ABSTRACT
Interleukin −22 (IL-22) is a member of interleukin-10 (IL-10) family cytokines that is produced by different types of lymphocytes included in both innate and adaptive immune systems. These lymphocytes include activated T cells, most notably Th17 and Th22 cells, as well as NK cells, γδ T cells, etc. IL-22 mediate its effects via the IL-22-IL-22R complex and subsequent Janus Kinase-signal transduces and activators transcription (JAK-STAT) signaling pathway. According to recent evidence, IL-22 played a critical role in the pathogenesis of many non-autoimmune diseases. In this review, we mainly discussed the recent findings and advancements of the role of IL-22 in several non-autoimmune diseases, such as acute lung injury, atherosclerosis and some bacterial infections, suggesting that IL-22 may have therapeutic potential for treating non-autoimmune diseases.
Introduction
Interleukin-22 (IL-22) is a recently described interleukin-10 (IL-10) family cytokine that is produced by T-helper (Th)-17 cells, γδ T cells, NKT cells and newly described innate lymphoid cells (ILCs).Citation1–Citation3
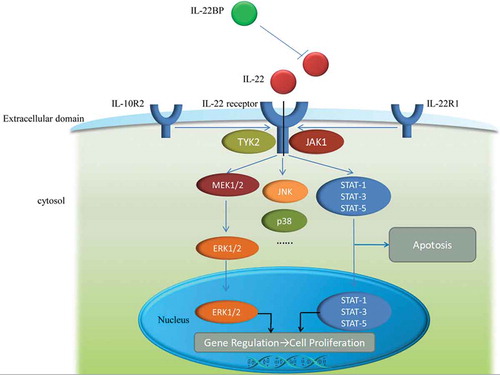
Figure 1. The mechanism of IL-22, IL-22receptor as well as IL-22BP and signaling pathway. IL-22receptor is consist of two parts, IL-10R2 and IL-22R1. The relationship among IL-22, IL-22receptor and IL-22BP is displayed in extracellular domain and the signaling pathways are shown in cytosol and nucleus.
IL-22 functions as a double-edged swor,Citation4 with anti-inflammatory and pro-inflammatory features. It not only leads to the occurrence of diseases, but also militates in the treatment of chronic inflammatory diseases such as T2DM and CAD.Citation4,Citation5
In addition, IL-22 is closely related to some substances in our body. For instance, interleukin-23 (IL-23) is a cytokine that regulates innate and adaptive immune responses by inducing the production of IL-17A, IL-17F, and IL-2,Citation6 but IL-22 is rapidly induced by Pathogen Recognition Receptors Stimulation in Bone-Marrow-derived Dendritic Cells in the absence of IL-23.Citation7 Furthermore, S100/Calgranulin has no direct effect on cholesterol efflux in macrophages, but rather promotes the secretion of IL-22, which then directly reduces cholesterol efflux in macrophages by decreasing the expression of ABCG1.Citation8 The beneficial effects of some nutritional supplements on autoimmune diseases have also been reported in rats, mice and humans. For instance, n-3 polyunsaturated fatty acids (PUFAs) stimulated Th17 cells to produce lower levels of inflammatory factors, including interleukin (IL)-17, IL-22, IL-23 and stimulated Treg cells to produce higher anti-inflammatory factors, such as Foxp3 on psoriasis.Citation9,Citation10
Moreover, IL-22 is released by leukocytes, but it specifically targets non-hematopoietic cells, thus providing a connection between immune system cells and parenchymal cells. Accumulating evidence showed that IL-22 was involved in the development of several autoimmune diseases and their pathogenesi,Citation11 such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), Sjogren’s syndrome (SS), psoriasis, inflammatory bowel disease (IBD), etc..Citation12–Citation16 However, dysregulated IL-22 leads to deleterious inflammation, involving in diseases such as psoriasis, intestinal inflammation and cancer.
Intriguingly, Chien-Huan Weng et alCitation17 have shown that IL-22 can mediate a number of effects linked to RA pathogenesis including driving the production of pro-inflammatory cytokines like IL-1β, IL-6, and TNFα, increasing the expression of matrix metalloproteinase (MMP)-9, and promoting osteoclastogenesis when people are exposed to cigarette smoke.
Recently, there has been an increased interest in understanding the role of IL-22 in health and disease, with the aim to explore the importance of this specific cytokine in therapy. Nowadays, there is a growing concern about the involvement of IL-22 in the formation of non-autoimmune diseases, especially in cardiovascular endothelialCitation18 as well as utility or inutility of IL-22 as a biomarker in humans.Citation19,Citation20 What’s more, increasing evidences have shown that IL-22 was involved in the regeneration of epithelial cells.Citation21–Citation24 In this paper, we mainly discussed the recent articles on IL22 in regeneration and progress of non-autoimmune diseases as well as its clinical significance and therapeutic potential.
The cellular sources of IL-22
Cellular sources and regulatory factor
Human and mouse IL-22 consists of 179 amino acids, showing an overall sequence identity with 25% of IL-10 in humans and 22% of IL-10 in mous.Citation25 Its structure is similar to the well-known immunosuppressive cytokine IL-10, and for which IL-22 is initially named as IL-10-related-Tcell-derived inducible factor. IL-22 can be produced by several different cytokines, and majorly produced by a broad variety of lymphocytes, such as Th17 cells and Th22 cells ().Citation1 Th17 cells are different from natural T-cell precursors, and Th17 themselves have the capacity to secrete a variety of cytokines such as IL-17 and IL-22.Citation26 In addition, murine Th17 cells are further characterized by the expression of retinoic acid receptor-related orphan receptor (ROR)γt.Citation27
Table 1. The major cell resource of IL-22.
