ABSTRACT
In China, progress to include the RV vaccine in the national immunization program (NIP) is slow. The only two vaccines, the Lanzhou lamb rotavirus vaccine (LLR) and Rotateq, are provided through the private market. This study aims to assess the health impact and cost-effectiveness of using three vaccines in the NIP, Rotateq, Rotarix, and LLR, compared to the status quo. A decision-tree Markov model was adopted to follow the 2019 birth cohort, and a societal perspective was used. Input parameters were based on the latest local data when possible. Outcomes included cases and deaths averted, quality-adjusted life years (QALYs) gained, and incremental cost-effectiveness ratios (ICER). Sensitivity analyses and scenario analyses to consider herd immunity and vaccine price reduction were performed. Including Rotateq in the NIP was projected to prevent 348 million RVGE cases (62.6% reduction) and 4251 deaths (72.6% reduction) compared to the status quo. Rotarix through the NIP would prevent 48.7% of cases and 63.2% of deaths, and LLR would avert 20.3% of cases and 22.4% of deaths. The ICERs per QALY gained were US$ 8833 for Rotateq through the NIP, US$ 9503 for Rotarix, and US$ 26,759 for LLR. In uncertainty analyses, the reduction of vaccine prices and the incorporation of herd immunity further improved the cost-effectiveness of the NIPs, especially Rotateq or Rotarix. In conclusion, introducing the RV vaccine in China’s NIP is expected to be cost-effective compared to the GDP per capita. Reducing vaccine prices and adopting vaccines with better efficacy would be the future focus.
Introduction
Rotavirus (RV) is the leading cause of severe acute gastroenteritis (GE) in children under five years of age (U5) worldwide and causes approximately 258 million cases, 2 million hospitalizations, and 128 thousand deaths annually.Citation1–7 As the improvements in water, sanitation, and hygiene haven’t substantially altered the incidence of RV disease, vaccination is considered one of the most efficient interventions to reduce morbidity and mortality from RV.Citation1,Citation2,Citation7,Citation8 Since 2009, the World Health Organization (WHO) has recommended all countries include RV vaccines in their national immunization programs (NIPs).Citation2,Citation9 Four RV vaccines (RotaTeq, Rotarix, Rotavac and ROTASIIL) have been prequalified by WHO; among them, Rotarix (RV1) and Rotateq (RV5) have been widely used internationally.Citation1,Citation2 As of January 2022, 114 countries have introduced rotavirus vaccines, and significant reductions in disease burden and effectiveness of RV vaccines has been observed.Citation10–13
China has a substantial disease and economic burden of RVGE, with an estimated 12 million cases in 2008 and 3200 deaths in 2013 among U5 children.Citation14,Citation15 China has the highest societal treatment costs for RVGE in Asia.Citation16 However, progress in immunization planning is relatively slow compared to other countries.Citation17,Citation18 Currently, only two RV vaccines are available in China: Lanzhou lamb rotavirus vaccine (LLR) by a domestic manufacturer, Lanzhou Institute of Biological Products (licensed in 2000) and RotaTeq (newly licensed in September 2018).Citation17,Citation18 Moreover, under the immunization financing mechanism in China, RV vaccines are classified as non-Expanded Program on Immunization (EPI) vaccines, and are provided through the private market at a high out-of-pocket prices ($26- 43/dose), resulting in a low vaccine coverage across the country and potential disparities in vaccination access and health outcomes.Citation19,Citation20 Without vaccination or timely treatment, RVGE could cause severe fluid and electrolyte imbalance and the development of fatal complications, leading to shock and even death.Citation1,Citation21,Citation22 Lack of RV vaccine in the NIP might contribute to disease burden and prolonged hospitalization, health care expenses, and social productivity loss.Citation1,Citation21,Citation22 To date, few studies in China have assessed the cost-effectiveness of including RV vaccine in the NIPs, and results are limited due to both the uncertainty in local parameters (vaccine efficacy, price, etc.) at the time of the study and the assumption of zero coverage in the status quo, which may overestimate the cost-effectiveness of the strategies of NIPs.Citation23–26
Given the availability of relevant evidence recently, particularly on the epidemiology of RVGE, vaccine profile, and coverage in the private market provision in China, and updates to economic evaluation models internationally, this study aims to evaluate the public health impact and cost-effectiveness of introducing rotavirus vaccine into the NIPs compared to the current provision by private market in China. By integrating the latest local data, if available, the results would contribute to evidence-based decision-making in China’s NIPs, as well as to the prevention and reduction of regional RVGE disease and economic burden.
Materials and methods
Study design and model structure
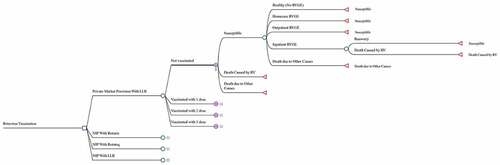
An economic evaluation was conducted to compare the impact and cost-effectiveness of the potential national rotavirus vaccination programs with the status quo, private market provision. The two RV vaccines prequalified by WHO, RotaTeq and Rotarix, and the domestic LLR were evaluated.Citation9,Citation18 LLR and Rotateq have been licensed in China, and Rotarix has been used internationally with a randomized controlled trial (RCT) demonstrating its efficacy among Chinese children.Citation18,Citation27 The study didn’t include the other two WHO prequalified vaccines, Rotavac and ROTASIIL, as they were predominantly used locally in India and were not available in the Chinese market.Citation9,Citation18 A decision tree-Markov state transition model was developed to estimate the impact of rotavirus vaccine on disease burden, quality-adjusted life years (QALYs), and costs for the 2019 birth cohort in China (). The model follows the 2019 Chinese birth cohort for 5 years with a cycle length of 6 months, and allows children to have more than once RVGE episode during the horizon. For each cycle, children will either stay healthy or develop an RVGE episode. Children with RVGE may require home-based care or seek outpatient or inpatient medical care. It is assumed that RVGE requiring home care or outpatient visits is not severe (Vesikari severity score <11), and that only hospitalized cases are severe (severity score ≥11) and may result in death. Children would develop partial immunity against subsequent RVGE when they have been vaccinated against rotavirus or when they are exposed to a natural rotavirus infection for the first time.Citation1,Citation7,Citation28
The analysis was conducted from a societal perspective using a lifetime time horizon for the cohort. All costs and impacts were discounted at 3% following WHO’s recommendations, and all costs were converted to 2019 US dollars (1 US$ = 6.5 RMB) with adjustment for inflation where necessary.Citation29,Citation30 The model was developed using TreeAge Pro 2019 (TreeAge Software, Inc., Williamstown, MA). The input parameters for the model are presented in .
Table 1. Model parameters and data source.
