ABSTRACT
An important strategy for addressing maternal and newborn risks of disease is through vaccinating pregnant women. We conducted a mixed-methods study including a narrative literature review of drivers of maternal vaccination and key informant interviews in Spain, Italy, and India to characterize different approaches to national maternal immunization programs. Fifty-nine respondents participated in the study conducted between November 2018 and January 2019. Policies in Spain and Italy both reflect a life-course approach to vaccination, but recommendations and how they ensure uptake differs. Italy was focused on tracking of progress and mandates to ensure compliance in all regions, while Spain, an early adopter, relied more on advocacy and building provider acceptance. India includes Td in their national program, but the political will and advocacy for other vaccines are not seen. Needs for improving rates of maternal vaccination include education of health-care providers and pregnant women, use of central registries to track progress, stronger global guidance for use of vaccines, and engagement of champions, particularly obstetrician-gynecologists (ob-gyns). Health security concerns can also be leveraged to build political priority and needed platforms to detect disease and deliver vaccines in some countries. Understanding what drives a country’s maternal immunization program decisions and the success of implementation is useful in designing strategies to share best practices and guide support to strengthen platforms for maternal vaccination.
Introduction
The global community has committed to ending preventable deaths in newborns and significantly reducing maternal mortality.Citation1 An important strategy for addressing maternal and newborn deaths is through immunizing pregnant women to prevent disease in both the mother and the newborn.Citation2–5 Pertussis, influenza, and tetanus are three diseases for which vaccines are currently available and recommended for pregnant women. The World Health Organization (WHO) recommends that countries give the highest priority to pregnant women if they are considering expansion of seasonal influenza programs,Citation6 but even then, many countries do not have national influenza vaccination policies or recommendations in place, particularly in low- and middle-income settings.Citation7–9 In Europe, where a goal of 75% influenza vaccination coverage of key risk groups including pregnant women has been established, no country achieved this target in the 2017/18 season and many countries did not report coverage.Citation10 In 2017, WHO updated tetanus toxoid (TT) recommendations to include tetanus-diphtheria (Td) vaccines for pregnant women,Citation11 and has a permissive recommendation for acellular pertussis-containing vaccines in pregnant women (e.g, national programs may consider 1 dose of tetanus, diphtheria, acellular pertusis (Tdap) in the 2nd or 3rd trimester in addition to vaccination of infants in high or increasing infant pertussis morbidity or mortality).Citation12 Nonetheless, a number of countries, including Italy,Citation12 Spain,Citation13 nine other countries in Europe,Citation14 United States (US),Citation15 New Zealand, Australia, Argentina, Brazil, the Bahamas, Chile, Costa Rica, El Salvador, Honduras, MexicoCitation16 have recommended Tdap vaccines in pregnancy, albeit at different schedules. Many countries report low coverage for Tdap in pregnant womenCitation17–20 and some have no reliable coverage data.Citation21,Citation22
There are a number of reported reasons for the low coverage rates for maternal vaccination. Safety is reported to be a leading concern amongst both pregnant women and providers in a number of countries.Citation23,Citation24 Provider recommendation was a predictor of acceptance in a number of countries.Citation23,Citation25 It is influenced by a series of risk-benefit determinations including financial and practical considerations, perception about the value of vaccine, and risks and steps taken to promote the benefits of vaccination.Citation26,Citation27 There is still a need to improve awareness of vaccine benefits and risks amongst providersCitation28 and pregnant women.Citation21,Citation27,Citation29 Lack of perceived need for maternal vaccination and low awareness about the dangers of VPDs in pregnancy also leads to low rates of acceptance.Citation30
Despite an understanding of the barriers seen across countries, there is still little understanding of the potential similarities and differences between countries. Better understanding the varying approaches toward maternal vaccination across countries can help in developing strategies to address similar issues and share best practices across countries. To determine if there were unique characteristics of countries’ maternal vaccination programs, we examined three countries in more detail.
Methodology
The project used a mixed-methods approach including a narrative literature review, desk research, and qualitative interviews to develop case studies to examine key factors influencing decision-making and uptake of maternal immunization.
Country selection
We selected three countries – Spain, Italy, and India – to provide a diversity of situation, approach, and performance in order to compare and contrast. Spain and Italy were selected to better understand countries with similar recommendations for vaccinating pregnant women but for different drivers of decisions and differing levels of uptake. India was selected to determine how a country's more limited national recommendations for maternal vaccination approached decision-making and implementation differently.