Various reports have shown that CD11c+dendritic cells (DCs) can produce IL-22.Citation28,Citation29 However, CD11c is a DC-specific marker, and is also expressed by other cell populations including NK cells and LTi cells. So, further research is required to demonstrate whether DCs represent another innate source of IL-22.
Besides, oral administration of Resiquimod (R848) triggers TLR-7 on CD11c+dendritic cells, inducing IL-23 expression followed by a burst of IL-22 secretion by innate lymphoid cells. This in turn leads to Reg3γ expression and restoration of colonization resistance against Vancomycin-resistant Enterococcus faecium (VRE).Citation30 Lorena Härdle et al. revealed that IL-22 mRNA was enhanced in tristetraprolin (TTP)-deficient splenocytes bound by AU-rich elements and isolated primary T cells and TTP directly controls the production of IL-22, a process counteracted by MEK1/2.Citation31
IL-22 and IL-22 receptors
Like many other cytokines in IL-10 family, IL-22 also has α-helical secondary structure.Citation32 The IL-22 receptor (IL-22R) belongs to class II cytokine receptor family and consists of two subgroups, IL-22R1 and IL-10R2 (also called IL-10RB).Citation32,Citation33 On the other hand, TNF-α enhances the effect of IL-22 on keratinocytes by increasing the expression of both IL-22 receptor and STAT3.Citation34 IL-22R1 is expressed in various non-immune tissues such as skin, lungs, kidneys, pancreas, etc. whereas IL-10R2 is widely expressed in innate lymphoid cells (ILCs).
Previous studies have demonstrated the functions of IL-38 and Th17 cells by blocking the IL-1R, IL-18R, and IL-36R pathways.Citation35 These evidences suggested that the influence of IL-38 on Th17 cells was similar to the blocking of IL-1R and IL-36R pathways, which suppressed IL-17 and IL-22 secretion. Van de Veerdonk et al. also reported that low concentrations of IL-38 were more effective than high concentrations in inhibiting IL-17 and IL-22 production as higher concentrations modestly increased IL-22.
IL-22 binding protein
There is a key regulator of IL-22 signaling, which is a soluble form of IL-22R1 subunit, and is known as IL-22 binding protein (IL-22BP) ().Citation24 Although the expression of IL-22BP has been primarily identified in DCs including CD103+DCs, which are also the producers of IL-23,Citation36 IL-22BP has also been confirmed and identified in epithelial cells as well as macrophages.Citation37–Citation39 Moreover, no clear induction of IL-22BP was detected when monocytes were differentiated in other conditions than granulocyte macrophages colony-stimulating factor (GM-CSF) and IL-4-supplemented media.Citation36 The critical role of endogenous IL-22 in promoting intestinal healing has been recently confirmed in mice deficient of IL-22BP, displaying enhanced tissue regeneration in response to mechanical intestinal injury.Citation40 However, Huber et al. clearly demonstrated that IL-22 can also promote experimental tumourigenesis in the intestine.
The signaling pathway of IL-22
IL-22 signaling is mediated through interferon receptor-related proteins CRF2-4 and IL-22R.Citation41 It forms cell surface complexes with IL-22R1 and IL-10R2 chains, resulting in signal transduction through IL-10R2. The IL-22/IL-22R1/IL-10R2 complex further activates intracellular kinases (JAK1, Tyk2, and MAP kinases) and transcription factors, especially signal transducer and activator of transcription (STAT) 3 (). On the other hand, IL-22 signaling is mediated by janus kinase (Jak) 1 and STAT 1, 3 and 5.Citation41 It can also induce IL-20 and IL-24 signaling when IL-22R1 pairs with IL-20R2.
According to a previous study in mouse model of ischemic acute kidney injury (AKI), the loss of endothelial STAT3 signaling significantly exacerbated kidney dysfunction, morphologic injury, and proximal tubular oxidative stress. Moreover, important proximal tubular adaptive mechanisms related to injury were diminished in association with decreased tissue mRNA levels of the epithelial cell survival cytokine IL-22.Citation42 A latter study further demonstrated that IL-22 signaling utilized Jak1 and Tyk2 to propagate downstream phosphorylation signals, including MAPK signaling pathways (ERK1/2, MEK1/2, JNK, and p38 kinase), STAT1, STAT3, and STAT5.Citation11,Citation43 Although STAT3-mediated signaling is a common pathway shared by IL-10 cytokine family members, IL-22 signaling showed several distinguishing properties. For instance, compared with IL-10 stimulation that induces phosphorylation of tyrosine residues on STAT3, IL-22 stimulation affects STAT3 phosphorylation on both tyrosine and serine residues, and strongly activates the ERK1/2 pathway.Citation43 As both IL-22 and IL-10 utilize IL-10R2, Jak1, and Tyk2, but the differences in signal transduction pathways may be to the differences between IL-22R1 and IL-10R1.Citation44
Shataakshi Dube et al. in a mouse ischemia-reperfusion model of AKI found that the endothelial cell STAT3 signaling limited the trafficking of leukocytes, which have the potential to exacerbate ischemic injury during AKI.Citation42 But Hania Kebir et al. have reported that IL-17 and IL-22 promoted transmigration of human ex vivo CD4+lymphocytes.Citation16 Modulation of endothelial cytokine production, regulation of endothelial cytokine response, and alteration of endothelial adhesion molecule expression may contribute to this observation and provide another fruitful avenue for future investigation.
What’s more, Wei Ren et al. have revealed that the expression of STAT3 in the pulmonary microvascular endothelial cells (PMVECs) after IL-22 interference was obviously increased and the intranuclear accumulation of STAT3 was enhanced, was and these processes were completely inhibited after the interference of AG490, an inhibitor of JAK.Citation45 Similar results were found in the study by Zhiyong Wu et al. The study revealed that IL22 demonstrated protective effects on lung injury through inhibiting AngII-induced PMVECs apoptosis and PMVEC barrier injury by activating the JAK2/STAT3 signaling pathway.