Model input parameters
Disease burden
The disease burden of RVGE was estimated based on the Global Burden of Disease (GBD 2019), the UN Interagency Group for Child Mortality Estimation (UN-IGME), the China National Maternal and Child Health Surveillance System (MCHS), systematic reviews and population-based surveillance in China.Citation31–35 The annual incidence of RVGE among U5 children was calculated based on the incidence of all-cause diarrhea in 2019 estimated by the GBD and the community detection rate of rotavirus in diarrhea (9.3%) in China.Citation31,Citation34 Using the method by the WHO Child Health Epidemiology Reference Group, the annual mortality rate of RVGE in 2019 was calculated by multiplying the under-five mortality rate by the proportion of deaths due to diarrhea (3.1%) from the MCHS and the RV detection rate in hospitalized diarrhea among U5 children (39.7%) from a systematic review.Citation5,Citation6 The age distribution of RVGE was obtained from population-based surveillance in northern and southern China.Citation36 To estimate the number of cases by settings, cases were first differentiated as care-seeking and not care-seeking. Those not seeking care were categorized as home care cases. Considering the severity of diarrhea caused by RV, the care-seeking rate for RV diarrhea (74.8%) was assumed as the highest rate among those for diarrhea from Hospital Utilization and Attitudes Surveys (HUAS).Citation37–40 Then, among those seeking cases, the ratio of the number of outpatient visits to hospitalization (149 vs. 14.4) from the surveillance in Beijing and Gansu provinces was applied to categorize outpatient and inpatient cases, adopting the methodology by Parashar et.al.Citation5,Citation24,Citation35
Very few studies have examined the utility of children with RV diarrhea, and most of them were conducted in developed countries, such as the UK, Canada, and Danish.Citation41–45 A study in Thailand reported the most updated results and was also the only one from developing countries.Citation45 Considering the similarity in rotavirus epidemiology, healthcare system, and health service utilization of diarrhea between China and Thailand, the utility values were derived from the study in Thailand to estimate QALYs.Citation35,Citation46,Citation47 The utility score for mild diarrhea was used for the homecare RVGE, and the score for moderate RVGE was used for outpatient RVGE and severe RVGE for inpatient RVGE. The average duration of RVGE and RVGE and its range for outpatients and inpatients were extracted from a hospital-based surveillance of rotavirus diseases conducted in 5 cities, including Beijing, Shanghai, Suzhou, Fuzhou, and Guangzhou, and the duration of homecare cases was assumed to be the same as that of outpatient cases.Citation15
Vaccine characteristics and natural immunity
For the three-dose Rotateq and two-dose Rotarix, the vaccination was assumed to be delivered in conjunction with the Diphtheria, Tetanus, and Pertussis (DTP) vaccine according to the schedule recommended by WHO.Citation2,Citation9 The three-dose LLR was assumed to be administered according to the schedule recommended by the manufacturer: one dose in the first year followed by annual booster dose in the second and third years.Citation48 By 2019, LLR was the most used vaccine in the private market, while RotaTeq was newly licensed in September 2018 with little availability and coverage information.Citation18,Citation20,Citation49 It was assumed that the coverage for Rotateq was 0% in the status quo. The coverage for each dose of LLR vaccine in the status quo were estimated from a facility-based survey in 2019, covering over 6,000 children in 10 provinces.Citation49 For RV vaccine coverage in the NIP using the three vaccines, respectively, the DTP vaccine coverage in China was used as a proxy.Citation50
The efficacy of Rotateq and Rotarix was obtained from randomized controlled trials (RCT) conducted in China.Citation27,Citation51 Based on the meta-regression of global RCTs, where efficacy was estimated by duration of follow-up, the partial-immunization efficacy of vaccines was estimated by obtaining the ratios of efficacy between incomplete and complete courses of vaccination in a low-mortality stratum setting to which China belongs.Citation52,Citation53 Data from RCTs on the efficacy and protection duration of LLR were lacking, but case-control studies reported the effectiveness of 1 dose of LLR in the first two years.Citation54,Citation55 The efficacy of LLR was estimated from the studies, and it was assumed that 1 dose provided protection for 2 years, 2 doses for 4 years, and 3 doses for the whole course of 5 years.Citation54,Citation55 Birth cohort studies have demonstrated the protection from natural infection for subsequent infections, and we estimated the natural acquired immunity against subsequent infections based on the study in Mexico, which is also in the low-mortality stratum setting as China.Citation28,Citation56,Citation57 The efficacy of vaccination and naturally acquired immunity against RVGE that required different types of care was distinguished based on the efficacy against RVGE of different severity. The efficacy against non-severe (Vesikari score <11) was used as the proxy for the efficacy against home care and outpatient RVGE, and the efficacy against severe (Vesikari score ≥11) was used as the proxy for the efficacy against inpatient RVGE. Adverse effects of vaccination were not incorporated based on the Cochrane review, clinical trials, and surveillance of adverse events following immunization in China.Citation8,Citation27,Citation51,Citation54,Citation55,Citation58 The impact of the herd immunity was incorporated in the uncertainty analyses.
Costs of health care and immunization
The health care costs for each RVGE included direct medical, direct non-medical, and indirect costs. Direct non-medical costs covered items such as transportation, accommodation and nonprescription medication. Indirect costs were associated with the loss of caregiver productivity. The costs of homecare case were estimated from the Hospital Utilization and Attitudes Surveys (HUAS) on diarrhea diseases in Zhejiang provinces, which covered 4400 children in areas of different socio-economic levels.Citation23,Citation59 The costs for inpatient and outpatient cases were obtained from the hospital-based active surveillance of RVGE among U5 children in Beijing and Gansu province.Citation35 The direct medical cost were adjusted using health care expenditures by region from the China Health Statistical Yearbook, and direct non-medical costs were adjusted using the consumer price index (CPI) from the China Statistical Yearbook.Citation60,Citation61 Using the human capital approach, lifetime productivity loss due to premature death was estimated using the national average annual wage rate for the average years of productive life (16 to 60 years) from census data and discounted to the year of vaccination.Citation62,Citation63
The price of LLR and Rotateq in the private market were obtained from the Chinese Center for Disease Control and Prevention (CDC).Citation64 The price of Rotarix, which has not yet approved in China, was estimated by multiplying the price of Rotateq by the ratio between the prices of Rotateq and Rotarix in the US market.Citation65 The costs of immunization delivery per dose, including the direct costs for governments to provide immunization services (e.g, personnel, cold chain, surveillance, communication activities, training, and supervision) and the indirect costs for households to seek vaccination (e.g., transportation and loss of caregiver productivity), were derived from the cost and investment survey of routine immunization services in 15 provinces by the Chinese CDC.Citation62,Citation66 The cost of routine immunization service per dose was used as the proxy for the three RV vaccines’ immunization delivery costs per dose.
Cost-effectiveness analysis
The cost-effectiveness results were presented as incremental cost-effectiveness ratios (ICERs), defined as the incremental costs, i.e., the cost of RV vaccination and the cost of disease, per RVGE case averted, per death averted and per QALY gained by the potential NIP strategies compared to the status quo. China has no policy or recommendation of thresholds for interpreting cost-effectiveness results, while one time and three-time the national gross domestic product (GDP) per capita were widely adopted.Citation23,Citation26,Citation67,Citation68 To reflect the conventional practice in China and the updated international opinion on the cost–effectiveness criteria, two thresholds were used for the evaluation: (1) the national GDP per capita in 2019 (US$ 10,276); (2) the threshold range proposed by Woods et al. for China (US$ 1151–4550), which accounted for the opportunity cost of the health expenditure and may be more appropriate to inform on resource allocation decisions.Citation68–72
Uncertainty analyses
Uncertainty analyses, including sensitivity analyses and scenario analyses, were performed to test the robustness of the model results and to assess the sources of model uncertainty. Deterministic sensitivity analyses (DSA) were conducted for all model parameters using the plausibility ranges specified in . For parameters with an unknown uncertainty range, the plausibility range was assumed to be 25% of the base value. Probabilistic sensitivity analysis (PSA) was conducted using Monte Carlo simulation (N = 1000 iterations) to assess the impact of changing multiple parameters simultaneously. The model uncertainty from the DSA and PSA were summarized using tornado diagrams and cost-effectiveness acceptability curves.