Narrative literature review and desk research
We conducted a narrative literature review and desk research to identify barriers and drivers of decision-making and implementation for national government programs to inform a semi-structured interview guide used in the qualitative component of the study. Previous research on drivers of vaccine introduction and uptakeCitation31,Citation32 also informed our research. We used the following search terms: “vaccination”[tiab] OR “immunization”[tiab] AND (“maternal immunization”[tiab] OR “maternal immunisation”[tiab] OR pregnan*[tiab]) AND (“implement*” OR “introduc*” OR “recommend*” OR polic* OR barrier OR gap OR challenge) AND (Spain OR Italy OR India). Results were limited to those published between 2005 and 2019, resulting in 237 articles. To further identify relevant articles, the references lists of pertinent articles were examined and any additional relevant articles were included. Inclusion criteria included 1) full-text availability; 2) published within the last 15 years; and 3) articles with a focus on maternal vaccination policies, barriers, and implementation. Exclusion criteria included articles that merely focused on morbidity and mortality associated with maternal immunization, without a focus on policy, barriers, or implementation. We scanned gray literature including government, partner, and professional association websites, media and other reports for country background, vaccine recommendations and other public policies, reports on vaccine uptake, and maternal vaccine stakeholders. Searches were conducted in English, Spanish, and Italian and supplemented by Google Translate.
Interviews
We used a snowball approach to identify key informants in each country comprised of experts from a vaccine technical, maternal health, program implementation, health economics & financing, and advocacy backgrounds. One-hour interviews were conducted in-person or by phone in English. Interviews were recorded and transcribed. No identifiers were included in the transcriptions to maintain confidentiality, only expertise and job type categorization. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board deemed this to be non-human subjects research.
Case study analysis
For the qualitative component of the study, a thematic analysis was performed on the interviews using ATLAS.ti to create a case study for each country. This data was plotted, and graphs designed using Graph Pad Prism Version 7.0. Tables were also developed, and results were compared across countries.
Results
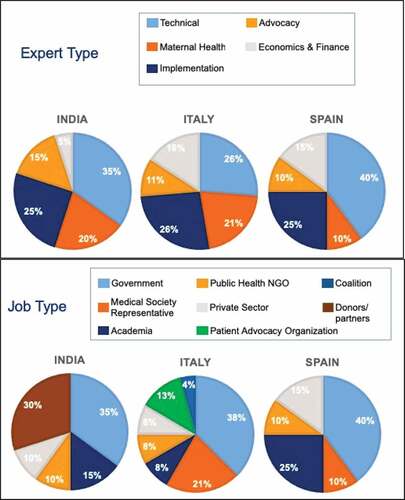
We reviewed 237 articles identified in the literature review, which covered the following themes: decision-making process; priority of life-course approach to vaccination, maternal vaccination infrastructure including processes, registries, and implementation; key players and stakeholders in decision-making and implementation; access to maternal immunization and degree of vaccine hesitancy; financing; and system-level barriers and facilitators of maternal vaccination. We conducted 59 key informant interviews (20 per country in Spain and India and 19 in Italy) November 2018 and January 2019, probing on the themes listed above. Results of the literature review, desk research, and interviews are shown below. The distribution of respondents is listed in .
Figure 1. Profile of key respondents interviewed. n = 20 in Spain and India, n = 19 in Italy. Some respondents categorized into multiple job types in Italy
Decision-making and recommendations
Recommendations
Both Spain and Italy had recommended influenza vaccines and Tdap in pregnancy.Citation14 Spain recommends Tdap from 27 weeks and influenza vaccine in any trimester,Citation33 since 2014Citation19 and influenza vaccination since 2005.Citation34 In 2017, Italy recommended Tdap at 27–36 weeks and influenza vaccine in the 2nd or 3rd trimester.Citation35,Citation36 In 2019, Italy updated influenza vaccine recommendations to any trimester.Citation37 India, a large lower-middle-income country, only recommended TT and had recently updated their recommendation to Td as part of the national program.Citation38 India does not nationally recommend influenza vaccine for pregnant women, even for pandemic preparedness.Citation39–41
Drivers of decisions
Based on findings both from the literature review and respondents, the main drivers of decision-making varied in each country (). For example, in India, low cost of TT/Td vaccine was cited as an enabler decisions and political priority (driven by a high infant mortality rate). Respondents in India also noted that other maternal vaccines had not been considered, likely because of cost, lack of advocacy and poor data. Italian respondents cited newborn mortality as the most frequent driver of national decisions to adopt vaccine while Spanish respondents frequently noted recommendations from other countries and wanting to be known as a leader in the region. Both Italy and Spain had a recent focus on the life-course as reflected in their national calendars. Another frequently mentioned factor in Spain was the shortage of adolescent diphtheria-tetanus-acellular pertussis vaccine and availability of Tdap.Citation42,Citation43 Lack of safety data in pregnant women was not mentioned as a driver of decision-making by respondents in any country. When prompted further, respondents noted its importance, but did not view this as preventing decisions. Several Indian respondents cited vaccine hesitancy as a barrier. A few respondents in India mentioned an overall lack of disease burden data in decision-making for pregnant women. In Italy, elections and the influence of a female physician's health minister were also mentioned as a driver of decisions.