In addition, SLURP1 (an autosomal recessive skin disorder) mRNA expression was significantly upregulated by IL-22 neither by IL-17 nor by TNF-α. The stimulatory effect of IL-22 was completely suppressed in the normal human epidermal keratinocytes treated with a STAT3 inhibitor or transfected with siRNA targeting STAT3. Because IL-22 induces the production of antimicrobial proteins in epithelial cells, the antibacterial activity of SLURP1 significantly suppressed the growth of Staphylococcus aureus.Citation46 Although SLURP1 is produced as an endogenous protective factor in most of the conditions discussed above, the data on the whole indicated that the modulatory potential of the IL-22/STAT3 axis is not saturated by endogenous IL-22. This consequently raised the expectations regarding the use of this cytokine in novel therapeutic strategies.Citation11
The signaling pathway of IL-22 can affect the multiple molecular transformation processes of the diseases, and therefore, manipulation of IL-22 and its signalling pathways,for example, administration of IL-22BP and expression regulation of IL-22receptor as well as STAT3, may have the potential to treat autoimmune and non-autoimmune diseases.
Effect of IL-22 on non-autoimmune diseases
A better understanding of IL-22 pathogenesis helps us to deal with the diseases more accurately, which in turn improves the prognosis of the disease. Therefore, the effect of IL-22 on disease mechanisms is still the direction we should strive for, especially on non-autoimmune diseases. The effects of IL-22 on non-autoimmune diseases conducted to date is following ():
Table 2. The characteristics of the various scientific and intervention studies about IL-22.
Lung diseases
Wei Ren et al. investigated the roles and mechanisms of IL-22 involved in the pathogenesis of aortic dissection (AD) complicated with acute lung injury (ALI) using the samples of mice and patients. The results revealed that IL-22 obviously suppressed the apoptosis of PMVECs mediated by angiotensin II and downregulated the expression and intranuclear transmission of STAT3, and completely inhibited by administration of AG490.Citation45
Similar to IL-10, recombinant IL-22 treatment inhibited the phenoconversion of alveolar epithelial cells into MFs, thus reducing the number of extracellular matrix producing cells in bleomycin-induced mouse model of lung fibrosis.Citation47 Administration of anti-IL-22 neutralizing antibody has improved pulmonary inflammation and ECM deposition in the same bleomycin-induced model of lung fibrosis. Minrui Liang et al. also revealed that administration of recombinant human IL-22 to alveolar epithelial cell line (A549 cells) ameliorated epithelial to mesenchymal transition (EMT) and partially reversed the impaired cell viability induced by BLM. Furthermore, blockage of IL-22 deteriorated pulmonary fibrosis, and elevated EMT marker [α-smooth muscle actin (α-SMA)] and overactivated Smad2.Citation47 Similar results were found in hypersensitive pneumonitis mouse model induced by repeated exposure to Bacillus subtilis, leading to lung fibrosis.Citation48,Citation60 Furthermore, IL-22 alone demonstrated no effect on epithelial or mesenchymal gene expression. However, IL-22+TGF-β1 promoted the expression of some EMT transcription factors, which led to a more profound cadherin shift, but this was observed only in cells obtained from severe asthmatics.Citation49
In addition, a strong IL-17 phenotype in patients with cystic fibrosis (CF) was compared to non-CF controls. The results revealed higher proportions of double positive IL-17+IL-22+cells and the IL-22+population in maturation of memory Th response to pathogen antigens,Citation61 which can be used as a possible tool for studying prognostication.
Skin diseases
IL-22−/− mice were used to investigate the in vivo role of IL-22 in acute skin wounding. Heather M. McGee et al. found that IL-22 can activate ECM gene expression in fibroblasts as well as differentiate myofibroblasts.Citation50 While the α-SMA mRNA expression was increased in response to wounding in both WT and IL-22−/− mice, suggesting that the α-SMA mRNA was significantly reduced in wounds of IL-22 null mice compared to WT wounds. Interestingly, their study uncovered a role of IL-22 in the amelioration of fibroblast function during skin wound repair.
Intestine diseases
Geethanjali Pickert et al. have reported that activation of intestinal epithelial STAT3 regulated immune homeostasis in the gut by promoting IL-22–dependent mucosal wound healing.Citation28 They also confirmed that IL-22 during acute colitis was produced by the ILCs and CD4 + T cells.