In scenario sensitivity analyses, the vaccine price per dose in the NIPs for the three RV vaccines was adjusted, and the impact of herd immunity was considered. The combined effect of the vaccination (direct and herd immunity effects) at different levels of RV vaccine coverage were estimated using regression estimates proposed by Wahl et al.Citation62,Citation73 The regression model was detailed in supplementary material 1. The price of RV vaccines would be reduced in the private market by 10%, 25%, and 50% to estimate the impact of the potential vaccine prices in the NIPs on cost-effectiveness outcomes, and identified the break-even price at which the incremental vaccination costs in the NIPs would be offset by the averted economic burden of the disease compared to the status quo. In addition, a threshold analysis was conducted to explore the price at which Rotarix would be as cost-effective as Rotateq at the current market price.
Results
Health benefits and economic impacts of rotavirus vaccination
shows the health benefits, economic impact, and ICERs of the three national rotavirus vaccination programs compared with the current provision by the private market. The status quo was projected to cause 5.57 million RVGE cases, including 1.41 million homecare cases, 3.80 million outpatient cases and 366 thousand inpatient cases, and 5855 deaths among the 2019 birth cohort over five years. In comparison, national rotavirus vaccination programs could substantially reduce the disease burden. Including Rotateq in the NIP would cause the disease burden of 2.09 million RVGE cases and 1604 deaths, reducing 62.6% of the total cases and 72.6% of the deaths and gaining additional 131,850 QALYs from the status quo. Rotarix through the NIP would cause the disease burden of 2.86 million RVGE cases and 2157 deaths, averting 48.7% of RVGE cases and 63.2% of the deaths and gaining additional 113,913 QALYs from the status quo. LLR through the NIP would avert 20.3% of RVGE cases and 22.4% of deaths with an additional 41,185 QALYs gained.
Table 2. Disease burden averted and cost-effectiveness of including RV vaccines into NIP compared with the status quo.
From a societal perspective, the total incremental cost of introducing Rotateq into the NIPs is projected to be 1164.69 million US$ compared to the status quo, with the additional cost of immunization (2185.24 million US$) being partially offset by the averted cost of treatments and the lost productivity from premature death (1020.55 million US$). The introduction of Rotarix into the NIPs could generate an incremental cost of 1082.53 million US$, while including LLR in the NIP with LLR could generate an incremental cost of 1102.08 million US$.
Cost-effectiveness analysis
Compared to the status quo, the ICERs of Rotateq through the NIP were US$ 334 per case averted, US$ 273,984 per death averted, and US$ 8833 per QALY gained. The ICER per QALY gained was US$ 9503 for Rotarix and US$ 26,759 for LLR. At a threshold of one-time GDP per capita (US$ 10,276), Rotateq and Rotarix through the NIP were cost-effective. But all the three RV vaccines were not cost-effective at the threshold range advised by Woods et al. (US$ 1151–4550) ().
Uncertainty analyses
In the deterministic sensitivity analysis, the most sensitive parameters for Rotateq or Rotarix through the NIP were vaccine efficacy against hospitalization and vaccine price. The most sensitive parameters for LLR were vaccine efficacy against outpatient or home care cases and protection of natural infection against hospitalization (). In the PSA, the probability of being cost-effective at a threshold of one-time GDP per capita was 38.7% for Rotateq, 34.3% for Rotarix, and 6.3% for LLR through the NIP. The probability of being cost-effective at the upper bound (US$ 4550) of the threshold range by Woods et al. was 9.3% for Rotateq, 7.9% for Rotarix, and 0.1% for LLR through the NIP ().
Figure 2. Tornado diagrams for one-way sensitivity analyses of NIP with Rotateq, Rotarix or LLR compared to the status quo.
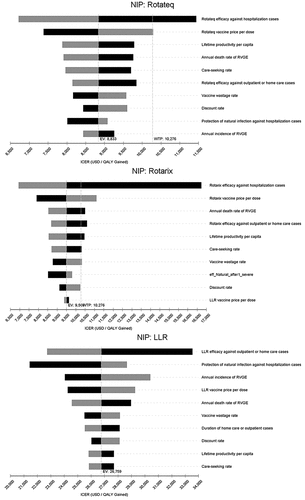
Figure 3. Cost-Effectiveness acceptability curves of the PSAs for the NIPs with Rotateq, Rotarix or LLR in the base case scenario and the scenario of considering herd immunity.
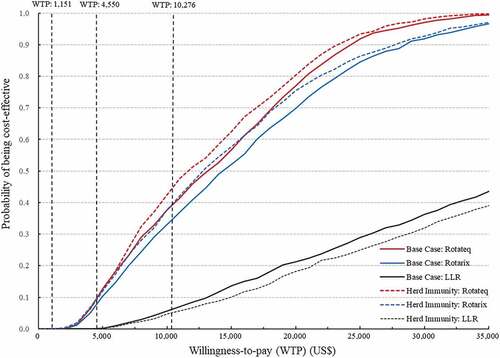
The scenario analysis showed that including the herd immunity in the model reduced the ICER of Rotateq to US$ 8434 per QALY, Rotarix to US$ 9097 per QALY, but slightly increased the ICER of LLR to US$ 29,210 per QALY (). In the PSA, after considering the herd immunity, the probability of being cost-effective at a one-time GDP per capita threshold increased to 44.0% for Rotateq and 38.5% for Rotarix, but decreased to 5.3% for LLR. The probability of being cost-effective at the upper bound (US$ 4550) of the threshold range by Woods et al. increased to 9.5% for Rotateq and 9.6% for Rotarix ().
Table 3. Scenario analyses of the NIPs with Rotateq, Rotarix or LLR at different vaccine prices in the base case scenarios and the scenario of considering herd immunity.
In addition, the scenario analysis explored the ICERs of the RV vaccines through the NIP at varied vaccine prices in the absence and presence of herd immunity in the model. In the base case without herd immunity, the break-even price for Rotateq is US$ 17.0 per dose, a reduction of 60.6% from the current price. The break-even price is US$ 25.3 per dose for Rotarix (a 59.4% reduction) and US$ 1.8 for LLR (a 93.2% reduction). When herd immunity is included in the model, the break-even price is US$ 17.5 per dose for Rotateq (a 59.4% reduction), US$ 26.0 per dose for Rotarix (a 58.3% reduction) and 1.3 US$ for LLR (a 95.1% reduction). Moreover, the threshold analysis showed that Rotarix at 59.7 US$ per dose (a 4.2% reduction) would be as cost-effective as Rotateq with the current market price in both the base case scenario and the scenario that included the herd immunity.
Discussion
To reduce the substantial RV disease burden, countries around the world, particularly in the western Pacific and southeast Asian regions, have advanced progress in evaluating rotavirus vaccines and introducing them into the NIPs.Citation10,Citation12 In China, the detection rate of rotavirus was 22.9% among outpatient diarrhea, and 39.7% among inpatient diarrhea.Citation74,Citation75 It was reported that the current prevalence characteristic of RV in China is similar to that of other countries before the introduction of RV vaccines.Citation74 This study presents the comprehensive cost-effectiveness of using three potential RV vaccines in the NIPs, integrating the latest local data available in China and accounting for the impact of private market provision and herd immunity. The inclusion of RV vaccines in the NIP could substantially reduce the disease burden of RV diarrhea in children under 5 years of age in China compared to the current situation that results in 5.57 million RVGE cases and 5855 deaths in the birth cohort. For example, Rotateq through the NIP could avert 62.6% of the total cases and 72.6% of the deaths, with the ICERs of US$ 8833 per QALY gained. The national vaccination program, especially using Rotateq or Rotarix showed to be cost-effective compared with the national GDP per capita. Moreover, the reduction of vaccine prices and the incorporation of herd immunity have further improved the cost-effectiveness, especially with Rotateq or Rotarix.