Table 1. Frequently cited drivers of maternal immunization decision-making
Stakeholders involved in decision making
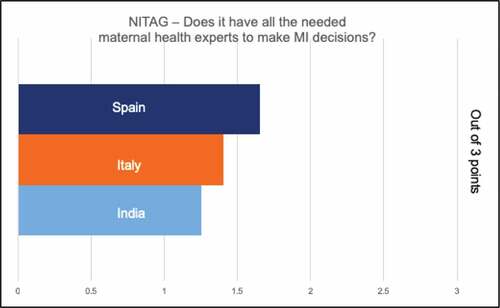
All countries had centralized decision-making in the form of a technical advisory group (NITAG), which respondents noted was often comprised of pediatricians. In India, some respondents relayed a concern that the voice of the ob-gyns was not strong despite their presence on the National Technical Advisory Group on Immunization (NTAGI). Most respondents in all countries did not feel there was a champion for maternal immunization to move the agenda forward. In general, respondents perceived the level of specific expertise in decision-making for pregnant women to be limited. When asked to rate adequacy of maternal immunization expertise for decision-making, Spanish representatives rated this highest, Italy next, and India lowest, noting only 1–2 representatives on the NITAG (). In addition, in India, lack of data in pregnant women was a frequently cited barrier to decision-making, including for “maternal immunization experts.”
Health security
Health security and outbreaks were mentioned by respondents in all countries, although not necessarily in response to a question regarding facilitators for decision-making. In India, the H1N1 outbreak did not result in a recommendation to adopt pandemic influenza vaccines (although distributed to health workers) and one respondent told us that pregnant women were not considered in the conversation. According to respondents in Spain, pertussis outbreaks drove a decision to act according to respondents and in Italy, outbreaks were reported by some as a driver of new or expanded policies.
Implementation: facilitators and barriers
Advocacy & provider buy-in
Advocacy was described as a prime facilitator of uptake in Spain and India. While health providers are viewed as important influencers of decisions and uptake, Spanish physicians were not consistently convinced on the need for vaccine. The lack of physician buy-in amongst some was noted as a significant challenge in Spain. In India, maternal health advocates and those representing marginalized populations (e.g., WHO, UNICEF, non-governmental organizations) were important, but many commented that providers, in general, were not making the recommendation, perhaps because they are not convinced due to a lack of local studies of vaccines in pregnant women. Some respondents questioned whether maternal health was a priority in India. Generally, ob-gyns were cited has having a strong influence on implementation at an individual level, but were not correspondingly viewed as strong champions at a national level.
Registries and monitoring in a centralized manner
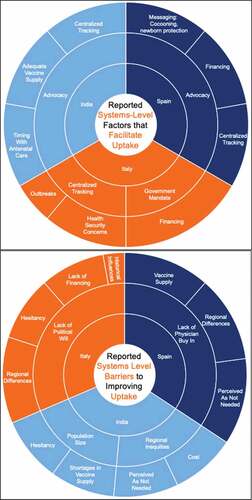
In Italy, respondents credited registries and mandates for their program’s ability to achieve high uptake (). Centralized tracking of coverage rates was mentioned by some respondents in all three countries as a facilitator for uptake. In Italy, respondents spoke about a big push to centralize previously regional vaccination calendars, implement national registries and mandate vaccination to ensure consistency across regions. Vaccines are mandatory for children and fines are levied for noncompliance. Although the same does not apply to adults, there is a requirement for the regions to offer vaccines. By contrast, a smaller number of respondents in Spain also suggested some degree of centralization through tracking of all regions. Vaccinations in Spain are not mandatory. A recent law in Spain (2016) focuses on disparities across regions and should help reduce some of the differences in coverage rate. Respondents in India explained that there were pilots to create electronic health records (EHRs), which could become part of a centralized dashboard, but they have not currently progressed. Some respondents also commented that the program for maternal immunization is implemented by the National Health Mission, yet decisions and procurement decisions were made under the Universal Immunization Programme (UIP). A couple of respondents mentioned that better coordination was needed.
Figure 3. System-level facilitators and barriers to improving uptake. Key: Inner circle: Factor mentioned by ~100% of respondents, Outer Circle: Other mentioned factors, Size is proportional to frequency that respondents mentioned it as a factor. Advocacy is referring to pediatric and new-born health experts advocating for vaccines to protect baby and mother. Ob-gyn’s seem to not play an influential role in uptake currently in the case study countries
Regional differences and inequities
Regional differences in implementation were mentioned in all three countries, although most common in India, where the concept of inequities was brought up frequently. This was not necessarily referring to TT, which enjoyed a very high coverage rate. Respondents in India spoke of the challenges in antenatal care (ANC), which was the responsibility of the National Health Mission and immunization, a part of the UIP. A few respondents recognized a need for greater coordination between the two programs, noting greater coordination would be essential when vaccines required specific timing. A few respondents noted that there were initiatives to help address inequities such as Intensified Mission Indradhanush (IMI), which has helped improve coverage, monitoring, and surveillance capacity in underperforming districts.Citation44 This initiative and focus on pregnant women helped India achieve 81% coverage of TT vaccine in 2018.Citation45 In Spain, although respondents were not aware of reliable coverage estimates, administrative data show high national average coverage for Tdap (79–80%in 2017 and 2018), and regional variation ranging from 57% to 93% in 2018.Citation46 Studies on acceptance and prescribing, however, suggest coverage rates of Tdap or influenza would be lower.Citation47–49 Italy reports on influenza vaccine coverage for pregnant women, but rates are extremely low.Citation10 Additional surveys report similarly low coverage and suggest that education and a lack of provider recommendation are important factors in low uptake.Citation50,Citation51
Financing and cost
Perception of affordability at the patient- and systems-level can be a major barrier to implementation and uptake. All countries reported cost as being influential, to some degree, in successful decision-making and implementation performances. In all three countries, if the vaccine is recommended, it is free for the patient and there are no out-of-pocket costs if the vaccine is received at a designated vaccination place.
