T, Van Herck K, Jilg W, Bauer T, Van Damme P. Two decades of hepatitis B vaccination in mentally retarded patients: effectiveness, antibody persistence and duration of immune memory. Vaccine. 2012;30(32):4757–61. doi:10.1016/j.vaccine.2012.05.044. PMID:22652400.
- Hudu SA, Malik YA, Niazlin MT, Harmal NS, Adnan A, Alshrari AS, Sekawi Z. Antibody and immune memory persistence post infant hepatitis B vaccination. Patient Preference Adherence. 2013;7:981–6. doi:10.2147/PPA.S49776. PMID:24101865.
- Saffar H, Saffar MJ, Ajami A, Khalilian AR, Shams-Esfandabad K, Mirabi AM. Long-term T-cell-mediated immunologic memory to hepatitis B vaccine in young adults following neonatal vaccination. Hepatitis Monthly. 2014;14(9):e22223. doi:10.5812/hepatmon.22223. PMID:25368659.
- McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54(3):801–7. doi:10.1002/hep.24442. PMID:21618565.
- Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8(3):e58029. doi:10.1371/journal.pone.0058029. PMID:23533578.
- Avdicova M, Crasta PD, Hardt K, Kovac M. Lasting immune memory against hepatitis B following challenge 10–11 years after primary vaccination with either three doses of hexavalent DTPa-HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 and 11–12 months of age. Vaccine. 2015;33(23):2727–33. doi:10.1016/j.vaccine.2014.06.070. PMID:24962750.
- Pinto M, Dawar M, Krajden M, Naus M, Scheifele DW. Will infant hepatitis B vaccination protect into adulthood?: Extended Canadian experience after a 2-, 4- and 6-month immunization schedule. Pediatric Infect Dis J. 2017;36(6):609–15. doi:10.1097/INF.0000000000001543.
- Thisyakorn U, Chokephaibulkit K, Kosalaraksa P, Benjaponpitak S, Pancharoen C, Chuenkitmongkol S. Immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine as a booster dose in 12- to 18-month-old children primed with 3 doses of 7-valent pneumococcal conjugate vaccine. Hum Vaccines Immunotherapeutics. 2014;10(7):1859–65. doi:10.4161/hv.28642.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics Med. 1998;17(8):857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8%3c857::AID-SIM777%3e3.0.CO;2-E.

![Figure 4. Individual anti-HBs titer profiles for participants with samples post-primary vaccination and at 9–10 years of age, irrespective of whether a sample was available at 12–18 months of age (top panel: DTaP-IPV-HB-PRP-T + PCV7 [N = 71]; bottom panel: DTaP-IPV-HB//PRP-T + PCV7 [N = 79]).](/cms/asset/34067004-6c1a-4283-b26c-716ad76186e7/khvi_a_1426418_f0004_b.gif)