ABSTRACT
To evaluate rotavirus (RV) disease burden and circulating strains of RV among Chinese children younger than 5-years old who had diarrhea from 2011 to 2018. PubMed, Web of Science, Embase, CNKI and WANFANG databases were systematically searched to identify studies that reported RV prevalence in mainland China. After data extraction, a fixed-effects model or a random-effects model was applied to estimate RV positivity and proportions of G and P types. Statistical analysis was conducted using R software. We initially reviewed 1323 studies, and identified 69 studies that were eligible. The overall proportion of RV gastroenteritis (RVGE) among children under 5-years old who presented with diarrhea and sought medical care was 34.0% (95% CI: 31.3, 36.8), and RV positivity was higher among inpatients (39.7%) than outpatients (23.9%). Western areas of China had the highest proportion of RVGE (42.7%), and RV positivity was highest for children who were 6 months-old to 2 years-old. The most prevalent G types were G3 (26.1%), G9 (17.5%), and G1 (12.8%), the most prevalent P type was P[8] (56.8%) and the most prevalent G-P combination was G9P[8] (20.9%). RV continues to be a main cause of acute gastroenteritis in Chinese children who are younger than 5 years old. Following the introduction of an RV vaccine in 2011, monitoring of the disease burden of RV diarrhea and circulating strains in China remain important for assessments of vaccine efficacy.
Introduction
Rotavirus (RV) is the leading cause of severe acute gastroenteritis in young children worldwide. Each year, there are more than 110 million cases of RV infections throughout the world, approximately 100 million children with RV gastroenteritis (RVGE) who require home care, and 4.5 million children who require hospital visits (2.5 million outpatient visits and 2 million hospitalizations).Citation1 Furthermore, RVGE is responsible for 215,000 deaths per year among children younger than 5-years old and is a huge public health burden, especially in developing countries.Citation2
A previous systematic review investigated the epidemiology, disease burden, and circulating strains of RV in children younger than 5-years old in China prior to 2011. The results indicated that the incidence of RV positivity in children with diarrhea was 42.6% for inpatients, 32.5% for outpatients, and 9.3% for children in the community.Citation3 Another systematic review that examined the period of 2000 to 2011 reported that the most common strains in Chinese children under 5-years old were G1P [8] (23.6%) and G2P [4] (11.8%).Citation4
However, a growing number of studies have reported potential changes in the infection patterns and distributions of different RV strains in China since 2011.Citation5–7 Therefore, we performed an updated systematic review and meta-analysis of the RV disease burden and comprehensively described the current epidemiology of RVGE in China after 2011. This study provides up-to-date information that will be important to health-care practitioners and policy makers.
Materials and methods
Search strategy
The phrase “Rotavirus and China” was used to search PubMed, Web of Science, and Embase for studies published in the English language from 2011 to October 30, 2018. CNKI and WANFANG were searched using term “Rotavirus” for studies published in the Chinese language during the same period.
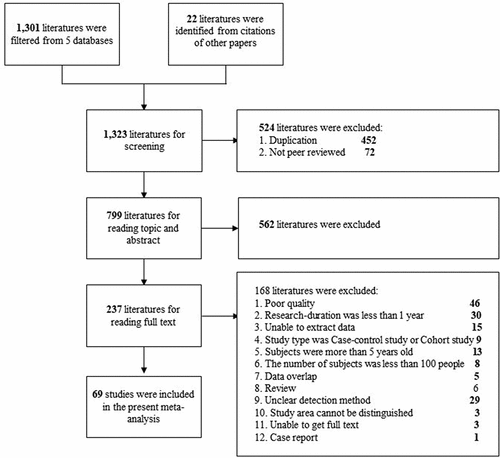
Selection began with a review of the title and abstract, and when this was insufficient, the full text was reviewed. Studies were also identified by review of the citation lists. The selection process was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. All duplications were removed and all searches were conducted by two independent investigators, with conflicts resolved by discussion.