India, more frequently than the other two countries, reported the cost of maternal vaccines as a main barrier to adopting and implementing new vaccines. Indeed, respondents reported that tetanus was recommended because of its low cost. Some respondents expressed that pertussis vaccination has not been brought to the agenda due to cost. They also perceived this to be the issue with influenza vaccination. Other respondents noted there was insufficient disease burden data to support decisions, however. Respondents reported that both access and affordability drive differences in uptake that are geographical, regional, rural-urban, poor-rich, and gender-related.
Respondents in Italy described the financing as both a facilitator and barrier to maternal immunization, noting an increase in spending for a new life-course vaccination plan, and cost-effectiveness data that showed that this spending would bring significant savings. However, cuts to healthcare needs and provider staffing resulting from austerity measures were reported by respondents as a potential concern regionally. Regional authorities expressed concerns that some areas may face rising demand for services under the new policy, while simultaneously struggling with declining numbers of physicians.
In Spain, respondents noted strong support for public financing of vaccination. Public financing of vaccines in calendar started in 2006 when the current menu of services offered to all under the Sistema Nacional de Salud.
Supply
Some respondents noted that vaccine supply played an important role in maternal immunization. In Spain, maternal pertussis immunization increased following shortages of pediatric DTaP vaccines (the country experienced pertussis outbreaks).Citation30 This contributed to the recommendation and political support for maternal Tdap.Citation32 In India, several respondents noted the size of the population played a role due to concerns over whether the country would even be able to supply vaccine.
Vaccine communication and hesitancy
Hesitancy was noted by some respondents as a barrier to uptake for both women and providers in IndiaCitation30,Citation52,Citation53 and Italy.Citation54–56 Hesitancy, although seen in Spain,Citation47,Citation48 did not emerge as a major barrier. The respondents mentioned the country also had a strong promotion of vaccination which could explain lower perceived levels of vaccine hesitancy. Some Spanish respondents also stated that lack of uptake could be ascribed to a lack of awareness. The issues with hesitancy described in Italy may stem from institutional distrust and political parties which tend to stoke these sentiments. Respondents, however, claimed that distrust and hesitancy around immunization died down during outbreaks. Regional variation in hesitancy was also reported and, in every country, respondents noted the important role of providers, particularly ob-gyns in convincing women to be vaccinated, although most felt they needed to be more proactive and that their influence could be strengthened.
Providers and access
Having easy access to vaccination providers can influence level of uptake. Physicians in Spain reported a lack of support at the administrative/implementation level and one respondent noted that Spain spends less on public health and prevention than other countries in the EU. Women do not always know where to access the vaccines as well. In Italy, the view that physicians have a lot of power was common, and due to regional variations, the value placed on vaccines may result in lower coverage rates in some places. The ob-gyns and general practitioners were named as important sources of information and pregnant women could get vaccine free of charge in any national clinic and in pharmacies for the case of influenza vaccine. In India, respondents remarked that women receive vaccines at antenatal care visits and through supplemental immunization activities (SIAs), particularly in high-risk districts. This, combined with a number of other strategies, helped India achieve maternal and neonatal tetanus elimination. There was near-universal mention of this as a source of pride, but some respondents felt that giving other maternal vaccines may still be a challenge if they must be given at a particular time in pregnancy.
Discussion
Both Italy and Spain appear to be vying for leadership in Europe in terms of their decision-making around life-course immunization, which has become an important driver in decisions around maternal immunization. Italy’s decision-making may have been facilitated by a strong champion, while implementation seems to rely on centralization of the vaccine program, legislation,Citation57 and tracking to ensure that years of disparity and hesitancy enabled by differing politics are eliminated. Tdap and influenza vaccines have only been offered to pregnant women since 2017.Citation36,Citation57 Awarenessamongst many providers and pregnant women is generally low, indicating significant opportunity for improvement.Citation58 Further, safety, while not shown to be a significant barrier for inclusion of existing vaccines in national programs, is a key driver of uptake amongst pregnant women, and will be an important driver for acceptance.Citation54,Citation58
Spain, on the other hand, has been an early adopter of immunization, reportedly following the United Kingdom, US, and Australia, as well as looking to Italy. Advocacy appears to be an important driver of vaccine decisions and uptake appears to strong, in at least some places, driven by provider recommendations and a high degree of parental trust in providers. Health security concerns (e.g., pertussis outbreaks) have also helped drive action and reinforced the need to further strengthen their system.