The model parameter, data sources, and assumptions may have a significant impact on the results of the economic evaluation. To estimate the disease burden, we have collected the most updated, representative, and local data from multiple sources. The all-cause mortality rate, the proportion of deaths due to diarrhea, and RV detection rate among community and hospitalized diarrhea were obtained from UN-IGME, MCHS, and the systematic review and meta-analysis in China, respectively, to promote national representativeness.Citation32–34–Citation75 In the absence of national surveillance, we obtained the incidence of all-cause diarrhea in 2019 from the GBD.Citation31 Based on global data from the scientific literature, population-representative surveys, and health care use records, the GBD adopted a Bayesian, hierarchical meta-regression tool as well as space-time information and covariates to produce modeled estimates, which have been used in study such as the global economic burden of infectious diseases and China’s vaccine economic evaluation.Citation3,Citation62,Citation76,Citation77 Besides, that the estimates from the GBD 2019 (0.96, 0.73–1.23 per child) was comparable with that (1.22, 0.53–1.97 per child) from a study that systematically reviewed China’s studies before 2012, considering the declined tread of diarrhea morbidity in China.Citation31,Citation78 Moreover, our sensitivity analysis showed that the incidence of RVGE was not the major sensitive parameter that impacted the study conclusion, especially for Rotateq and Rotarix, suggesting it is an acceptable model input. To obtain the ratio of outpatient visits to hospitalization, we have reviewed the studies on rotavirus disease burden and epidemiology in China (Supplementary Materials 2). Among the 4 papers found, a similar ratio of about 10 was reported from regions of different socio-economic levels and tiers of hospitals.Citation79–81 We chose the population-based surveillance by Zhang et al., as it reflected the most updated rotavirus epidemiology and healthcare utilization in regions with higher socio-economic (Beijing) and lower socio-economic levels (Gansu) and included the largest number of hospitals to date to ensure the representativeness.Citation35 Also, the sensitivity analyses show that the ratio of outpatient visits vs. hospitalization was not the major sensitive parameter for the three RV vaccines, suggesting it is a consistent and acceptable model input.
Similarly, we have conducted a review to collect the cost of illness for rotavirus diarrhea among children under 5 in China (Supplementary Materials 3). Among the 11 studies found, 6 were conducted after 2010, and most of the 6 studies were based on the surveillance in Beijing and Gansu Province.Citation78–80–Citation82–88 We obtained the cost data based on the population-based surveillance by Zhang et al., the updated and the only study that covered the regions of different socio-economic levels.Citation78–80–Citation82–88 Compared with China’s previous studies, the cost reported by Zhang et al., such as that of the hospitalization, was in the cost range among other studies conducted in rural or urban settings.Citation78–80–Citation82–88 It was also comparable with the cost estimates of all-cause diarrhea in China modeled by Baral et.al. in 2015 (Etc., the total cost of outpatient visit: 144.9 vs. 112.1; total cost of hospitalization: 372.3 vs. 453.9).Citation89 Moreover, we have made adjustment, extrapolating the local data to the nation. The direct medical costs were adjusted using health care expenditures by region, and direct non-medical costs were adjusted using the consumer price index (CPI).Citation60,Citation61 The sensitivity analysis also showed that the costs of health care did not significantly impacting our conclusion.
As the utilities may vary among countries and regions with different socio-economic levels, disease epidemiology, healthcare system, and health service utilization pattern, our study obtained QALY weights from the only study in the developing country, Thailand.Citation45 First, the RV epidemiology is similar in China and Thailand, with a relatively high morbidity and low mortality rate.Citation35,Citation75,Citation90 For example, the hospitalization rate of RVGE was estimated about 11.3 per 1000 children in Thailand and 14.4/1000 in China, and G9P[8] and G3P[8] are the most dominant RV genotype in Thailand (47.3%) and China (40.4%).Citation35,Citation75,Citation90 Second, with the universal health coverage and public hospital system, both countries have promoted health service accessibility, especially primary healthcare, enabling individuals to seek care at nearby health units.Citation91,Citation92 Last, surveys reported similar care-seeking behaviors of parents for their children with diarrhea, with a care-seeking rate of about 60% in Thailand and 58–74% in China.Citation37–Citation39-40–Citation46 In the absence of local data, QALY weights based on the study in Thailand provided a reasonable estimation.
In addition, the impact of vaccination schedules of different RV vaccines on the immunization delivery cost needed to be clarified. In our study, the cost of routine immunization service per dose was used as the proxy for the three RV vaccines’ immunization delivery costs per dose.Citation66 As recommended by WHO, we assumed Rotateq or Rotarix to be delivered in conjunction with the Diphtheria, Tetanus, and Pertussis (DTP) vaccine.Citation2,Citation9 For LLR that would be given annually for three doses, the first, second, and third dose could by delivered in conjunction with the DTP vaccine, Japanese encephalitis vaccine, and meningococcal vaccine, respectively, based on China’s national immunization program schedule.Citation93 WHO position paper has addressed that simultaneous administration of RV vaccines with these vaccines included in the NIP has not been shown to interfere with the protective immune responses or safety profiles of the respective vaccines.Citation2,Citation9 So, the RV vaccination would not require additional visits besides the current routine visits and would not result in a significantly higher immunization delivery cost. Each RV vaccine’s total immunization delivery cost in the NIP depends on the number of doses required.
