ABSTRACT
In light of their quick development and low risk, mRNA vaccines are gradually replacing traditional vaccines. In order to characterize the patent landscape of mRNA vaccines, this study collated mRNA vaccine–related applications that have been registered since 1962. Accordingly, the 1852 patent families were discussed in relation to their temporal distribution, geographic scope, organizational assignees, and co–patenting activities. mRNA vaccines were shown to demonstrate promise in infectious disease, cancer immunotherapy, and allergic disease, with a focus on lipid nanoparticles. Notably, these vaccines are being developed against a backdrop of fierce industrial competition and intensive collaboration with a rise in applications. The findings of this study highlighted cutting–edge inventions, key players, and collaboration dynamics among institutions. By understanding the landscape of mRNA vaccine patents, researchers and those in industry may better comprehend the latest trends in this area, which may also assist relevant decision–making by academics, government officials, and industrial leaders.
1. Introduction
In the late 18th century, Edward Jenner developed the world’s first smallpox vaccine, marking the beginning of the fight against viruses using vaccines.Citation1 The emergence of vaccines has resulted in profound improvements in human health. However, as vaccine science developed, traditional vaccines have demonstrated that they are unable to fully meet certain human requirements. Specifically, vaccines that can be produced at speed and scale, are affordable, and are capable of efficiently dealing with complex and mutating viruses are needed, which resulted in the advent of messenger RNA (mRNA) vaccines. Instead of injecting antigens into the body, this new generation of vaccines utilizes injected mRNA in order to help produce antigens in the body. mRNA vaccines can greatly reduce associated production costs and production cycles, thus steering vaccinology in a new direction.Citation2
In 1990, Wolff et al. demonstrated that proteins can be produced in living tissues from mRNA transcribed in vitro. Retrospectively, this finding marked the initial step in synthesizing a vaccine from mRNA.Citation3 In the following decades, mRNA vaccines entered a period of rapid development. Following the outbreak of COVID-19 in 2020, and by leveraging significant technological innovation and progress of the mRNA vaccine platform over the last decade, COVID-19 mRNA vaccines were successfully developed at unprecedented speeds and were approved for emergency use in many countries. Moreover, the development of COVID-19 mRNA vaccines continues to be in full swing internationally. Pfizer–BioNTech’s BNT162b2 mRNA COVID-19 vaccine received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) on 11 December 2020, becoming the first approved mRNA COVID-19 vaccine. A week later, Moderna’s mRNA-1273 vaccine was also granted EUA by the FDA. The two vaccines were officially approved by the FDA on 23 August 2021 and 31 January 2022, respectively.Citation4 More recently, on 31 January 2022, two professors at the Massachusetts Institute of Technology developed a method to deliver mRNA vaccines orally.Citation5
Since the onset of the COVID-19 pandemic, rapid mRNA vaccine development had the benefits and practicability of mRNA delivery as its vaccination strategy, fostering new hope for vaccine development for a variety of other diseases. For instance, at the end of January 2022, Moderna and the International AIDS Vaccine Initiative (IAVI) announced that the first mRNA AIDS vaccine was inoculated in humans as a trial. Meanwhile, mRNA vaccines for other diseases are also being studied.Citation6 Unlike other vaccine technologies, mRNA vaccines can be developed rapidly and have unique advantages. As additional findings from human clinical trials are brought to light, prospects of mRNA vaccines may be further realized. Therefore, mRNA vaccines will remain a vital technology in the future.
Technological advances have previously been shown to lead to new patents, which are usually granted for novel technological inventions. Patents are important as they provide a short but exclusive right to exercise full control over the technology and exclude others from its use. mRNA vaccine–related technologies have been discussed broadly in journal and preprint literature under the context of the pandemic; however, patents have received relatively little attention and analysis. As a useful source but stealth research of information, patents may be easily ignored. Unlike paper, which can be freely accessed worldwide, patents have better protection policies to prevent others from copying the corresponding technologies without the permission of the patent assignee. Accordingly, using patents to analyze mRNA vaccines can provide additional information to scientific literature.