India, by contrast, has not adopted either influenza or pertussis vaccines for pregnant women,Citation59 instead following WHO recommendations for tetanus immunization (Td vaccine). Indian respondents were proud of achieving maternal and neonatal tetanus elimination and high rates of immunization coverage in pregnant women.Citation60 Despite high neonatal priority and political priority for maternal health, maternal vaccines other than Td, however, are not on the political agenda.Citation61 Even during the H1N1 outbreak in 2009, NTAGI did not consider vaccination of pregnant women and deployed vaccine only to health workers.Citation62 Respondents observed few Indian champions for maternal immunization, although there is a representation of the Federation of Obstetrics and Gynecologic Societies of India (FOGSI) and maternal health experts on NTAGI. Stronger advocacy efforts, including at the state level, are likely needed to move the agenda, which can include not only consideration of new vaccines, but the data that will be necessary to make decisions about new vaccines in pregnant women. Although safety was not mentioned by respondents in this study as a barrier to vaccine recommendations in India, previous experience indicates disease burden, vaccine safety and efficacy, and economic evaluations will all be needed to move toward a decision.Citation63,Citation64
For countries such as India, where decisions have not been made to adopt maternal vaccines beyond Td, sharing experiences about the benefit of maternal immunization programs in other countries, may be useful. Stronger global policies around influenza and pertussis vaccination will likely be needed for countries to adopt them, but the first steps of improving surveillance and developing infrastructure that enables stronger integration with antenatal care will be needed at early stages.Citation22 Additionally, global tracking of progress in both recommendations and implementation can help create global.prioritization. Local data will also assist in making the case to policy-makers; however, it should be of high quality and not delay action if a reasonable case can be made using regional data.
Ob-gyns and midwives are important sources for advice, but their voice at the national level for maternal immunization may not be fully leveraged. Potentially, this could be addressed through a deliberate effort to engage ob-gyn and midwifery leadership in the NITAG discussions and empower them to speak up through planned interactions with government and scientists to advocate for needed local data and evidence informed decisions. An organized voice and global and local champions can help raise political priority and action across all countries.Citation65
Limitations and strengths of this study
This study was conducted with a small number of countries and the findings may not represent the full extent of drivers and barriers for maternal immunization. Although we were able to compare and contrast decision-making and implementation in study countries, this does not enable us to describe potential country archetypes. The study does provide, however, important insights upon which further research can be based to understand potential strategies for addressing gaps or leveraging certain approaches to improve vaccine decision-making and uptake.
Conclusion
Understanding what drives a country’s maternal vaccine program decisions, whether implementation is successful or faces challenges, is useful in designing strategies to share best practices and guide support to strengthen platforms for maternal vaccination. Champions are important to build confidence in maternal vaccination programs and understanding of the need for platforms to educate both providers and expectant mothers, deliver vaccines, and accurately track vaccine uptake. Health security concerns, goals of reducing newborn mortality, or broader life-course immunization and health goals can be leveraged where appropriate. Global monitoring of progress and stronger global guidance to strengthen platforms for maternal vaccination according to the needs of the country may help raise political priority.
Disclosure of potential conflicts of interest
LPD has received funding from GSK to conduct this study.
Acknowledgments
The author thanks the study participants in each of the countries who generously provided their time and expertise; Alexandra Michel for her support and careful review; Patricia Pascual-Iliakis and Ivo Vojtek (GSK group of companies) for their helpful input and championing of this project.
Additional information
Funding
References
- UN. Transforming our world: the 2030 agenda for sustainable development. New York (NY: United Nations); 2015.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis. 2013;26(3):248–53. doi:10.1097/QCO.0b013e3283607a58.
- Chu HY, Englund JA. Maternal immunization. Clin Infect Dis. 2014;59(4):560–68. doi:10.1093/cid/ciu327.
- Beigi RH, Fortner KB, Munoz FM, Roberts J, Gordon JL, Han HH, Glenn G, Dormitzer PR, Gu XX, Read JS, et al. Maternal immunization: opportunities for scientific advancement. Clin Infect Dis. 2014;59(Suppl 7):S408–14. doi:10.1093/cid/ciu708.
- Steedman MR, Kampmann B, Schillings E, Al Kuwari H, Strategies DA. To Boost Maternal Immunization To Achieve Further Gains In Improved Maternal And Newborn Health. Health Aff (Millwood). 2016;35(2):309–16. doi:10.1377/hlthaff.2015.1090.
- Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012; 87: 461–76.
- Members of the Western Pacific Region Global Influenza Surveillance Response System. Seasonal influenza vaccine policies, recommendations and use in the World Health Organization's Western Pacific Region. Western Pac Surveill Response J. 2013;4(3):51–59. doi:10.5365/WPSAR.2013.4.1.009
- Neuzil KM, Bresee JS, de la Hoz F, Johansen K, Karron RA, Krishnan A, Madhi SA, Mangtani P, Spiro DJ, Ortiz JR, et al. Data and product needs for influenza immunization programs in low- and middle-income countries: rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine. 2017;35(43):5734–37. doi:10.1016/j.vaccine.2017.08.088.
- Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM, Danovaro-Holliday MC, Heffelfinger JD, Tevi-Benissan C, Teleb NA, et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34(45):5400–05. doi:10.1016/j.vaccine.2016.07.045.
- European Centre for Disease Prevention and Control. Seasonal influenza vaccination and antiviral use in EU/EEA Member States – overview of vaccine recommendations for 2017–2018 and vaccination coverage rates for 2015–2016 and 2016–2017 influenza seasons. Stockholm, Sweden: ECDC; 2018.
- World Health Organization. Tetanus vaccines: WHO position paper, February 2017 - Recommendations. Vaccine. 2018;36(25):3573–75. doi:10.1016/j.vaccine.2017.02.034.
- Bechini A, Tiscione E, Boccalini S, Levi M, Bonanni P. Acellular pertussis vaccine use in risk groups (adolescents, pregnant women, newborns and health care workers): a review of evidences and recommendations. Vaccine. 2012;30(35):5179–90. doi:10.1016/j.vaccine.2012.06.005.
- Moreno-Perez D, Alvarez Garcia FJ, Alvarez Aldean J, Cilleruelo Ortega MJ, Garces Sanchez M, Garcia Sanchez N, et al. Immunisation schedule of the Spanish association of paediatrics: 2019 recommendations. An Pediatr (Barc). 2019;90(56):e1–e9.
- European centre for disease prevention and control. ECDC Vaccine Scheduler.
- Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: updated Recommendations of the advisory committee on immunization practices - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(3):77–83. doi:10.15585/mmwr.mm6903a5.
- WHO. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. World Health Organization; 2019.
- Brillo E, Tosto V, Giardina I, Buonomo E. Maternal tetanus, diphtheria, and acellular pertussis (Tdap) and influenza immunization: an overview. J Matern Fetal Neonatal Med. 2019;1–30.
- Ghaswalla P, Poirrier JE, Packnett ER, Irwin DE, Gray SR, Buck PO. Maternal immunization in the U.S.: A nationwide retrospective cohort study. Am J Prev Med. 2019;57(3):e87–e93. doi:10.1016/j.amepre.2019.04.013.
- Fernandez-Cano MI, Espada-Trespalacios X, Reyes-Lacalle A, Manresa Dominguez JM, Armadans-Gil L, Campins-Marti M, et al. Vaccination coverage against pertussis in pregnant women of Catalonia in the first year of implementation of the immunisation program. Enferm Infecc Microbiol Clin. 2017;35:550–55.
- Ledent E, Gabutti G, de Bekker-grob EW, Alcazar Zambrano JL, Campins Marti M, Del Hierro Gurruchaga MT, Fernández Cruz MJ, Ferrera G, Fortunato F, Torchio P, et al. Attributes influencing parental decision-making to receive the Tdap vaccine to reduce the risk of pertussis transmission to their newborn - outcome of a cross-sectional conjoint experiment in Spain and Italy. Hum Vaccin Immunother. 2019;15(5):1080–91. doi:10.1080/21645515.2019.1571890.
- Moniz MH, Beigi RH. Maternal immunization. Clinical Experiences, Challenges, and Opportunities in Vaccine Acceptance. Hum Vaccin Immunother. 2014;10:2562–70.
- Ortiz JR, Neuzil KM. Influenza immunization in low- and middle-income countries: preparing for next-generation influenza vaccines. J Infect Dis. 2019;219(Supplement_1):S97–S106. doi:10.1093/infdis/jiz024.
- Lutz CS, Carr W, Cohn A, Rodriguez L. Understanding barriers and predictors of maternal immunization: identifying gaps through an exploratory literature review. Vaccine. 2018;36(49):7445–55. doi:10.1016/j.vaccine.2018.10.046.
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015;33(47):6420–29. doi:10.1016/j.vaccine.2015.08.046.
- Bethancourt CN, Wang TL, Bocchini JA Jr. Vaccination during pregnancy: first line of defense for expecting mothers and vulnerable young infants. Curr Opin Pediatr. 2017;29:737–43.
- Mehrotra A, Fisher AK, Mullen J, Rodriguez L, Jiles AJ, Albert AP, Randall LA, Frew PM. Provider insight on surmounting specialty practice challenges to improve Tdap immunization rates among pregnant women. Heliyon. 2018;4(5):e00636. doi:10.1016/j.heliyon.2018.e00636.