Consistent with the previous studies in China, which all conducted before 2016, this study suggests the potential cost-effectiveness of the national RV vaccination program, especially using Rotateq or Rotarix.Citation23–26 When comparing results within studies, various aspects of factors should be noted, including the input parameters, model settings and threshold for interpreting evaluations.,Citation29,Citation94,Citation95 Previous studies have assumed the vaccine prices, one of the most important model parameters, were at a low level (US$1.19 to US$30) compared to the current private market prices.Citation23–26 As for the model settings, previous studies assumed zero vaccine coverage in the status quo.Citation23–26 However, LLR has been approved for use for twenty years, and the recent investigation after 2018 has reported a first-dose coverage of 32.8% in six provinces, making the private market provision an non-neglectable scenario for comparison.Citation20 In addition, our model further considered the significant protection by natural immunity against sequential RV infections, which has been demonstrated by epidemiology studies with birth cohorts and integrated in recent economic evaluations.Citation28,Citation96,Citation97 These updates would therefore lead to a relatively conservative cost-effectiveness result in our study compared to previous studies. Researchers have suggested tailored thresholds for economic evaluations to reflect the opportunity cost of health interventions in different settings of countries.Citation70,Citation71 For example, Thailand has adopted a threshold of 1.2 times GDP per capita for economic evolution.Citation98 In our study, Rotateq or Rotarix through the NIP would be cost-effective at a threshold of GDP per capita but not cost-effective at a more stringent threshold advised by Woods et al.Citation69 This suggests that though having significant potential, the inclusion of RV vaccines into the NIP may not reach a clear decision at this time point if other vaccination programs or health interventions, such as sanitation, were to be considered by the policymakers under a limited budget.Citation70 More measures are needed to promote the RV vaccination, such as adjusting the vaccine price. The systematic review of RV vaccines showed that the mass vaccination programs tended to be cost-saving or cost-effective in low-and-middle income countries but not in high-income countries, which were mainly due to the high vaccine prices or low to no subsidies in the high-income settings.Citation94 Similarly, vaccine prices were also identified as one of the most sensitive parameters in our one-way analyses. The landscape of vaccines in China has highlighted that the contract prices for vaccines in the private market are often higher than prices for comparable vaccines in the US, Europe, and UNICEF.Citation19 Furthermore, the RV vaccines are among the costly vaccines ($26- 43/dose) in the Chinese private market, approximately ten times the price in European countries, GAVI Vaccine Alliance-eligible countries, and UNICEF ($0.8–5.1/dose).Citation19,Citation64,Citation99 The LLR vaccine is reported to be used mainly in areas with good economic conditions, with extremely limited use in poor areas.Citation100 Given the limited budget constraints on investment in vaccination and the relatively low contract prices of vaccines in the NIPs ($0.1–5.7), a reduction in future contract prices for vaccines may be needed to include RV vaccine in the NIP.Citation19 The practice is possible, as our scenario analyses demonstrated that the ICERs values for Rotateq and Rotarix would be halved at a 25% reduction in price, approximating the upper bound of the cost-effective threshold range by Woods et al.Citation69 The break-even prices, which would help achieve significant public health impact, would be approximately 40% of the current prices. Manufacturers are also likely to benefit through large demands in economies of scale, with a dramatic increase in vaccine coverage from around 20% to over 90%.Citation101
In addition to the direct protection of the vaccine on individuals receiving vaccination, the indirect effects, including herd immunity, were indicated to play a role in the population-level impact of vaccination.Citation102,Citation103 Recentstudies have attempted to incorporate the herd immunity into the economic evaluations, such as those in the United States, Canada, Japan, and the Netherlands, and the methods have developed from assuming an increase in vaccine effectiveness to generating relational expressions of coverage rate and effectiveness including herd immunity for the static model, or adopting dynamic models.Citation104,Citation105 Considering the lack of epidemiological data on rotavirus in China, the relational expressions informed by empirical studies in other countries, including low- and middle-income countries, were adopted in our scenario analyses.Citation73 Including herd immunity further improves the cost-effectiveness of Rotateq or Rotarix in comparison with the private market provision with LLR. This is mainly due to the higher efficacies of the two imported vaccines (58.1–78.9%) compared to the domestic LLR (34.9–39%), leading to a larger increment in vaccine effective coverage (VEC) as indicated by the relational expressions.Citation27,Citation51,Citation54,Citation55 However, the indirect effect would be less significant when the vaccine coverage reaches over 90% in the mass vaccination scenario instead of at the range between 20–60% in the status quo, making LLR through the NIP less cost-effective compared to the private market provision after including herd immunity in the analyses.
Since 2009, China has been strengthening the surveillance networks for viral diarrhea, including sentinel surveillance and population-based surveillance to provide epidemiological evidence.Citation106,Citation107 Regarding the unclear impact of the domestic LLR vaccine, which due to both the lack of RCT and the complicated and delayed schedule for administrating compared with that recommend by the WHO, a novel improved vaccine, the oral human-lamb reassortant trivalent (G2-G3-G4) vaccine has been developed recently.Citation108 Moreover, the national expert advisory committee has been established in 2017 to inform evidence-based decision-making and adjustment.110 All of these practices would help to promote progress in considering the inclusion of RV vaccines into the NIP. This study highlights the significant public health impact and cost-effectiveness of incorporating RV vaccines in the NIP in China, especially those with better vaccine characteristics (e.g., efficacy) and low prices.
Our study has several limitations. First, the efficacy of domestic LLR vaccine and duration of protection at each dose were estimated from real-world studies on its effectiveness. Second, due to the lack of reports on RV epidemiology in China, the way in which the impact of the herd immunity effect was included in the model was based on the estimation from empirical studies in other countries. The herd immunity may have a relatively different impact in China, as the vaccine coverage in the private market may vary across regions and population groups at different socio-economic levels. Third, some of the model inputs, including the ratio of outpatients visit vs. hospitalization and cost of health care, were based on regional surveillance and surveys, which may hinder the representativeness. Last, the current projection was developed based on the latest data from the ‘pre-COVID-19’ era, while the COVID-19 pandemic may have influenced the transmission and prevention of enteric pathogen infections, as well as the cost-effectiveness of the vaccination. With future updates on local data, studies are needed to better evaluate the impact of vaccination by considering the dynamic transmission of the RV, quantifying its impact on health inequality, and comparing the vaccination programs with other sectors such as water and sanitation investment.
Conclusion
The national rotavirus vaccination program in China is expected to be cost-effective in comparison with the current provision by private market, reducing substantial disease and economic burden among children under 5 years of age. Reducing vaccine prices, adjusting the current NIP schedule to include rotavirus vaccination, and adopting or developing the vaccine with better characteristics, such as Rotateq or Rotarix, would be the focus of future efforts to promote the introduction of rotavirus vaccine.
Supplementary Material
Download MS Word (32.1 KB)Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2090162
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. The immunological basis for immunization series: module 21: rotavirus vaccines. Geneva: World Health Organization; 2020.
- World Health Organization. Rotavirus vaccines: WHO position paper—January 2013. Weekly Epidemiological Rec. 2013;88(5):1–13.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958. doi:10.1001/jamapediatrics.2018.1960.
- World Health Organization. Rotavirus. [accessed 2022 Feb 10]. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/rotavirus.
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–572. doi:10.3201/eid0905.020562.
- Clark A, Black R, Tate J, Roose A, Kotloff K, Lam D, Blackwelder W, Parashar U, Lanata C, Kang G, et al. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. Plos One. 2017;12(9):e0183392. doi:10.1371/journal.pone.0183392.
- Parashar UD, Cortese MM, Offit PA. 52 - Rotavirus Vaccines. In: Plotkin S, Orenstein W, Offit P, Edwards K, editors. Plotkin’s Vaccines. 7th ed. Philadelphia, PA:Elsevier; 2018. p. 950–969.e11.
- Soares Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019;(10). doi:10.1002/14651858.CD008521.pub4.
- World Health Organization. Rotavirus vaccines: WHO position paper—January 2021. Weekly Epidemiological Rec. 2019;96(28): 301-219.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School. VIEW-Hub. [accessed 2022 Feb 10]. www.view-hub.org.
- Parashar UD, Johnson H, Steele AD, Tate JE. Health impact of rotavirus vaccination in developing countries: progress and way forward. Clin Infect Dis. 2016;62(Suppl 2):S91–5. doi:10.1093/cid/civ1015.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey J, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the global rotavirus surveillance network. Lancet Glob Health. 2019;7(7):e893–e903. doi:10.1016/S2214-109X(19)30207-4.
- Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097–2107. doi:10.1016/j.vaccine.2015.03.016.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- Jin H, Wang B, Fang Z, Duan Z, Gao Q, Liu N, Zhang L, Qian Y, Gong S, Zhu Q, et al. Hospital-Based study of the economic burden associated with rotavirus diarrhea in eastern China. Vaccine. 2011;29(44):7801–7806. doi:10.1016/j.vaccine.2011.07.104.