In addition to these anti-apoptotic and pro-proliferative mechanisms, IL-22 mediated further protection at host/environment interfaces by enhancing mucus production and by activating the antibacterial host defense mechanisms, such as the IL-23/IL-22/antimicrobial peptide (AMP) pathwa.Citation62
Caroline A. Lindemans et al. have found that recombinant IL-22 directly targeted intestinal stem cells (ISCs), augmenting the growth of both murine and human intestinal organoids, increasing proliferation, and promoting ISC expansion. Furthermore, treatment with IL-22 in vivo after murine allogeneic bone marrow transplantation (BMT) enhanced recovery of ISCs, increased epithelial regeneration, and reduced intestinal pathology and mortality from graft vs. host disease.Citation22
Cardiac diseases
Short-term homing experiments demonstrated that smooth muscle cell-derived IL-17C plays a pro-atherogenic role by supporting the recruitment of Th17 cells to atherosclerotic lesions.Citation63 But high-density lipoprotein (HDL) also has the potential to suppress the immune response of Th1 and Th17 by modulating DC maturation and function.Citation64,Citation65 Accumulating evidence suggested that the balance of Th cells remained important, i.e., imbalance of Th1/Th2 may result in atherosclerosis.Citation66 It has been reported that the Th1 cells were predominant in ACSCitation67,Citation68 and unstable angina pectoris (UA).Citation69,Citation70 Similarly, a Th17/Tregs imbalance has been associated with plaque destabilization and its progression.Citation71,Citation72 In addition, Th22 cells were positively correlated with IL-22 as well as Th17 cells in patients with acute myocardial infarction (AMI) and UACitation73 and IL-22 level was significant higher in patients with AMI and UA compared to control individuals.Citation4 Blockage of IL-22R1 eliminated the protective role of IL-22 against lysophosphatidylcholine- (LPC-), high glucose-, and combined stimuli-induced injury on human umbilical vein endothelial cells.Citation4 According to the above mentioned reports, Lei Zhang et al. have speculated that Th22 cells may participate in the process of ACS by IL-22 secretion. In addition, the peripheral blood Th22 number, aryl hydrocarbon receptor (AHR) expression, and plasma IL-22 levels were significantly higher in patients with acute coronary syndromes (ACS) compared with the control groups.Citation74 However, no further research on the underlying relationship among Th17, Th22 and IL-22 has been done in atherosclerosis.
Moreover, Yujie Guo et al. have shown that compared to control mice at the same time points, chronic myocarditis and dilated cardiomyopathy (DCM) mice have higher percentage of splenic Th22 cells, higher plasma IL-22 levels, increased cardiac IL-22R, as well as increased collagen typeI-A1 (COL1-A1), collagen type III-A1 (COL3-A1) and matrix metalloproteinase-9 (MMP9) expression. Treatment of chronic myocarditis mice with an anti-IL-22 antibody decreased the survival rate and exacerbated myocardial fibrosis. Besides, the percentage of splenic Th22 cells, plasma IL-22 levels and cardiac IL-22R expression were also decreased in anti-IL-22 antibody treatment group as compared to IgG and PBS treated groups of chronic myocarditis mice.Citation51 These results revealed that IL-22-producing Th22 cells played a protective role in chronic myocarditis and dilated cardiomyopathy by inhibiting myocardial fibrosis.
In ischemia-induced mouse model of heart fibrosis, impaired mobilization of bone marrow-derived endothelial progenitor cells that are crucial in neovascularization and tissue repair were observed in the heart of IL-10-knock-out mice compared to wild type controls.Citation75 IL-10 treatment of the mice enhanced the survival of endothelial progenitor cells, leading to a better myocardial recovery.Citation60,Citation75 However, further studies should be conducted to demonstrate significant difference between the role of IL-22 and IL-10.
Interestingly, these results revealed that there was no association of the generation of aortic smooth muscle by IL-22 with apoptosis of the endothelial cells.Citation18
Liver diseases
Previous studies have suggested that the activation of innate immunity also stimulated Kupffer cells to produce the hepatoprotective cytokine IL-6 and the anti-inflammatory cytokine IL-10 during alcoholic liver injury. Accumulating evidences have showed that IL-22, similar to IL-6 and IL-10, is another important hepatoprotective cytokine that protects against acute and chronic alcoholic liver injury by binding to a complex composed of IL-10R2 and IL-22R receptor chains on the surfaces of hepatocytes.Citation52–Citation54,Citation76,Citation77
IL-22 exerts its functions via interacting with IL-10R2 and IL-22R1 on cell surface, and the former one is ubiquitously expressed on all types of liver cells, whereas the latter one is expressed primarily on epithelial cells, such as hepatocytes.Citation77 Thus, IL-22 targets only hepatocytes that express both IL-10R2 and IL-22R, and plays important role in the hepatoprotection and liver regeneration.Citation52–Citation54 In addition, the protective role of IL-22 in T cell hepatitis was further confirmed in IL-22-deficient miceCitation54 and IL-22 transgenic mice.Citation53 Co-treatment with IL-22 may diminish corticosteroid- or TNF-α inhibitor-induced bacterial infection and may prevent the inhibition of liver regeneration. This is because IL-22 has antimicrobial effect that promotes liver regeneration.Citation53
Infectious
Jennifer A. Juno et al. showed that in vitro studies of influenza infection have revealed novel effector functions of iNKT cells including IL-22 production and modulation of myeloid-derived suppressor cells, but ex vivo characterization of human iNKT cells during influenza infection are lacking.Citation78 Similarly, Paget et al. have recently reported that activation of DC TLR7 and RIG-I during murine H3N2 infection resulted in IL-1b and IL-23-mediated signals that induce iNKT IL-22 secretion,Citation55 while IL-22 production did not affect the viral replication, and protected the epithelial cells from damage in vitro.
What’s more, Qing Kong et al. have demonstrated that treatment of acute viral myocarditis (AVMC) mice with anti-IL-22 antibody exacerbated the severity of viral myocarditis and neutralization of IL-22 significantly promoted cardiac viral replication.Citation56 However, Epstein-Barr virus-induced gene 3 (EBI3) negatively regulated the expression of IL-17, IL-22 and RORγt as well as the protective immunity against L. monocytogenes.Citation79
Nutrition
Andrea Sommer et al. have reported that the differentiation of IL-22-producing T cells mediated by supernatants of vitamin D-treated DCs was dependent on TNF-α, IL-6 and IL-2.Citation57 This indicated that vitamin D promotes the aspects of both pro-inflammatory and anti-inflammatory immune responses in humans.
Furthermore, a positive correlation was observed in patients with cows’ milk allergy (CMA) between blood eosinophil numbers and plasma concentrations of IL-4, IL-9, IL-17A and IL-22. Treatment with cows’ milk elimination diet decreased IL-4, IL-9, IL-13 and IL-22 and increased IL-17A in plasma,Citation80 suggesting it as important targets for future interventions.