Inclusion and exclusion criteria
The populations, interventions, comparators, outcomes, timing, and setting (PICOTS) criteria for study inclusion were used. All included studies examined populations of children with acute gastroenteritis who were less than 5-years old and lived in China; examined at least 100 cases; assessed RV positivity among those with acute gastroenteritis or reported RV strain distribution (G or P types); included epidemiological data from 2011 to 2018 and had a study duration of 12 months or more; had a retrospective or prospective design; and detected RV in fecal samples using at least one of the following laboratory tests on fecal samples: enzyme-linked immunosorbent assay (ELISA), polyacrylamide gel electrophoresis (PAGE), reverse-transcriptase polymerase chain reaction (RT-PCR), latex agglutination, gold immunoassay, indirect immunofluorescence, electronic microscopy, or immune-chromatography. Studies that did not meet these criteria were excluded.
Data collection
Standardized and structured tables were used to extract basic study characteristics (author, publishing year, city/province, sample size, and research period); patient characteristics (age, sex, and setting); and disease endpoints of interest (RV positivity in acute gastroenteritis, and RV strain distribution). Age-stratified RV positivity was also collected if reported. When multiple studies examined the same population, only the most complete and recent study was included. Data extraction was conducted independently by two researchers, and conflicting opinions were resolved by consensus. A third investigator provided arbitration when necessary.
Assessment of study quality
The risk of bias of each included studies was assessed by two investigators using the tool developed by Linhares et al.Citation8 This tool was based on a checklist of essential items in the STROBE statementCitation9 and two methodological studies.Citation10,Citation11 A third investigator resolved disagreements by discussion.
Statistical analysis
The R software package was used to calculate RV positivity and strain distribution of G and P types and G/P combinations, with 95% confidence intervals (CIs). If the I2 value from a Q test was greater than 50%, then a fixed-effects model was used. Otherwise, a random-effects model was used.
Subgroup analyses of RV positivity rate, with stratification by age, geographic region, and care setting, were performed using meta-regression. Given the climatic differences among different regions, subgroup analysis of southern and northern China was also performed, in which northern China was defined as areas north of the Qinling Mountains and the Huaihe River, and other areas as southern China. Furthermore, according to the differences of the economic conditions, the eastern, central, and western areas were also classified, using the standards of the National Bureau of Statistics. Patient setting was categorized as inpatient, outpatient, mixed (inpatient + outpatient), and unknown (not reported). Age grouping was based on categories reported in each study. Sensitivity analysis was conducted for the overall RV positive rate based on study quality (high, medium, or low).
Results
Eligible studies
We initially reviewed 1323 studies (). We excluded 452 studies due to duplication and 72 studies because they were not peer-reviewed. We then excluded another 562 studies because of irrelevance to the topic, based on review of the titles and abstracts, and also excluded 237 studies after reading the full texts. Thus, we included 69 studies that met our inclusion criteria in the final analysis. These eligible studies were conducted in 22 provinces, mostly in the southern and western areas, and there were fewer studies in the coastal and northern regions. Most studies used ELISA (38) or the gold immunoassay (25) to detect RV. Latex agglutination, PAGE, RT-PCR, and immunofluorescence were used in some studies. Only two studies applied two detection methods; one study used ELISA and PAGE, and the other study used gold immunoassay and ELISA.
RV positivity overall and in different care settings
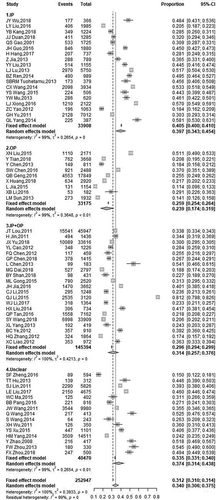
A total of 252,947 children with gastroenteritis who were under 5-years old were examined in the 69 studies (). The overall RV positivity was 34.0% (95% CI: 31.3, 36.8), and was higher in the inpatient setting (39.7%; 95% CI: 34.2, 45.6) than in the outpatient setting (23.9%; 95% CI: 18.1, 30.9). The RVGE positivity was 31.4% (95% CI: 27.1, 36.0) for the mixed inpatient + outpatient setting and 37.4% (95% CI: 32.3, 42.9) for unknown care setting.