- Kawai K, O’-Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30(7):1244–1254. doi:10.1016/j.vaccine.2011.12.092.
- Kirkwood CD, Steele AD. Rotavirus vaccines in China. JAMA Network Open. 2018;1(4):e181579. doi:10.1001/jamanetworkopen.2018.1579.
- Yangtze River Delta Immunization Integration Working Group, Chinese Medical Association Infectious Diseases Branch Children′s Infection and Hepatology Group Expert consensus on the prevention, diagnosis and treatment of rotavirus gastroenteritis (RVGE) in children (2020). Chin J Prev Med. 2020;54(4):392-405. (in Chinese).
- Zheng Y, Rodewald L, Yang J, Qin Y, Pang M, Feng L, Yu H. The landscape of vaccines in China: history, classification, supply, and price. BMC Infect Dis. 2018;18(1). doi:10.1186/s12879-018-3422-0.
- Liu Y, Yue C, Li Y, Wang Y, Gao S, Wang Z, Xie X, Zhao H, Wang D, Liang X, et al. Analysis of vaccination situation of orial live attenuated rotavirus vaccine (LLR strain) among children in 6 provinces of China. Chin J Prev Med. 2018;52(3):282–286. (in Chinese). doi:10.4103/0366-6999.223851.
- Aldemir-Kocabaş B, Karbuz A, Özdemir H, Tural-Kara T, Tapısız A, Belet N, Güriz H, Çiftçi E, İ̇nce E. Complications with rotavirus: a single center experiences. Turk J Pediatr. 2016;58(6):602–608. doi:10.24953/turkjped.2016.06.005.
- Meloni A, Locci D, Frau G, Masia G, Nurchi AM, Coppola RC. Epidemiology and prevention of rotavirus infection: an underestimated issue? J Matern Fetal Neonatal Med. 2011;24(Suppl 2):48–51. doi:10.3109/14767058.2011.601920.
- Sun S, Gao Y, Yin J, Zhuang G. A cost-effectiveness analysis on universal infant rotavirus vaccination strategy in China. Chin J Epidemiol. 2016;37(2):238–242. (in Chinese). doi:10.3760/cma.j.issn.0254-6450.2016.02.018.
- Liu N, Yen C, Fang Z, Tate JE, Jiang B, Parashar UD, Zeng G, Duan Z-J. Projected health impact and cost-effectiveness of rotavirus vaccination among children <5 years of age in China. Vaccine. 2012;30(48):6940–6945. doi:10.1016/j.vaccine.2012.05.084.
- Wang XY, Riewpaiboon A, von Seidlein L, Chen XB, Kilgore PE, Ma JC, Qi S-X, Zhang Z-Y, Hao Z-Y, Chen J-C, et al. Potential cost-effectiveness of a rotavirus immunization program in rural China. Clin Infect Dis. 2009;49(8):1202–1210. doi:10.1086/605632.
- Cui S, Tobe RG, Mo X, Liu X, Xu L, Li S. Cost-Effectiveness analysis of rotavirus vaccination in China: projected possibility of scale-up from the current domestic option. BMC Infect Dis. 2016;16(1):677. doi:10.1186/s12879-016-2013-1.
- Li R, Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, Mo Z-J, Liao X-Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life. Hum Vaccines Immunother. 2014;10(1):11–18. doi:10.4161/hv.26319.
- Velázquez FR. Protective effects of natural rotavirus infection. Pediatr Infect Dis J. 2009;28(3):S54–S56. doi:10.1097/INF.0b013e3181967c03.
- World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes. 2nd ed. Geneva: World Health Organization; 2019.
- The World Bank. Inflation, consumer prices (annual %) - China | Data. [accessed 2022 Feb 11]. https://www.vacmic.com/htm/20163/13_984.htm.
- Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME); 2020. [accessed 2022 Feb 10]. http://ghdx.healthdata.org/gbd-results-tool.
- UN Inter-agency Group for Child Mortality Estimation. [accessed 2022 Feb 10]. https://childmortality.org/data/.
- Liu Z, Liu X, He C, Miao L, Kang L, Li X, Zhu J, Li Q, Huang Y, Wang YP, et al. [Analysis of mortality and leading causes of death in Chinese children under 5-year-old between 2010 and 2016]. Chin J Prev Med. 2019;53(4):411–414. (in Chinese). doi:10.3760/cma.j.issn.0253-9624.2019.04.016.
- Liu N, Xu Z, Li D, Zhang Q, Wang H, Duan Z. Update on the disease burden and circulating strains of rotavirus in China: a systematic review and meta-analysis. Vaccine. 2014;32(35):4369–4375. doi:10.1016/j.vaccine.2014.06.018.
- Zhang J, Liu H, Jia L, Payne DC, Hall AJ, Xu Z, Gao Z, Chang Z, Jiang B, Parashar UD, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing Municipality and Gansu Province, China. Pediatr Infect Dis J. 2015;34(1):40–46. doi:10.1097/INF.0000000000000505.
- Wang J, Zhou H, Mo Z, Wang S, Hao Z, Li Y, Zhen S-S, Zhang C-J, Zhang X-J, Ma J-C, et al. Burden of viral gastroenteritis in children living in rural China: population-based surveillance. Int J Infect Dis. 2019;90:151–160. doi:10.1016/j.ijid.2019.10.029.
- Lin Y, Wang J, Fu X, Wang X, Shen Z, Jiang W, Xu R, Shen G. Investigation on the burden of diarrhoeal disease among community population in Jiaxing city. Chin J Public Health Manage. 2013;29(2):158–160. (in Chinese).
- Zhao S, Luo K, Hu S, Deng Z, Ouyang Y, Zhang X, Long Q, Yao Z, Gao L, Li J. Epidemiological characteristics of diarrhea and behaviors of seeking medical advice in rural residents in Hunan. China Trop Med. 2018;18(1):41–44. (in Chinese).
- Yang Z Disease burden of acute gastroenteritis during autumn and winter among children under 5 years old in Henan Suixian [ Master Thesis]. Beijing, China: Chinese Center for Disease Control and Prevention; 2017. (in Chinese)
- Yu J Research on Etiological spectrum and incidence estimation of diarrhoeal diseases [ Doctor Thesis]. Beijing, China: Chinese Center for Disease Control and Prevention; 2015. (in Chinese)
- Brisson M, Senecal M, Drolet M, Mansi JA. Health-Related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. Pediatr Infect Dis J. 2010;29(1):73–75. doi:10.1097/INF.0b013e3181b41506.
- Martin A, Cottrell S, Standaert B. Estimating utility scores in young children with acute rotavirus gastroenteritis in the UK. J Med Econ. 2008;11(3):471–484. doi:10.3111/13696990802321047.
- Marlow R, Finn A, Trotter C. Quality of life impacts from rotavirus gastroenteritis on children and their families in the UK. Vaccine. 2015;33(39):5212–5216. doi:10.1016/j.vaccine.2015.07.012.
- Hoffmann T, Iturriza M, Faaborg-Andersen J, Kraaer C, Nielsen CP, Gray J, Hogh B. Prospective study of the burden of rotavirus gastroenteritis in Danish children and their families. Eur J Pediatr. 2011;170(12):1535–1539. doi:10.1007/s00431-011-1465-y.
- Rochanathimoke O, Riewpaiboon A, Postma MJ, Thinyounyong W, Thavorncharoensap M. Health related quality of life impact from rotavirus diarrhea on children and their family caregivers in Thailand. Expert Rev Pharmacoecon Outcomes Res. 2018;18(2):215–222. doi:10.1080/14737167.2018.1386561.