High serum levels of IL-22 were positively rather than inversely associated with several cardiometabolic risk factors.Citation19 However, the mechanisms by which inflammation contributes to the obesity-linked metabolic dysfunctions were incompletely understood. Increased secretion of pro-inflammatory cytokines may directly influence the metabolic functions of insulin-responsive tissues/cells such as adipocytes and myocytes, causing insulin resistance in these tissues/cells.81 Currently, a key challenge is to determine whether IL-22 can be used as a therapy for obesity and as an overnurishment in humans.
Others
IL-22 levels were elevated in the lacrimal fluids of patients with dry eye disease (DED) and were inversely correlated with the severity of disease. Yong Woo Ji et al. have reported that loss of function analyses using IL-22 knock-out mice demonstrated that IL-22 is essential for suppression of ocular surface infiltration of Th17 cells and inhibition of DED induction.Citation58
Minling Hu et al. demonstrated that IL-22 protected rat insulinoma cells from palmitate-induced oxidative and ER stress through autophagy.Citation59 Palmitate induced increased levels of reactive oxygen species (ROS), overexpression of glucose-regulated protein 78 (GRP78) and C/EBP homologous protein (CHOP), whereas these effects were partly reversed by treatment with IL-22. Moreover, the effects were partly suppressed by treating the cells with 3-methyladenine (3-MA), an autophagy inhibitor.
However, although the number of experiments on non-autoimmune diseases is still too small to confirm significant results and negative results, the therapeutic potential of IL-22 cannot be ignored, especially in cardiovascular system. For instance, nowadays more and more researches on macrophage and DC have been conducted and IL-22BP mRNA expression was strongly induced during DC differentiation. So maybe there is a crosstalk between exosomes and IL-22, which can be applied to treatment of atherosclerosis.
Conclusion
Overall, preclinical studies supported therapeutic administration of seemingly well-tolerated recombinant IL-22 for the treatment of an array of acute diseases manifested in epithelial tissues. However, the feasibility of prolonged administration of this cytokine is expected to be restricted due to potent complications (such as the tumourigenic potential of the IL-22/STAT3 axis).Citation11
Although the effect of IL-22 on non-autoimmune diseases remains to be discussed, several in vitro and in vivo models have supported IL-22 as a potential target for the treatment of non-autoimmune diseases, especially cardiovascular diseases. However, the properties of IL-22 largely depends on the microenvironment surrounding the inflammatory factor,Citation24 showing many uncertainties and it is the biggest obstacle for the development of therapeutics, especially in human system. Further studies should be conducted to investigate the underlying correlations between IL-22 and other diseases in our body, and remained a big challenge for researchers.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Yue Zheng
Yue Zheng and Tong Li searched the articles and Y. Zheng wrote this article.
References
- Brockmann L, Giannou AD, Gagliani N, Huber S. Regulation of th17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis [J]. Int J Mol Sci. 2017;18(5):1033. doi:10.3390/ijms18051033.
- Kumar P, Thakar MS, Ouyang W, Malarkannan S. Il-22 from conventional nk cells is epithelial regenerative and inflammation protective during influenza infection [J]. Mucosal Immunol. 2013;6(1):69–82. doi:10.1038/mi.2012.49.
- Sedda S, Marafini I, Figliuzzi MM, Pallone F, Monteleone G. An overview of the role of innate lymphoid cells in gut infections and inflammation [J]. Mediators Inflamm. 2014;2014:235460. doi:10.1155/2014/235460.
- Gong F, Wu J, Zhou P, Zhang M, Liu J, Liu Y, Lu X, Liu Z. Interleukin-22 might act as a double-edged sword in type 2 diabetes and coronary artery disease [J]. Mediators Inflamm. 2016;2016:8254797. doi:10.1155/2016/8254797.
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Il-22 but not il-17 provides protection to hepatocytes during acute liver inflammation [J]. Immunity. 2007. doi:10.1016/j.immuni.2007.07.023.
- Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. Il-22 production is regulated by il-23 during listeria monocytogenes infection but is not required for bacterial clearance or tissue protection [J]. PLoS One. 2011;6(2):e17171. doi:10.1371/journal.pone.0017171.
- Fumagalli S, Torri A, Papagna A, Citterio S, Mainoldi F, Foti M. Il-22 is rapidly induced by pathogen recognition receptors stimulation in bone-marrow-derived dendritic cells in the absence of il-23 [J]. Sci Rep. 2016;6:33900. doi:10.1038/srep33900.
- Chellan B, Yan L, Sontag TJ, Reardon CA, Hofmann Bowman MA. Il-22 is induced by s100/calgranulin and impairs cholesterol efflux in macrophages by downregulating abcg1 [J]. J Lipid Res. 2014;55(3):443–454. doi:10.1194/jlr.M044305.
- Qin S, Wen J, Bai XC, Chen TY, Zheng RC, Zhou GB, Ma J, Feng JY, Zhong BL, Li YM. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the il-17/il-23 axis [J]. Mol Med Rep. 2014;9(6):2097–2104. doi:10.3892/mmr.2014.2136.
- Peterson RA. Regulatory t-cells: diverse phenotypes integral to immune homeostasis and suppression [J]. Toxicol Pathol. 2012;40(2):186–204. doi:10.1177/0192623311430693.
- Pan HF, Li XP, Zheng SG, Ye DQ. Emerging role of interleukin-22 in autoimmune diseases [J]. Cytokine Growth Factor Rev. 2013;24(1):51–57. doi:10.1016/j.cytogfr.2012.07.002.
- Li LJ, Gong C, Zhao MH, Feng BS. Role of interleukin-22 in inflammatory bowel disease [J]. World J Gastroenterol. 2014;20(48):18177–18188. doi:10.3748/wjg.v20.i48.18177.