RV positivity in different age groups
Analysis of different age groups () indicated the highest RV positivity in the 12 to 23 month-old group (40.1%, 95% CI: 33.4, 47.2), followed by the 6 to 11 month-old group (33.1%, 95% CI: 24.1, 43.6). The lowest positivity rates were in the 0 to 5 month-old group (17.7%) and the 48 to 59 month-old group (22.9%).
Table 1. RV positivity in Chinese children of different age groups from 2011 to 2018
RV positivity in different regions of China
Analysis of infection rates in different regions of China () indicated the highest RV positivity rates were in central China (38.9%) and western China (37.0%). RV positivity was lowest in the eastern region (31.6%), except when compared to nationwide or multi-provincial studies. RV positivity was 35.4% in the northern region and 33.7% in the southern region.
Table 2. RV positivity in Chinese children in different geographic regions from 2011 to 2018
Prevalences of different G and P types
The most prevalent G type was G3 (26.1%), followed by G9 (17.5%), G1 (12.8%), G2 (5.51%), and mixed G type (7.08%) (). G8 (0.49%) and G4 (2.36%) were the rarest G types. P[8] was the main circulating P type (56.8%), followed by P[4] (10.1%). The proportions of P[10] (2.66%), P[6] (2.17%), and P[9] (0.78%) were relatively small. Analysis of combined G and P types () indicated the most common types were G9P[8] (20.9%), G3P[8] (19.5%), and G1P[8] (10.6%).
Table 3. Proportions of G and P types of RV in Chinese children from 2011 to 2018
Table 4. Proportions of G-P type RV combinations in Chinese children from 2011 to 2018
Sensitivity analysis
To ensure the stability of the included studies, the study group evaluated the quality of 69 studies and classified them according to three levels: high quality, medium quality, and low quality. After two rounds of evaluation, 44 studies were rated as high quality, and 22 as medium quality and 3 as low quality. Considering the stability of the results, we performed a sensitivity analysis for overall RV positivity. Specifically, we deleted two studies in turn, one with low quality and the other with maximum sample size. The results of overall positivity were changed into 33.8% and 33.9% respectively, which indicated that the results of the present study were still relatively stable.
Discussion
RV infection is a major public health problem worldwide. Since its discovery in 1974, the WHO has considered RV one of the main pathogens responsible for acute gastroenteritis in infants and young children.Citation12 RV infection is associated with substantial mortality and a high rate of hospitalization.Citation13 RV vaccination is one of the most effective preventive strategies. Countries that introduced RV vaccines and had high coverage rates have reported substantial declines in the incidence of RVGE.Citation14–17
In our study, the overall RV positivity in children with diarrhea who were under 5-years old and seeking medical care from 2011 to 2018 was 34.0%, 2.7% less than reported in a previous study for an earlier time period.Citation3 A sentinel-hospital based surveillance system (China Viral Diarrhea Surveillance Network, CVDSN), which was established in 2006, reported a similar slight numerical reduction of RV positivity. This system focused on viral diarrhea and included infections by RV and other common viral pathogens causing acute gastroenteritis.Citation7,Citation18 Unpublished data from the CVDSN for the period of 2011 to 2018 indicated that RV positivity decreased from 33.4% to 27.3% (χ2 = 252.8, P < .01).
The first RV vaccine was administered in China during 2001, and other licensed RV vaccines are gradually being introduced. To date, there are two RV vaccines available on the private market in China: LLR, G10P[15], a monovalent RV vaccine (Lanzhou lamb RV vaccine, Lanzhou institute of Biological Products, Lanzhou, China) and RV5, a human-bovine reassortant pentavalent vaccine (Merck & Co., Inc., Kenilworth, NJ, USA) which was introduced in 2018. LLR is used in children aged 2 months to 3 years as a single annual oral dose for 3 years. A post-marketing study reported that one dose of the LLR vaccine conferred a 43.8% (95% CI: 34.7, 51.7%) reduction of RVGE among children who were 2 to 35-months old.Citation19 Approximately 600 to 700 million doses of LLR were administered annually in China from 2011 to 2018.Citation20 The LLR vaccine is primarily used in areas with good economic conditions,Citation21 and its use in poorer areas has been extremely limited. It is still too early to determine if the LLR vaccine contributed to the slight reduction of RV positivity in China.