- Maneekarn N, Khamrin P. Rotavirus associated gastroenteritis in Thailand. VirusDisease. 2014;25(2):201–207. doi:10.1007/s13337-014-0201-4.
- Zhang J, Duan Z, Payne DC, Yen C, Pan X, Chang Z, Liu N, Ye J, Ren X, Tate JE, et al. Rotavirus-Specific and overall diarrhea mortality in Chinese children younger than 5 years. Pediatr Infect Dis J. 2015;34(10):e233–e237. doi:10.1097/INF.0000000000000799.
- Lanzhou Institute of Biological Products Co. LTD. Lanzhou Lamb rotavirus vaccine. [accessed 2022 Feb 10]. https://www.vacmic.com/htm/20163/13_984.htm/.
- Lai X, Rong H, Ma X, Hou Z, Li S, Jing R, Zhang H, Peng Z, Feng L, Fang H, et al. Willingness to pay for seasonal influenza vaccination among children, chronic disease patients, and the elderly in China: a national cross-sectional survey. Vaccines. 2020;8(3):405. doi:10.3390/vaccines8030405.
- Cao L, Wang HQ, Zheng JS, Yuan P, Cao LS, Zhang GM. National immunization coverage survey in China after integrated more vaccines into EPI since 2008. Chin J Vaccines Immunization. 2012;18(5):419–424+478. (in Chinese).
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, Wei D, Fu B, Liao X, Chu J, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35(43):5897–5904. doi:10.1016/j.vaccine.2017.08.081.
- Burnett E, Parashar UD, Tate JE. Real-World effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Global Health. 2020;8(9):e1195–e1202. doi:10.1016/S2214-109X(20)30262-X.
- Pempa,, Luz A, Luangasanatip N, Kingkaew P, Adhikari D, Isaranuwatchai W, Choiphel D, Pecenka C, Debellut F. Economic evaluation of rotavirus vaccination in children of Bhutan. Vaccine. 2020;38(32):5049–5059. doi:10.1016/j.vaccine.2020.05.035.
- Zhen S, Li Y, Wang S, Zhang X, Hao Z, Chen Y, Wang D, Zhang Y-H, Zhang Z-Y, Ma J-C, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case–control design. Emerging Microbes Infections. 2015;4(1):1–6. doi:10.1038/emi.2015.64.
- Li J, Zhang Y, Yang Y, Liang Z, Tian Y, Liu B, Gao Z, Jia L, Chen L, Wang Q, et al. Effectiveness of Lanzhou lamb rotavirus vaccine in preventing gastroenteritis among children younger than 5 years of age. Vaccine. 2019;37(27):3611–3616. doi:10.1016/j.vaccine.2019.03.069.
- Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, Mølbak K, Sommerfelt H. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis. 2002;186(5):593–597. doi:10.1086/342294.
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DWG, et al. Protective effect of natural rotavirus infection in an Indian Birth Cohort. N Engl J Med. 2011;365(4):337–346. doi:10.1056/NEJMoa1006261.
- Zhang L, Li K, Du W, Li Y, Fan C, Yu W, Cao L, Cao L, Yin Z. Surveillance of adverse events following immunization in China, 2019. Chin J Vaccines Immunization. 2021;27(4):438–445. (in Chinese).
- Chai C Study on epidemic characteristics and disease burden of diarrhea [ Master Thesis]. Zhejiang, China: Zhejiang University; 2010. (in Chinese)
- National Bureau of Statistics of China. China statistics yearbook 2014. Beijing: China Statistics Press; 2014.
- Ministry of health of China. China health statistical yearbook 2014. Beijing: Peking Union Medical College Press; 2014.
- Zhang H, Garcia C, Yu W, Knoll MD, Lai X, Xu T, Jing R, Qin Y, Yin Z, Wahl B, et al. National and provincial impact and cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in China: a modeling analysis. BMC Med. 2021;19(1). doi:10.1186/s12916-021-02049-7.
- National Bureau of Statistics of China. Tabulation on the 2010 population census of the People’s Republic of China. [accessed 2022 Feb 11]. http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm.
- Zhuang J, Wagner AL, Laffoon M, Lu Y, Jiang Q. Procurement of category 2 vaccines in China. Vaccines (Basel). 2019;7(3):97. doi:10.3390/vaccines7030097.
- Vaccines for Children Program (VFC) | Current CDC Vaccine Price List | CDC. [accessed 2022 Feb 11]. https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
- Yu W, Lu M, Wang H, Rodewald L, Ji S, Ma C, Li Y, Zheng J, Song Y, Wang M, et al. Routine immunization services costs and financing in China, 2015. Vaccine. 2018;36(21):3041–3047. doi:10.1016/j.vaccine.2018.04.008.
- Su S, Wong WC, Zou Z, Cheng DD, Ong JJ, Chan P, Ji F, Yuen M-F, Zhuang G, Seto W-K, et al. Cost-Effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Global Health. 2022;10(2):e278–e287. doi:10.1016/S2214-109X(21)00517-9.
- Pan XF, Griffiths UK, Pennington M, Yu H, Jit M. Systematic review of economic evaluations of vaccination programs in mainland China: are they sufficient to inform decision making? Vaccine. 2015;33(46):6164–6172. doi:10.1016/j.vaccine.2015.09.081.
- Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi:10.1016/j.jval.2016.02.017.
- Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny M, Hill SR. Cost–effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930. doi:10.2471/BLT.15.164418.
- Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. doi:10.2471/BLT.14.138206.
- Department Of Population And Employment Statistics, National Bureau Of Statistics. China population and employment statistics yearbook 2020. Beijing: China Statistics Press; 2020.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, et al. Burden of streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e757. doi:10.1016/S2214-109X(18)30247-X.
- Geng Q, Lai S, Yu J, Zhang Z, Yang W, Li Z, Wu J, Yang W. Epidemiological characteristics of rotavirus caused diarrhea in children aged< 5 years in 26 provinces in China, 2011 to 2014. Dis Surveillance. 2016;31(6):463–470. (in Chinese).
- Li J, Wang H, Li D, Zhang Q, Liu N. Infection status and circulating strains of rotaviruses in Chinese children younger than 5-years old from 2011 to 2018: systematic review and meta-analysis. Hum Vaccin Immunother. 2021;17(6):1811–1817. doi:10.1080/21645515.2020.1849519.
- Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–941. doi:10.1016/S1473-3099(16)00146-8.
- Lai X, Wahl B, Yu W, Xu T, Zhang H, Garcia C, Qin Y, Guo Y, Yin Z, Knoll MD, et al. National, regional, and provincial disease burden attributed to streptococcus pneumoniae and Haemophilus influenzae type b in children in China: modelled estimates for 2010–17. Lancet Reg Health West Pac. 2022;22:100430. doi:10.1016/j.lanwpc.2022.100430.
- Cui P Study on disease burden of rotavirus diarrhea and epidemiologic characteristic of intussusception among children in China [ Master Thesis]. Beijing, China: Chinese Center for Disease Control and Prevention; 2016. (in Chinese)
- Fang Z, Zhang L, Tang J, Zhang Q, Hu H, Xie H, Zheng L. Study on rotavirus diarrhea among children in Lulong County, Hebei Province, China. Chin J Virol. 2005;21(1):21–26. (in Chinese).