- Perriard G, Mathias A, Enz L, Canales M, Schluep M, Gentner M, Schaeren-Wiemers N, Du Pasquier RA. Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes [J]. J Neuroinflammation. 2015;12:119. doi:10.1186/s12974-015-0335-3.
- Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Mirabelli G, Cannarile F, Cipriani P, Giacomelli R, Gerli R. T regulatory and t helper 17 cells in primary sjogren’s syndrome: facts and perspectives [J]. Mediators Inflamm. 2015;2015:243723. doi:10.1155/2015/125380.
- Yang X, Zheng SG. Interleukin-22: A likely target for treatment of autoimmune diseases [J]. Autoimmun Rev. 2014;13(6):615–620. doi:10.1016/j.autrev.2013.11.008.
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human th17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation [J]. Nat Med. 2007;13(10):1173–1175. doi:10.1038/nm1651.
- Weng CH, Gupta S, Geraghty P, Foronjy R, Pernis AB. Cigarette smoke inhibits rock2 activation in t cells and modulates il-22 production [J]. Mol Immunol. 2016;71:115–122. doi:10.1016/j.molimm.2016.01.013.
- Gorzelak-Pabis P, Chalubinski M, Wojdan K, Luczak E, Borowiec M, Broncel M. Il-22 modulates inflammatory properties of human primary aortic smooth muscle cells [J]. Adv Clin Exp Med. 2017;26(3):461–466.
- Herder C, Kannenberg JM, Carstensen-Kirberg M, Huth C, Meisinger C, Koenig W, Peters A, Rathmann W, Roden M, Thorand B. Serum levels of interleukin-22, cardiometabolic risk factors and incident type 2 diabetes: Kora f4/ff4 study [J]. Cardiovasc Diabetol. 2017;16(1):17. doi:10.1186/s12933-017-0624-5.
- Muhl H, Scheiermann P, Bachmann M, Hardle L, Heinrichs A, Pfeilschifter J. Il-22 in tissue-protective therapy [J]. Br J Pharmacol. 2013;169(4):761–771. doi:10.1111/bph.12196.
- Akiyama T, Tateishi R, Akiyama N, Yoshinaga R, Kobayashi TJ. Positive and negative regulatory mechanisms for fine-tuning cellularity and functions of medullary thymic epithelial cells [J]. Front Immunol. 2015;6:461. doi:10.3389/fimmu.2015.00461.
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration [J]. Nature. 2015;528(7583):560–564. doi:10.1038/nature16460.
- Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing rorgammat+ innate lymphoid cells [J]. Immunology. 2011;132(4):453–465. doi:10.1111/j.1365-2567.2011.03410.x.
- Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology [J]. Annu Rev Immunol. 2015;33:747–785. doi:10.1146/annurev-immunol-032414-112123.
- Dumoutier L, van Roost E, Ameye G, Michaux L, Renauld JC. Il-tif/il-22: genomic organization and mapping of the human and mouse genes [J]. Genes Immun. 2000;1:488–494. doi:10.1038/sj.gene.6363716.
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing cd4+ effector t cells develop via a lineage distinct from the t helper type 1 and 2 lineages [J]. Nat Immunol. 2005;6(11):1123–1132. doi:10.1038/ni1254.
- Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection [J]. Int Immunol. 2009;21(5):489–498. doi:10.1093/intimm/dxp021.
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. Stat3 links il-22 signaling in intestinal epithelial cells to mucosal wound healing [J]. J Exp Med. 2009;206(7):1465–1472. doi:10.1084/jem.20082683.
- van Maele L. Tlr5 signaling stimulates the innate production of il-17 and il-22 by cd3(neg)cd127(+) immune cells in spleen and mucosa [J]. 2010;185(2):1177–1185. doi:10.4049/jimmunol.1000115.
- Abt MC, Buffie CG, Susac B, Becattini S, Carter RA, Leiner I, Keith JW, Artis D, Osborne LC, Pamer EG. Tlr-7 activation enhances il-22-mediated colonization resistance against vancomycin-resistant enterococcus [J]. Sci Transl Med. 2016;8(327):327ra25. doi:10.1126/scitranslmed.aaf0746.
- Hardle L, Bachmann M, Bollmann F, Pautz A, Schmid T, Eberhardt W, Kleinert H, Pfeilschifter J, Muhl H. Tristetraprolin regulation of interleukin-22 production [J]. Sci Rep. 2015;5:15112. doi:10.1038/srep15112.
- Wolk K, Sabat R. Interleukin-22: A novel t- and nk-cell derived cytokine that regulates the biology of tissue cells [J]. Cytokine Growth Factor Rev. 2006;17(5):367–380. doi:10.1016/j.cytogfr.2006.09.001.
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. Il-22 increases the innate immunity of tissues [J]. Immunity. 2004;21(2):241–254. doi:10.1016/j.immuni.2004.07.007.
- Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al. Il-22 and il-20 are key mediators of the epidermal alterations in psoriasis while il-17 and ifn-gamma are not [J]. J Mol Med (Berl). 2009;87(5):523–536. doi:10.1007/s00109-009-0457-0.
- van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V, et al. Il-38 binds to the il-36 receptor and has biological effects on immune cells similar to il-36 receptor antagonist [J]. Proc Natl Acad Sci USA. 2012;109(8):3001–3005. doi:10.1073/pnas.1121534109.
- Martin JC, Beriou G, Heslan M, Chauvin C, Utriainen L, Aumeunier A, Scott CL, Mowat A, Cerovic V, Houston SA, et al. Interleukin-22 binding protein (il-22bp) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid [J]. Mucosal Immunol. 2014;7(1):101–113. doi:10.1038/mi.2013.28.