We also observed that RV positivity in children with diarrhea was greater among inpatients than outpatients (40% vs. 30%), similar to the 2014 systemic review.Citation3 Other research also reported that RV positivity and more severe diarrhea were more common among inpatients than outpatients.Citation7 This may be because of the more serious condition of inpatients, so that examinations are more extensive and thorough, and RV positivity is more common. Thus, as the proportion of RV outpatients increases, the overall RV positivity rate will decline, even when RV positivity remains unchanged for inpatients and outpatients. In addition, parents now tend to take their children to hospitals when they have less severe diarrhea, and this lowers the risk of hospitalization. Several studies in China showed decreasing rates of hospitalization of children due to diarrhea.Citation3,Citation7,Citation22 Given the limited number of studies in our analysis, we were unable to conduct subgroup analysis of inpatients and outpatients. Thus, we cannot determine whether differences in the proportions of inpatients and outpatients in different regions of China contributed to the decline of RV positivity.
Our analysis suggested geographical differences of RV infections in China. In particular, the proportion of RV-associated diarrhea in children was greatest in central China, in contrast to the results of the 2014 meta-analysis.Citation3 Although the economic conditions in western China has improved significantly during recent years, we found little difference in RV positivity between the central and western regions, but these regions still had greater RV positivity than the eastern region, which has the best economic status. This suggests that economic status affects RV positivity. Better economic status often means a cleaner and healthier living environment and the availability of more money for health care. Similar to the findings of previous studies, RV positivity was still higher in northern China than southern China. Compared with data presented in the 2014 meta-analysis,Citation3 RV positivity in northern China decreased from 40.6% to 35.4% and that in western China decreased from 43.3% to 37%, although the 95% CIs overlapped.
RV-associated diarrhea most commonly occurs in infants and young children between the ages of 6 months and 2 years. Infants younger than 6 months and those with repeated infections mostly have atypical clinical manifestations.Citation23 In agreement, we observed that RV positivity was highest in the 6 to 11 month-old group and the 12 to 23 month-old group, and that children in these age groups accounted for 94% of all episodes of RV-associated diarrhea. As children grow older, RV positivity decreases. Our results are thus consistent with those of the WHO, and suggest that the RV vaccine should be administered to children before they are 2-years old.Citation13
Diverse types of RVs can infect humans. According to the Rotavirus Classification Working Group (RCWG), there are now 36 G types (G1–G36) and 51 P types (P[1]–P[51]).Citation24 The most prevalent G and P types are G1, G2, G3, G4, G9, P[4], and P[8] and the most common combinations of G and P types are G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8].Citation25,Citation26 The distributions of different RV types varies widely throughout the world and also changes over time.Citation27 The greatest diversity of RV strains are in Africa and Asia.Citation28
Analysis of RV isolates in detected in China after 2006 indicated that G3 was the most prevalent G type, followed by G1, G2, and G4,Citation3 but the most recent research showed that G9 was increasing.Citation29–31 The most prevalent P types in China are P[8] and P[4].Citation7,Citation32–34 We found some changes in the major circulating strains of RV compared to the 2014 meta-analysis. In particular, the previous study reported a predominance of G3 and G1 from 1980 to 2011, but the present study showed that the main G types were G3 and G9. The proportion of G9 increased from 3.3% (1980–2011) to 17.5% (2011–2018). Although G3 remained the predominant strain, its proportion decreased from 39.3% (1980–2011) to 26.3% (2011–2018). These results may thus reflect a shift from G3 to G9 in China from 2011 to 2018. During the same period of time (2011–2018), the predominant strains circulating elsewhere in the world were quite different. In East Asia (Korea and Taiwan), G9P[8] was the predominant genotype. In the Middle East and some European countries (France, Italy, and Turkey), there was an increase of G9P[8] and this became the main circulating strain.Citation35–40 However, in Africa (Ethiopia and Ghana) and the U.S., G12P[8] was the predominant strain, although G1P[8] was more common during earlier periods.Citation41–43 In Latin America and Ireland, the predominant strain was G2P[4], possibly because of the extensive RV vaccination in these regions.Citation44–46
There were some limitations in our study. First, because the research period was 2011 to 2018, there were insufficient data to analyze changes in RVGE over time. Most of the included studies collected data during 2 or more years, and we could not obtain the original data to analyze RV dynamics. Second, although we conducted quality control analysis, our results may be unstable. However, our sensitivity analysis and quality evaluation indicated the results were reliable. Lastly, although we considered examining the effects of economic status during data extraction, the relevant dataset was too small and we could not provide a meaningful analysis of this topic.