- Bergin N, Murtagh J, Philip RK. Maternal vaccination as an essential component of life-course immunization and its contribution to preventive neonatology. Int J Environ Res Public Health. 2018;15.
- Vojtek I, Dieussaert I, Doherty TM, Franck V, Hanssens L, Miller J, Bekkat-Berkani R, Kandeil W, Prado-Cohrs D, Vyse A, et al. Maternal immunization: where are we now and how to move forward? Ann Med. 2018;50(3):193–208. doi:10.1080/07853890.2017.1421320.
- Pathirana J, Nkambule J, Black S. Determinants of maternal immunization in developing countries. Vaccine. 2015;33(26):2971–77. doi:10.1016/j.vaccine.2015.04.070.
- Sundaram N, Purohit V, Schaetti C, Kudale A, Joseph S, Weiss MG. Community awareness, use and preference for pandemic influenza vaccines in Pune, India. Hum Vaccin Immunother. 2015;11(10):2376–88. doi:10.1080/21645515.2015.1062956.
- Ozawa S, Privor-Dumm LA, Nanni A, Durden E, Maiese BA, Nwankwo CU, et al. Evidence-to-policy gap on hepatitis A vaccine adoption in 6 countries: literature vs. Policymakers’ Beliefs. Vaccine. 2014;32:4089–96.
- Privor-Dumm L, Vasudevan P, Kobayashi K, Gupta J. Archetype analysis of older adult immunization decision-making and implementation in 34 countries. Vaccine. 2020;38(26):4170–82. doi:10.1016/j.vaccine.2020.04.027.
- Salud C. CALENDARIO COMÚN DE VACUNACIÓN A LO LARGO DE TODA LA VIDA Calendario recomendado año 2020. Spain; 2020.
- Tuells J, Rodriguez-Blanco N, Duro Torrijos JL, Vila-Candel R, Nolasco Bonmati A. Vaccination of pregnant women in the Valencian Community during the 2014-15 influenza season: a multicentre study. Rev Esp Quimioter. 2018;31:344–52.
- Salute M. Piano Nazionale Prevenzione Vaccinale 2017-2019. Italy; 2017.
- Bonanni P, Chiamenti G, Conforti G, Maio T, Odone A, Russo R, Scotti S, Signorelli C, Villani A. The 2016 Lifetime Immunization Schedule, approved by the Italian scientific societies: A new paradigm to promote vaccination at all ages. Hum Vaccin Immunother. 2017;13(11):2531–37. doi:10.1080/21645515.2017.1366394.
- Ministero della Salute. Vaccinazioni raccomandate per le donne in età fertile e in gravidanza. Aggiornamento novembre 2019. 2019.
- Ministry of Health and Family Welfare GoINHM. Tetanus and adult diphtheria operational guidelines.New Delhi (India).
- Purohit V, Kudale A, Sundaram N, Joseph S, Schaetti C, Weiss MG. Public health policy and experience of the 2009 H1N1 influenza pandemic in Pune, India. Int J Health Policy Manag. 2018;7(2):154–66. doi:10.15171/ijhpm.2017.54.
- India FoOaGSo. Vaccination in Women. 2014.
- Balasubramanian S, Shah A, Pemde HK, Chatterjee P, Shivananda S, Guduru VK, Soans S, Shastri D, Kumar R. Indian academy of pediatrics (IAP) advisory committee on vaccines and immunization practices (ACVIP) recommended immunization schedule (2018-19) and update on immunization for children aged 0 through 18 years. Indian Pediatr. 2018;55(12):1066–74. doi:10.1007/s13312-018-1444-8.
- Fernandez-Cano MI, Armadans Gil L, Campins Marti M. Cost-benefit of the introduction of new strategies for vaccination against pertussis in Spain: cocooning and pregnant vaccination strategies. Vaccine. 2015;33(19):2213–20. doi:10.1016/j.vaccine.2015.03.045.
- Benes O Dealing with vaccine shortages: current situation and ongoing activities Impact of shortages and solutions set up by countries In: V-pDaIVDWHOROf, ed. Meeting of the Strategic Advisory Group of Experts on Immunization (2016). Europe.
- Gurnani V, Haldar P, Aggarwal MK, Das MK, Chauhan A, Murray J, Arora NK, Jhalani M, Sudan P. Improving vaccination coverage in India: lessons from Intensified Mission Indradhanush, a cross-sectoral systems strengthening strategy. BMJ. 2018;363:k4782. doi:10.1136/bmj.k4782.
- Njuguna HN, Yusuf N, Raza AA, Ahmed B, Tohme RA. Progress toward maternal and neonatal tetanus elimination - Worldwide, 2000-2018. MMWR Morb Mortal Wkly Rep. 2020;69(17):515–20. doi:10.15585/mmwr.mm6917a2.