Various researchers have recently investigated mRNA vaccine patents. Specifically, Cecilia et al.Citation7 summarized research pertaining to English–language patents for mRNA vaccines in the United States, Europe and Patent Cooperation Treaty between 2010 and 2020. Mario et al.Citation8 visualized scientific terms related to mRNA vaccines for COVID-19 by conducting a network analysis in 2021. In addition, Dan et al.Citation9 expounded on the collaborations and challenges in pioneer and key player companies who have focused on mRNA–based therapies in 2021, thus predicting their development trends. Furthermore, additional avenues of investigation are worthy to be explored; hence, this study seeks to provide a more detailed and in–depth analysis of mRNA vaccine patent research in order to describe the global mRNA vaccine patent landscape.
This study presents a comprehensive overview of the landscape of mRNA patents, which differs from existing literature due to its extended search strategy and integrated analysis from temporal, organizational, spatial and technical perspectives over the past six decades. Notably, the information derived from this study may support relevant decision–making and can serve as a reference, allowing key stakeholders to more intuitively discern and comprehend mRNA vaccine development while understanding its development trends. Thus, it is hypothesized that patents would demonstrate a tight technological relationship and strong technology flow.
2. Materials and methods
A series of search terms related to “mRNA vaccine” was used to search the titles, abstracts, and claims in the Derwent World Patents Index (DWPI) for patents with a publication date prior to 31 December 2021 using the Derwent Innovation platform (https://clarivate.com/products/derwent-innovation/). Detailed patent information based on the DWPI patent family was then collected, which was comprised of basic patents filed in the original country and subsequent equivalent patents on the same invention filed in other countries or offices. Once the corresponding information was obtained, it was analyzed in order to describe the patents’ time trends, filing countries, patent ownership, co–patents, technological categories, and citation networks and milestones. This data was also used to investigate the development dynamics of mRNA vaccines, the development of this technology in various countries, institutional cooperation, and patent citations. Preferred Reporting Items for Systematic Reviews and Meta–Analyses (PRISMA) was used as reference to simultaneously collect all patent documents worldwide and exclude any unrelated data. In addition, items were reported according to the Reporting Items for Patent Landscapes (RIPL) checklist (Supplementary Figure S1).Citation10,Citation11
This study adopted a network analysis perspective so as to analyze the patterns and dynamics of global knowledge flows in the technological domain under consideration. In economics and regional science, network analytical approaches have recently garnered increased interest in regard to the dynamics of global research, innovation networks and knowledge flows.Citation12,Citation13 Adopting a network perspective shifts the emphasis from individual patents to the citation relationships between them. In order to facilitate analysis on the citations between patents, a series of patent citation networks was constructed, in which nodes represented patents while directed edges and arrows denoted citation relationships and directions. Moreover, patents were clustered together to form an independent network component, in which nodes had relatively frequent internal connections. The structures and features within patent citation networks comprehensively depict the patterns of technology flows and evolution, embodying and reflecting the current development trend and future directions of development of the technology in question. This study also applied various descriptive approaches in order to illustrate patent indicators as well as the associations between them. For a more detailed explanation on methods, please refer to the methodology section in the supplementary files.
3. Results
3.1. Data overview
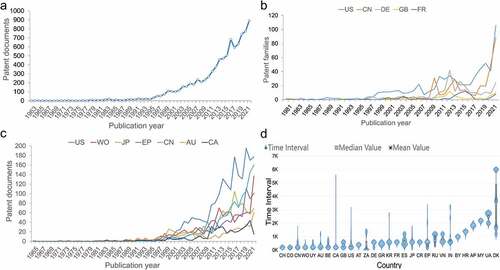
A total of 9,613 patent documents were collected to carry out the statistical analysis, in which percentages of invalid, under examination and valid cases were 40.35%, 16.88% and 42.77%, respectively. Expired or lapsed patents from failure to pay patent maintenance fees were found to account for 13.49% of invalid patents and 6.04% of all patents. Over a period of 60 years, mRNA vaccine patent publication exhibited a rising trend as a whole: it entered a take–off stage in 1994, increasing from 15 patents filed in 1994 to 892 filings in 2021 (). Notably, a substantial increase was present since 2019.