In conclusion, the present meta-analysis, which examined RV infections in Chinese children from 2011 to 2018, indicated that RV continues to be a main cause of acute gastroenteritis in this population. As the availability of RV vaccines continues to increase, it is important to continue monitoring the incidence and disease burden of RV infections in China.
List of abbreviations
| RV | = | Rotavirus |
| RVGE | = | Rotavirus gastroenteritis |
| ELISA | = | Enzyme-linked immunosorbent assay |
| PAGE | = | Polyacrylamide gel electrophoresis |
| RT-PCR | = | Reverse-transcriptase polymerase chain reaction |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
JL, HW and NL conceived and designed the research; JL, HW, DL and QZ collected data and conducted the research; JL, HW, DL, QZ, and NL analyzed and interpreted the data; JL, HW and DL wrote the initial paper; QZ and NL revised the paper; and NL had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgments
We thank Yongyu Su from MSD China for obtaining funding, advice on manuscript revision, and help with proofreading. Editorial assistance was provided by Medjaden Bioscience Limited, and this assistance was funded by MSD China.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62:S96–s105.
- Liu N, Xu Z, Li D, Zhang Q, Wang H, Duan ZJ. Update on the disease burden and circulating strains of rotavirus in China: a systematic review and meta-analysis. Vaccine. 2014;32:4369–75. doi:10.1016/j.vaccine.2014.06.018.
- Kawai K, O’Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30:1244–54. doi:10.1016/j.vaccine.2011.12.092.
- Liu X, Meng L, Li J, Liu X, Bai Y, Yu D, Ren X, Liu H, Shen X, Wang P, et al. Etiological epidemiology of viral diarrhea on the basis of sentinel surveillance in children younger than 5 years in Gansu, northwest China, 2009-2013. J Med Virol. 2015;87:2048–53. doi:10.1002/jmv.24283.
- Lou JT, Xu XJ, Wu YD, Tao R, Tong MQ. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol. 2011;50:84–87. doi:10.1016/j.jcv.2010.10.003.
- Yu J, Lai S, Geng Q, Ye C, Zhang Z, Zheng Y, Wang L, Duan Z, Zhang J, Wu S, et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009-2015. J Infect. 2019;78:66–74. doi:10.1016/j.jinf.2018.07.004.
- Linhares AC, Stupka JA, Ciapponi A, Bardach AE, Glujovsky D, Aruj PK, Mazzoni A, Rodriguez JA, Rearte A, Lanzieri TM, et al. Burden and typing of rotavirus group A in Latin America and the Caribbean: systematic review and meta-analysis. Rev Med Virol. 2011;21:89–109.
- White RG, Hakim AJ, Salganik MJ, Spiller MW, Johnston LG, Kerr L, Kendall C, Drake A, Wilson D, Orroth K, et al. Strengthening the reporting of observational studies in epidemiology for respondent-driven sampling studies: “STROBE-RDS” statement. J Clin Epidemiol. 2015;68:1463–71. doi:10.1016/j.jclinepi.2015.04.002.
- Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302:1136–40. doi:10.1136/bmj.302.6785.1136.
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–76.
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–06. doi:10.3201/eid1202.050006.
- World Health Organization. Rotavirus vaccines. WHO position paper – January 2013. Weekly epidemiological record. 2013;88:49–64.
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125:e208–213. doi:10.1542/peds.2009-1246.
- Hemming-Harlo M, Markkula J, Huhti L, Salminen M, Vesikari T. Decrease of rotavirus gastroenteritis to a low level without resurgence for five years after universal rotateq vaccination in Finland. Pediatr Infect Dis J. 2016;35:1304–08. doi:10.1097/INF.0000000000001305.
- Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine. 2011;29:4663–67. doi:10.1016/j.vaccine.2011.04.109.
- Chang WC, Yen C, Wu FT, Huang YC, Lin JS, Huang FC, Yu HT, Chi CL, Lin HY, Tate JE, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J. 2014;33:e81–86.
- Gong XH, Wu HY, Li J, Xiao WJ, Zhang X, Chen M, Teng Z, Pan H, Yuan ZA. Epidemiology, aetiology and seasonality of infectious diarrhoea in adult outpatients through active surveillance in Shanghai, China, 2012-2016: a cross-sectional study. BMJ Open. 2018;8:e019699. doi:10.1136/bmjopen-2017-019699.
- Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31:154–58. doi:10.1016/j.vaccine.2012.10.078.
- Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun L, Tang Y, Yang B, Hu D, Huang C. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One. 2014;9:e101734. doi:10.1371/journal.pone.0101734.
- Fu C, Dong Z, Shen J, Yang Z, Liao Y, Hu W, Pei S, Shaman J. Rotavirus gastroenteritis infection among children vaccinated and unvaccinated with rotavirus vaccine in Southern China: A population-based assessment. JAMA Netw Open. 2018;1:e181382. doi:10.1001/jamanetworkopen.2018.1382.
- Zhang J, Liu H, Jia L, Payne DC, Hall AJ, Xu Z, Gao Z, Chang Z, Jiang B, Parashar UD, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing municipality and Gansu Province, China. Pediatr Infect Dis J. 2015;34:40–46. doi:10.1097/INF.0000000000000505.
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2009;58:1–25.
- Dzeshka MS, Shahid F, Shantsila A, Lip GYH. Hypertension and atrial fibrillation: an intimate association of epidemiology, pathophysiology, and outcomes. Am J Hypertens. 2017;30:733–55. doi:10.1093/ajh/hpx013.
- Patton JT. Rotavirus diversity and evolution in the post-vaccine world. Discov Med. 2012;13:85–97.
- Yen C, Tate JE, Patel MM, Cortese MM, Lopman B, Fleming J, Lewis K, Jiang B, Gentsch J, Steele D, et al. Rotavirus vaccines: update on global impact and future priorities. Hum Vaccin. 2011;7:1282–90. doi:10.4161/hv.7.12.18321.
- World Health Organization. Global rotavirus information and surveillance bulletin. Reporting Period 2010. 2011. [accessed at 2021 Feb 5]. https://www.who.int/immunization/sage/3_Final_RV_bulletin_Jan_Dec_2010_Data_nov11.pdf
- Zaman K, Yunus M, El Arifeen S, Azim T, Faruque AS, Huq E, Hossain I, Luby SP, Victor JC, Dallas MJ, et al. Methodology and lessons-learned from the efficacy clinical trial of the pentavalent rotavirus vaccine in Bangladesh. Vaccine. 2012;30(Suppl 1):A94–100. doi:10.1016/j.vaccine.2011.07.117.
- Gupta S, Chaudhary S, Bubber P, Ray P. Epidemiology and genetic diversity of group A rotavirus in acute diarrhea patients in pre-vaccination era in Himachal Pradesh, India. Vaccine. 2019;37:5350–56. doi:10.1016/j.vaccine.2019.07.037.
- Omari JZ. Characterization of rotavirus among children under five years with gastroenteritis at Kenyatta National Hospital, Kenya. Thesis for Master of Science in Molecular Medicine. Kenya. College of Health Science, Jomo Kenyatta University of Agriculture and Technology; 2019.
- Koçak AA, Aydın M, Matsumoto T, Yahiro T, Dalgıç B, Bozdayi G, Ahmed K. Emergence of rotavirus G9 in 2012, as the dominant genotype in Turkish children with diarrhea, in a university hospital in Ankara. Rev Rom Med Lab. 2019;27:209–18.
- Tian Y, Chughtai AA, Gao Z, Yan H, Chen Y, Liu B, Huo D, Jia L, Wang Q, MacIntyre CR. Prevalence and genotypes of group A rotavirus among outpatient children under five years old with diarrhea in Beijing, China, 2011-2016. BMC Infect Dis. 2018;18:497. doi:10.1186/s12879-018-3411-3.