- Ministerio de Sanidad CyB, Gobierno de Espana. TdaP coverage rates in pregnant women by autonomous community. 2018.
- Rodriguez-Blanco N, Tuells J, Vila-Candel R, Nolasco A. Adherence and concordance of influenza and pertussis vaccination coverage in pregnant women in Spain. Int J Environ Res Public Health. 2019;16.
- Rodriguez-Blanco N, Tuells J. Knowledge and attitudes about the flu vaccine among pregnant women in the valencian community (Spain). Medicina (Kaunas). 2019;55.
- Vilca LM, Martinez C, Burballa M, Campins M. Maternal care providers’ barriers regarding influenza and pertussis vaccination during pregnancy in Catalonia, Spain. Matern Child Health J. 2018;22(7):1016–24. doi:10.1007/s10995-018-2481-6.
- Napolitano F, Napolitano P, Angelillo IF. Seasonal influenza vaccination in pregnant women: knowledge, attitudes, and behaviors in Italy. BMC Infect Dis. 2017;17(1):48. doi:10.1186/s12879-016-2138-2.
- Vilca LM, Cesari E, Tura AM, Di Stefano A, Vidiri A, Cavaliere AF, Cetin I. Barriers and facilitators regarding influenza and pertussis maternal vaccination uptake: A multi-center survey of pregnant women in Italy. Eur J Obstet Gynecol Reprod Biol. 2020;247:10–15. doi:10.1016/j.ejogrb.2020.02.007.
- Wagner AL, Masters NB, Domek GJ, Mathew JL, Sun X, Asturias EJ, et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines (Basel). 2019;7.
- Wiot F, Shirley J, Prugnola A, Di Pasquale A, Philip R. Challenges facing vaccinators in the 21 st century: results from a focus group qualitative study. Hum Vaccin Immunother. 2019;15(12):2806–15. doi:10.1080/21645515.2019.1621147.
- D’Alessandro A, Napolitano F, D’Ambrosio A, Angelillo IF. Vaccination knowledge and acceptability among pregnant women in Italy. Hum Vaccin Immunother. 2018;14(7):1573–79. doi:10.1080/21645515.2018.1483809.
- de Martino M. Dismantling the taboo against vaccines in pregnancy. Int J Mol Sci. 2016;17.
- Gualano MR, Bert F, Voglino G, Buttinelli E, D’Errico MM, De Waure C, Di Giovanni P, Fantini MP, Giuliani AR, Marranzano M, et al. Attitudes towards compulsory vaccination in Italy: results from the NAVIDAD multicentre study. Vaccine. 2018;36(23):3368–74. doi:10.1016/j.vaccine.2018.04.029.
- Di Pietro A, Visalli G, Antonuccio GM, Facciola A. Today’s vaccination policies in Italy: the National plan for vaccine prevention 2017-2019 and the Law 119/2017 on the mandatory vaccinations. Ann Ig. 2019;31:54–64.
- Marchetti F, Vilca LM, Cetin I. Insights and expectations for Tdap vaccination of pregnant women in Italy. J Matern Fetal Neonatal Med. 2019;1–8. doi:10.1080/14767058.2019.1659240.
- Cowling BJ, Caini S, Chotpitayasunondh T, Djauzi S, Gatchalian SR, Huang QS, Koul PA, Lee P-I, Muttalif AR, Plotkin S, et al. Influenza in the Asia-Pacific region: findings and recommendations from the global influenza initiative. Vaccine. 2017;35(6):856–64. doi:10.1016/j.vaccine.2016.12.064.
- Maternal and neonatal tetanus elimination: validation survey in 4 States and 2 union territories in India, May 2015. Wkly Epidemiol Rec. 2015; 90: 589–608.
- Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, Breedveld FC, D’Amelio R, Dougados M, Kapetanovic MC, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. doi:10.1136/annrheumdis-2019-215882.
- Goel MK, Goel M, Khanna P, Mittal K. Pandemic influenza A (H1N1) 2009 vaccine: an update. Indian J Med Microbiol. 2011;29(1):13–18. doi:10.4103/0255-0857.76517.
- Joshi J, Das MK, Polpakara D, Aneja S, Agarwal M, Arora NK. Vaccine safety and surveillance for adverse events following immunization (AEFI) in India. Indian J Pediatr. 2018;85(2):139–48. doi:10.1007/s12098-017-2532-9.
- Kochhar S, Edwards KM, Ropero Alvarez AM, Moro PL, Ortiz JR. Introduction of new vaccines for immunization in pregnancy - Programmatic, regulatory, safety and ethical considerations. Vaccine. 2019;37(25):3267–77. doi:10.1016/j.vaccine.2019.04.075.
- Shiffman J, Smith S. Generation of political priority for global health initiatives: a framework and case study of maternal mortality. Lancet. 2007;370(9595):1370–79. doi:10.1016/S0140-6736(07)61579-7.