Figure 1. Publication trend of mRNA vaccine patents. (a) the number of mRNA vaccine patents invented by publication year since 1962. (b) mRNA vaccine patent families by publication year in the top five region of patent assignees. (c) mRNA vaccine patent documents by publication year in the top seven jurisdiction’s patent offices where patents were filed. The cumulative numbers involving regions and patent offices are shown in Supplementary Figures 2 and 3, respectively. (d) the time interval between the patent publication time and the application time (country/region code and name are shown in Supplementary Table S1).
3.2. Geographical characteristics
According to the analysis of the number of mRNA vaccine patents in the region where the assignee was located, the United States was shown to be the global leader with 688 patent families, followed by China (336) and Germany (232), which accounted for 72% of the global total. In terms of global distribution, North America, Europe, East Asia and other economically developed regions were regions that were mainly distributed, where the above three countries were located (Supplementary Figure S3). shows the relationship between the number of patent families in the top five countries for patent filings and the year in which they were published. The number of patent families in these five countries was shown to generally increase in the past before significantly increasing between 2019 and 2021.
When analyzing countries or organizations that own mRNA vaccine patents, the United States, China, and the World Intellectual Property Organization (WIPO) were found to have the largest number of patents with 2,121, 1,136, and 1,071 patents, respectively, which was followed by Japan, the European Patent Office (EPO), and Australia (Supplementary Figure S2). The relationship between the number of patent documents and their publication year in the top seven jurisdictions of mRNA vaccine patents is shown in . The general trend across all countries demonstrated a rise in the number of patent applications. This analysis further depicted that the United States has been the global leader, though it experienced a notable decline in 2015 and 2017. Meanwhile, Japan jumped to second place in 2016, while China achieved second in 2020 after a period of rapid growth. In addition, the United States, EPO, and China all saw sharp increases in the number of patents filed in 2019.
The distribution of the time interval between the publication date and application date of each country is shown in , which demonstrated that Switzerland had the smallest average time interval at 92 days, indicating the fastest average publication rate, followed by Colombia and China. Meanwhile, Denmark was shown to have an average filing time interval of 3,449 days, which was the slowest average publication rate among all countries. Furthermore, Canada’s maximum time interval differed from the minimum time interval by 5,261 days, the largest span among any country.
3.3. Patent assignees and collaboration
The institutional collaboration network of patent assignees was found to have 305 nodes (assignees) and 300 edges (cooperative relationship between the co–assignees) (). In this network, the node size represented the number of times the research center engaged in cooperation (the larger the nodes, the more times it has cooperated with others), while the thickness of the edges represented the intensity of cooperation. As evident in , the top three research centers based on the number of collaborations were found to be the French National Institute for Health and Medical Research (10 times), BioNTech AG (10 times) and GlaxoSmithKline PLC (9 times). Evidently, the intensity of cooperation between BioNTech AG and TRON Translational Oncology Mainz was shown to be the highest among all survey centers.
Figure 2. Collaboration networks of mRNA vaccine patents. (a) Network of cooperative relationships between institutions. The nodes represent the assignee, and the edges represent the cooperative relationship between the co–assignees. The institutional collaboration network does not include the individual assignee and labels names of top active institutions. (b) Regional collaboration network. Colored nodes denote regions in which assignees were located, including individual patent assignees, while edges correspond with collaborations based on co–assignee relations. Node size is scaled to the number of patent families (also given as a numerical value of the degree), while the thickness of edges represents collaboration frequency (also given as a numerical value of the weighted degree).