- Dian Z, Wang B, Fan M, Dong S, Feng Y, Zhang AM, Liu L, Niu H, Li Y, Xia X. Completely genomic and evolutionary characteristics of human-dominant G9P[8] group A rotavirus strains in Yunnan, China. J Gen Virol. 2017;98:1163–68. doi:10.1099/jgv.0.000807.
- Xue R, Tian Y, Zhang Y, Zhang M, Li Z, Chen S, Liu Q. Diversity of group A rotavirus of porcine rotavirus in Shandong province China. Acta Virol. 2018;62:229–34. doi:10.4149/av_2018_216.
- Than VT, Jeong S, Kim W. A systematic review of genetic diversity of human rotavirus circulating in South Korea. Infect Genet Evol. 2014;28:462–69. doi:10.1016/j.meegid.2014.08.020.
- Wu FT, Bányai K, Jiang B, Liu LT, Marton S, Huang YC, Huang LM, Liao MH, Hsiung CA. Novel G9 rotavirus strains co-circulate in children and pigs, Taiwan. Sci Rep. 2017;7:40731. doi:10.1038/srep40731.
- Almalki SSR. Circulating rotavirus G and P strains post rotavirus vaccination in Eastern Mediterranean Region. Saudi Med J. 2018;39:755–66. doi:10.15537/smj.2018.8.21394.
- Kaplon J, Grangier N, Pillet S, Minoui-Tran A, Vabret A, Wilhelm N, Prieur N, Lazrek M, Alain S, Mekki Y, et al. Predominance of G9P[8] rotavirus strains throughout France, 2014–2017. Clin Microbiol Infect. 2018;24:660.e661-660.e664. doi:10.1016/j.cmi.2017.10.009.
- Tapisiz A, Bedir Demirdag T, Cura Yayla BC, Gunes C, Ugraş Dikmen A, Tezer H, Baran Aksakal N, Bozdayi G, Ozkan S. Rotavirus infections in children in Turkey: A systematic review. Rev Med Virol. 2019;29:e2020. doi:10.1002/rmv.2020.
- Ianiro G, Micolano R, Di Bartolo I, Scavia G, Monini M. Group A rotavirus surveillance before vaccine introduction in Italy, September 2014 to August 2017. Euro Surveill. 2019;15:24.
- Ogden KM, Tan Y, Akopov A, Stewart LS, McHenry R, Fonnesbeck CJ, Piya B, Carter MH, Fedorova NB, Halpin RA, et al. Multiple introductions and antigenic mismatch with vaccines may contribute to increased predominance of G12P[8] rotaviruses in the United States. J Virol. 2019:93(1).
- Damtie D, Melku M, Tessema B, Vlasova AN. Prevalence and genetic diversity of rotaviruses among under-five children in ethiopia: a systematic review and meta-analysis. Viruses. 2020;12(1):62.
- Lartey BL, Damanka S, Dennis FE, Enweronu-Laryea CC, Addo-Yobo E, Ansong D, Kwarteng-Owusu S, Sagoe KW, Mwenda JM, Diamenu SK, et al. Rotavirus strain distribution in Ghana pre- and post- rotavirus vaccine introduction. Vaccine. 2018;36:7238–42. doi:10.1016/j.vaccine.2018.01.010.
- Yandle Z, Coughlan S, Dean J, Tuite G, Conroy A, De Gascun CF. Group A rotavirus detection and genotype distribution before and after introduction of a national immunisation programme in Ireland: 2015-2019. Pathogens. 2020;9(6).
- Santos VS, Nóbrega FA, Soares MWS, Moreira RD, Cuevas LE, Gurgel RQ. Rotavirus genotypes circulating in Brazil before and after the national rotavirus vaccine program: a review. Pediatr Infect Dis J. 2018;37:e63–e65. doi:10.1097/INF.0000000000001770.
- Degiuseppe JI, Stupka JA. Genotype distribution of group A rotavirus in children before and after massive vaccination in Latin America and the Caribbean: systematic review. Vaccine. 2020;38:733–40. doi:10.1016/j.vaccine.2019.11.017.