- Wei CC, Ho TW, Liang WG, Chen GY, Chang MS. Cloning and characterization of mouse il-22 binding protein [J]. Genes Immun. 2003;4(3):204–211. doi:10.1038/sj.gene.6363947.
- Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, Dillon SR, Gao Z, Gilbert T, Madden K, et al. A soluble class ii cytokine receptor, il-22ra2, is a naturally occurring il-22 antagonist [J]. Proc Natl Acad Sci USA. 2001;98(17):9511–9516. doi:10.1073/pnas.171303198.
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, cloning, and characterization of a novel soluble receptor that binds il-22 and neutralizes its activity [J]. J Immunol. 2001;166(12):7096–7103.
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr., Murphy AJ, et al. Il-22bp is regulated by the inflammasome and modulates tumorigenesis in the intestine [J]. Nature. 2012;491(7423):259–263. doi:10.1038/nature11535.
- Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (il)-22, a novel human cytokine that signals through the interferon receptor-related proteins crf2-4 and il-22r [J]. J Biol Chem. 2000;275(40):31335–31339. doi:10.1074/jbc.M005304200.
- Dube S, Matam T, Yen J, Mang HE, Dagher PC, Hato T, Sutton TA. Endothelial stat3 modulates protective mechanisms in a mouse ischemia-reperfusion model of acute kidney injury [J]. J Immunol Res. 2017;2017:4609502. doi:10.1155/2017/5974574.
- Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (il-22) activates the jak/stat, erk, jnk, and p38 map kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from il-10 [J]. J Biol Chem. 2002;277(37):33676–33682. doi:10.1074/jbc.M204204200.
- Dumoutier L, de Meester C, Tavernier J, Renauld JC. New activation modus of stat3: A tyrosine-less region of the interleukin-22 receptor recruits stat3 by interacting with its coiled-coil domain [J]. J Biol Chem. 2009;284(39):26377–26384. doi:10.1074/jbc.M109.007955.
- Ren W, Wang Z, Wu Z, Hu Z, Dai F, Chang J, Li B, Liu H, Ruan Y. Jak2/stat3 pathway was associated with the protective effects of il-22 on aortic dissection with acute lung injury [J]. Dis Markers. 2017;2017:1917804. doi:10.1155/2017/1917804.
- Moriwaki Y, Takada K, Nagasaki T, Kubo N, Ishii T, Kose K, Kageyama T, Tsuji S, Kawashima K, Misawa H. Il-22/stat3-induced increases in slurp1 expression within psoriatic lesions exerts antimicrobial effects against staphylococcus aureus [J]. PLoS One. 2015;10(10):e0140750. doi:10.1371/journal.pone.0140750.
- Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, Qiu J, Zhou X, Guan M, Xue Y, et al. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis [J]. Mediators Inflamm. 2013;2013:209179. doi:10.1155/2013/209179.
- Simonian PL, Wehrmann F, Roark CL, Born WK, O’Brien RL, Fontenot AP. Gammadelta t cells protect against lung fibrosis via il-22 [J]. J Exp Med. 2010;207(10):2239–2253. doi:10.1084/jem.20100061.
- Johnson JR, Nishioka M, Chakir J, Risse P-A, Almaghlouth I, Bazarbashi AN, Plante S, Martin JG, Eidelman D, Hamid Q. Il-22 contributes to tgf-β1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells [J]. Respir Res. 2013. doi:10.1186/1465-9921-14-118.
- McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA, Horsley V. Il-22 promotes fibroblast-mediated wound repair in the skin [J]. J Invest Dermatol. 2013;133(5):1321–1329. doi:10.1038/jid.2012.463.
- Guo Y, Wu W, Cen Z, Li X, Kong Q, Zhou Q. Il-22-producing th22 cells play a protective role in cvb3-induced chronic myocarditis and dilated cardiomyopathy by inhibiting myocardial fibrosis [J]. Virol J. 2014;11:230. doi:10.1186/s12985-014-0230-z.
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (il-22) plays a protective role in t cell-mediated murine hepatitis: il-22 is a survival factor for hepatocytes via stat3 activation [J]. Hepatology. 2004;39(5):1332–1342. doi:10.1002/hep.20184.
- Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression [J]. Hepatology. 2011;54(1):252–261. doi:10.1002/hep.24339.
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation [J]. Immunity. 2007;27(4):647–659. doi:10.1016/j.immuni.2007.07.023.
- Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, et al. Interleukin-22 is produced by invariant natural killer t lymphocytes during influenza a virus infection: potential role in protection against lung epithelial damages [J]. J Biol Chem. 2012;287(12):8816–8829. doi:10.1074/jbc.M111.304758.
- Kong Q, Wu W, Yang F, Liu Y, Xue Y, Gao M, Lai W, Pan X, Yan Y, Pang Y, et al. Increased expressions of il-22 and th22 cells in the coxsackievirus b3-induced mice acute viral myocarditis [J]. Virol J. 2012;9:232. doi:10.1186/1743-422X-9-232.
- Andrea Sommer MF. Vitamin d regulates cytokine pattern secreted by dendritic cells to promote differentiation of il-22-producing t cells [J]. PLoS One. 2015; 10(6): e0130395.
- Ji YW, Mittal SK, Hwang HS, Chang EJ, Lee JH, Seo Y, Yeo A, Noh H, Lee HS, Chauhan SK, et al. Lacrimal gland-derived il-22 regulates il-17-mediated ocular mucosal inflammation [J]. Mucosal Immunol. 2017;10(5):1202–1210. doi:10.1038/mi.2016.119.