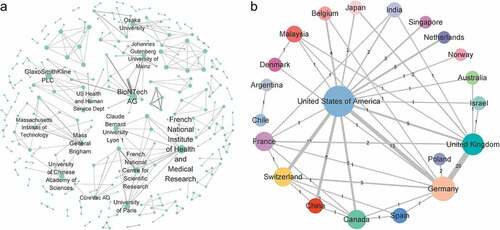
Based on the region of the assignee, the regional collaboration network was then calculated and plotted (). Clearly, the United States was shown to have an important role in international cooperation. Specifically, the United States engaged in collaborative studies with 85% of countries (i.e., international collaborations with centers from those countries) and participated in 56% of collaborative studies (64 times). In addition, Germany and the UK cooperated most frequently among all countries (20 times).
The research unit was then ranked with the most patents in order to identify the top 20 (). In this ranking, Sanofi SA was found to have the absolute lead (116 items), with its number of patent applications being twice that of second place (67 items), followed by CureVac AG, Moderna Therapeutics, BioNTech AG, and Enanta Pharmaceuticals.
Table 1. Ranking of top 20 patent assignees.
3.4. Technological characteristics
According to each patent’s filing time, a time heat map of International Patent Classification (IPC) codes was drawn (). In 2021, the most popular IPCs were found to be A61P003114 and A61K0039215, followed by A61K003900. A61P003114 represented anti–infectives used to treat RNA viruses, whereas the latter two were preparations containing antigens or antibodies to treat coronavirus.
Figure 3. Technological categories of mRNA vaccine patents. (a) the top 20 international patent classification (IPC) code since the 1980 annual active figure (detailed information about the IPC code are available at Supplementary table S2). (b) a landscape by ThemeScape using information included in title abstract and claims of the mRNA vaccine patents. The red dots represent the most recent patents issued between 2017 and 2021.
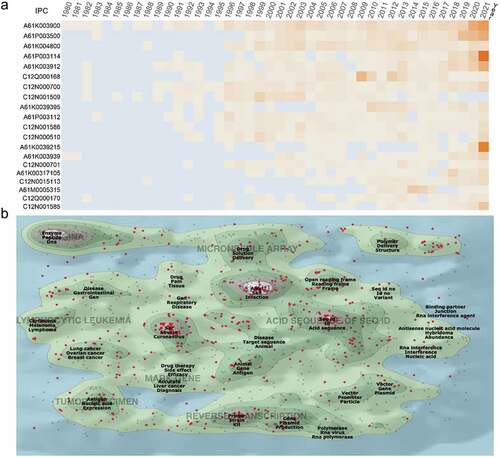
The patent landscape depicted fields of activity that were represented by peaks in the latest patent markers, including “Alkyl Salt Infection,” “Enzyme Peptide DNA,” and “Seq id Id Acid sequence” (). The most recent cluster of patents on the map, which were issued between 2017 and 2021, was found to be related to “severe acute respiratory coronavirus,” which is considered an emerging field.
3.5. Citation network and milestone patents
The global citation network consisted of 1767 nodes and 7097 edges (). The largest community (colored in blue) accounted for 13.7% of the nodes, while the second community (in magenta) accounted for 11.0% and the third community (in yellow) accounted for 9.3%. The blue patent community was found to possess many large nodes critical to the entire network, thus demonstrating the notable contribution of the patent cluster to the field.
Figure 4. Patent citation network of mRNA vaccines. (a) Global Citation network. Nodes represent patents and edges represent reference relationships. The node size is based on the connection out–degree (i.E., times of citation). (b) Time slice chart of the citation network. The patent was divided into 12 time slices according to the time when it was published, with one slice for 2010 and its predecessor, and one slice for each year from 2011 to 2021.