- Tang J, Fang Z, Hu H, Ye Q, Gao F, Liu C, Wang Y, Zhao X. Study on disease burden and character of rotavirus diarrhea among children in Lulong county of China. Chin J Child Health Care. 2007;15(2):179–180. (in Chinese).
- Lou J, Xu X, Wu Y, Tao R, Tong M. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol. 2011;50(1):84–87. doi:10.1016/j.jcv.2010.10.003.
- Zhang L Rotavirus Surveillance and Molecular Epidemiology among children with acute diarrhea in six regions of China [ Doctoral Thesis]. Beijing, China: Chinese Center for Disease Control and Prevention; 2005. (in Chinese)
- Wu S, Wang B, Jin H, Wang N. Surveillance and estimates of disease burden on group a rotavirus gastroenteritis. J Southeast Univ (Med Sci Ed). 2003;22(2):80–83. (in Chinese).
- Xiong L, Lin S, Li H. Study on the incidence and economic burden of rotavirus diarrhea in Longgang district of Shenzhen. Mod Prev Med. 2010;37(12):2332–2333. (in Chinese).
- Li J, Cui P, Tao Y, Jin Y, Liu N. Economic burden of rotavirus diarrhea in children under 5 years old in Nanjing. Int J Virol . 2019;26(4):229–232. (in Chinese).
- Liu H, Study on disease burden of rotavirus diarrhea among children under five years old in Beijing and Gansu [ Master Thesis]. Beijing, China: Chinese Center for Disease Control and Prevention; 2013. (in Chinese)
- Jia L, Liu X, Li H, Liang Y, Gao Z, Wang Q. Study on the economic burden of rotavirus diarrhea among children under 5 years old in Beijing. Chin J Vaccines Immunization. 2015;21(5):543–546+551. (in Chinese).
- Liu H, Liu X, Liu D, Zhang J, Meng L. Analysis on economic burden evaluation and epidemiology of rotavirus diarrhea among children under five years old. Chin Primary Health Care. 2014;28(6):90–92. (in Chinese).
- Baral R, Nonvignon J, Debellut F, Agyemang SA, Clark A, Pecenka C. Cost of illness for childhood diarrhea in low- and middle-income countries: a systematic review of evidence and modelled estimates. BMC Public Health. 2020;20(1). doi:10.1186/s12889-020-08595-8.
- Jampanil N, Kumthip K, Yodmeeklin A, Kanai Y, Okitsu S, Kobayashi T, Ukarapol N, Ushijima H, Maneekarn N, Khamrin P, et al. Epidemiology and genetic diversity of group a rotavirus in pediatric patients with acute gastroenteritis in Thailand, 2018–2019. Infect Genet Evol. 2021;95:104898. doi:10.1016/j.meegid.2021.104898.
- Sumriddetchkajorn K, Shimazaki K, Ono T, Kusaba T, Sato K, Kobayashi N. Universal health coverage and primary care, Thailand. Bull World Health Organ. 2019;97(6):415–422. doi:10.2471/BLT.18.223693.
- Yip W, Fu H, Chen AT, Zhai T, Jian W, Xu R, Pan J, Hu M, Zhou Z, Chen Q, et al. 10 years of health-care reform in China: progress and gaps in universal health coverage. Lancet. 2019;394(10204):1192–1204. doi:10.1016/S0140-6736(19)32136-1.
- National Health Commission Of China. National immunization program schedule and instructions (2021). [accessed 2022 May 15]. cdcp.gd.gov.cn/attachment/0/416/416672/3437452.pdf.
- Haider S, Chaikledkaew U, Thavorncharoensap M, Youngkong S, Islam MA, Thakkinstian A. Systematic review and meta-analysis of cost-effectiveness of rotavirus vaccine in low-income and lower-middle-income countries. Open Forum Infect Dis. 2019;6(4). doi:10.1093/ofid/ofz117.
- Kotirum S, Vutipongsatorn N, Kongpakwattana K, Hutubessy R, Chaiyakunapruk N. Global economic evaluations of rotavirus vaccines: a systematic review. Vaccine. 2017;35(26):3364–3386. doi:10.1016/j.vaccine.2017.04.051.
- Rochanathimoke O, Riewpaiboon A, Praditsitthikorn N, Tharmaphornpilas P, Jiamsiri S, Thavorncharoensap M, Postma MJ. Economic evaluation of rotavirus vaccination: an important step of the introduction to the national immunization program in Thailand. Expert Rev of Pharmacoecon Outcomes Res. 2021;21(4):811–819. doi:10.1080/14737167.2021.1932468.
- Saokaew S, Prasitsuebsai W, Bibera GL, Kengkla K, Zhang X, Oh K, Lee C. Economic evaluation of human rotavirus vaccine in Thailand. Infect Dis Ther. 2019;8(3):397–415. doi:10.1007/s40121-019-0246-1.
- Rotavirus vaccine (RV) price data | UNICEF Supply Division. [accessed 2022 Feb 11]. https://www.unicef.org/supply/documents/rotavirus-vaccine-rv-price-data.
- Fu C, Dong Z, Shen J, Yang Z, Liao Y, Hu W, Pei S, Shaman J. Rotavirus gastroenteritis infection among children vaccinated and unvaccinated with rotavirus vaccine in Southern China. JAMA Network Open. 2018;1(4):e181382. doi:10.1001/jamanetworkopen.2018.1382.
- Gavi, The Vaccine Alliance. Key concepts: economics of vaccine production. [accessed 2022 Feb 11]. https://www.who.int/immunization/programmes_systems/financing/analyses/en/briefcase_vacproduction.pdf
- Mast TC, Wang FT, Su S, Seeger JD. Evidence of herd immunity and sustained impact of rotavirus vaccination on the reduction of rotavirus-related medical encounters among infants from 2006 through 2011 in the United States. Pediatr Infect Dis J. 2015;34(6):615–620. doi:10.1097/INF.0000000000000702.
- Bennett A, Bar-Zeev N, Cunliffe NA. Measuring indirect effects of rotavirus vaccine in low income countries. Vaccine. 2016;34(37):4351–4353. doi:10.1016/j.vaccine.2016.07.001.
- Kurosawa T, Watanabe H, Takahashi K. Cost-utility analysis of rotavirus vaccines including the latest evidence and data as of June 2020 in Japan. Pediatr Infect Dis J. 2021;40(2):162–168. doi:10.1097/INF.0000000000002938.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine. 2017;35(49 Pt B):6828–6841. doi:10.1016/j.vaccine.2017.10.024.
- Yu J, Lai S, Geng Q, Ye C, Zhang Z, Zheng Y, Wang L, Duan Z, Zhang J, Wu S, et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009–2015. J Infect. 2019;78(1):66–74. doi:10.1016/j.jinf.2018.07.004.
- Wang L, Zhou S, Wang X, Lu Q, Shi L, Ren X, et al. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1);2464.
- Xia S, Du J, Su J, Liu Y, Huang L, Yu Q, Xie Z, Gao J, Xu B, Gao X, et al. Efficacy, immunogenicity and safety of a trivalent live human-lamb reassortant rotavirus vaccine (LLR3) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2020;38(46):7393–7400. doi:10.1016/j.vaccine.2020.04.038.
- The high-level seminar on evidence-based decision-making of national immunization Program was successfully held in Beijing. [accessed 2022 Feb 11]. https://www.chinacdc.cn/yw_9324/201712/t20171220_156991.html.