- Hu M, Yang S, Yang L, Cheng Y, Zhang H. Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in ins-1 cells through activation of autophagy [J]. PLoS One. 2016;11(1):e0146818. doi:10.1371/journal.pone.0146818.
- Sziksz E, Pap D, Lippai R, Beres NJ, Fekete A, Szabo AJ, Vannay A. Fibrosis related inflammatory mediators: role of the il-10 cytokine family [J]. Mediators Inflamm. 2015;2015:764641. doi:10.1155/2015/125380.
- Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, et al. Patients with cystic fibrosis have inducible il-17+il-22+ memory cells in lung draining lymph nodes [J]. J Allergy Clin Immunol. 2013;131(4):1117–29, 1129 e1-5. doi:10.1016/j.jaci.2012.05.036.
- Ngo VL, Abo H, Maxim E, Harusato A, Geem D, Medina-Contreras O, Merlin D, Gewirtz AT, Nusrat A, Denning TL. A cytokine network involving il-36γ, il-23, and il-22 promotes antimicrobial defense and recovery from intestinal barrier damage [J]. Proc Natl Acad Sci. 2018. doi:10.1073/pnas.1718902115.
- Butcher MJ, Waseem TC, Galkina EV. Smooth muscle cell-derived interleukin-17c plays an atherogenic role via the recruitment of proinflammatory interleukin-17a+ t cells to the aorta [J]. Arterioscler Thromb Vasc Biol. 2016;36(8):1496–1506. doi:10.1161/ATVBAHA.116.307892.
- Takata K, Imaizumi S, Zhang B, Miura S, Saku K. Stabilization of high-risk plaques [J]. Cardiovasc Diagn Ther. 2016;6(4):304–321. doi:10.21037/cdt.2015.10.03.
- Tiniakou I, Drakos E, Sinatkas V, van Eck M, Zannis VI, Boumpas D, Verginis P, Kardassis D. High-density lipoprotein attenuates th1 and th17 autoimmune responses by modulating dendritic cell maturation and function [J]. J Immunol. 2015;194(10):4676–4687. doi:10.4049/jimmunol.1402870.
- Shimada K, Park JK, Daida H. T helper 1/t helper 2 balance and hmg-coa reductase inhibitors in acute coronary syndrome: statins as immunomodulatory agents? [J]. Eur Heart J. 2006;27(24):2916–2918. doi:10.1093/eurheartj/ehl376.
- Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced t-helper-1 lymphocyte activation patterns in acute coronary syndromes [J]. J Am Coll Cardiol. 2005;45(12):1939–1945. doi:10.1016/j.jacc.2005.03.040.
- Cavusoglu E, Marmur JD, Hojjati MR, Chopra V, Butala M, Subnani R, Huda MS, Yanamadala S, Ruwende C, Eng C, et al. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome [J]. Am J Med. 2011;124(8):724–730. doi:10.1016/j.amjmed.2011.02.040.
- Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the t-cell repertoire in patients with unstable angina [J]. Circulation. 1999;100(21):2135–2139.
- Yamashita H, Shimada K, Seki E, Mokuno H, Daida H. Concentrations of interleukins, interferon, and c-reactive protein in stable and unstable angina pectoris [J]. Am J Cardiol. 2003;91(2):133–136.
- Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The th17/treg imbalance in patients with acute coronary syndrome [J]. Clin immunol. 2008;127(1):89–97.
- Potekhina AV, Pylaeva E, Provatorov S, Ruleva N, Masenko V, Noeva E, Krasnikova T, Arefieva T. Treg/th17 balance in stable cad patients with different stages of coronary atherosclerosis [J]. Atherosclerosis. 2015;238(1):17–21. doi:10.1016/j.atherosclerosis.2014.10.088.
- Zhang L, Wang T, Wang XQ, Du RZ, Zhang KN, Liu XG, Ma DX, Yu S, Su GH, Li ZH, et al. Elevated frequencies of circulating th22 cell in addition to th17 cell and th17/th1 cell in patients with acute coronary syndrome [J]. PLoS One. 2013;8(12):e71466. doi:10.1371/journal.pone.0071466.
- Lin YZ, Wu BW, Lu ZD, Huang Y, Shi Y, Liu H, Liu L, Zeng QT, Wang X, Ji QW. Circulating th22 and th9 levels in patients with acute coronary syndrome [J]. Mediators Inflamm. 2013;2013:635672. doi:10.1155/2013/635672.
- Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium [J]. Circ Res. 2011;109(11):1280–1289. doi:10.1161/CIRCRESAHA.111.248369.
- Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease [J]. J Gastroenterol Hepatol. 2012;27(Suppl 2):89–93. doi:10.1111/j.1440-1746.2011.07003.x.
- Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22 [J]. Semin Immunopathol. 2010;32(1):17–31. doi:10.1007/s00281-009-0188-x.
- Juno JA, Keynan Y, Fowke KR. Invariant nkt cells: regulation and function during viral infection [J]. PLoS Pathog. 2012;8:e1002838. doi:10.1371/journal.ppat.1002838.
- Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, et al. Epstein-barr virus-induced gene 3 negatively regulates il-17, il-22 and rorgamma t [J]. Eur J Immunol. 2008;38(5):1204–1214. doi:10.1002/eji.200838145.
- Barros KV, Flor Silveira VL, Laranjeira MS, Wandalsen NF, Passeti S, de Oliveira R, Munekata RV, Noakes PS, Miles EA, Calder PC. Evidence for involvement of il-9 and il-22 in cows’ milk allergy in infants [J]. Nutrients. 2017;9(10):1048. doi:10.3390/nu9101048.
- Wu H, Ballantyne CM. Inflammation versus host defense in obesity [J]. Cell Metab. 2014;20(5):708–709. doi:10.1016/j.cmet.2014.10.013.