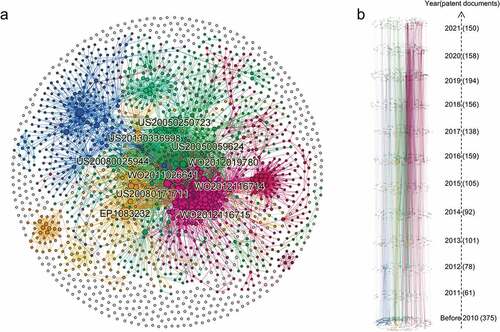
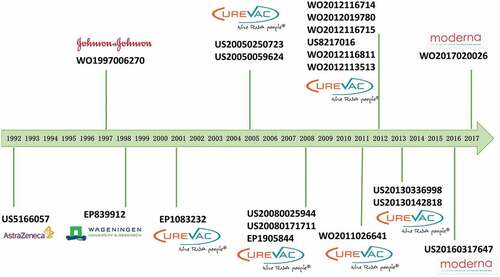
visualizes the time slice of the citation network coupled with the evolution process of clusters. By analyzing mRNA patent citations, half of the top 10 most–cited patents were shown to be filed in the United States, followed by four in WIPO and only one in EPO (). In terms of release time, most were concentrated around 2010 (), which was when major mRNA vaccine companies established or developed mRNA programs. The most cited patent in this field (EP1083232) put forward a method for transferring mRNA into cells and organisms. Meanwhile, the subsequent two entries in the list of most cited patents (US20080025944 and US20050250723) were found to be related to an immunostimulating agent comprising at least one chemically modified RNA. In particular, the latter also demonstrated that the immunostimulating agent and vaccine, according to the invention, were specifically employed against infectious diseases or cancers. Among the top 10 cited patents, patents US20050059624 and US20080171711 were mRNA mixtures for tumoral disease vaccination. Meanwhile, patents WO2012116714, WO2012116715 and US20130336998 were vaccines comprising mRNA encoding antigens for the prophylaxis and treatment of viral, bacterial or protozoological infectious diseases, autoimmune diseases, allergies or cancer (the former for the elderly and the latter two for newborns and infants). Patent WO2012019780 pertained to a new nucleic acid sequence used to increase the expression of proteins in treating cancers and allergies. Patent WO2011026641 referred to a novel polymeric carrier molecule used as a vaccine for treating tumors, viral, bacterial, protozoological infectious diseases, autoimmune diseases and also allergies or allergy–related diseases.
4. Discussion
4.1. Temporal implication
Since the discovery of mRNA in 1961, research on mRNA vaccines has been promoted in numerous countries.Citation14 Notably, very few patents for mRNA vaccines existed before 1989 due to the globally widespread use of traditional vaccines. In 1989, mRNA that was designed to be packaged in nanoliposomes was successfully transfected into cells for the first time.Citation15 The following year, “naked” mRNA was successfully injected into muscle cells in mice.Citation3,Citation16 These studies were the first to demonstrate that mRNA transcribed in vitro could transmit genetic information and be expressed in living tissue to produce specific proteins.Citation15,Citation16,Citation17 During this time, mRNA vaccines entered a new era of development, and the corresponding number of patents began to grow exponentially. The subsequent establishment of BioNTech in 2008 and Moderna in 2010 also accelerated the development of the industry. In 2012, the Defense Advanced Research Projects Agency (DARPA) commenced funding industry research into RNA vaccines and drugs, which led to further research into mRNA vaccines. Following the COVID-19 pandemic that began in the end of 2019, many countries are now competing to develop COVID-19 vaccines. mRNA vaccines stand out in this regard due to their safety, efficacy, and expedited research and development times. After Chinese scientists genetically sequenced the novel coronavirus in January 2020, many researchers began to invest in research and development of mRNA vaccines; this upsurge in R&D led to a substantial increase in the number of mRNA vaccine patents filed after 2019.Citation18
4.2. Geographical implication
By conducting an analysis on the country in which the patentee resides, most patent families were found to be distributed in North America, Europe and East Asia, which was highly correlated with levels of economic as well as scientific and technological development in this region. Among them, traditional developed countries, such as the United States and Germany, and economic powers such as China, were represented. Less developed regions such as Africa were found to have relatively few patent families (one in the whole of Africa). Here, patent R&D capability was shown to be closely related to national economic strength and scientific and technological capabilities (see Supplementary Figure S3). The United States ranked first in terms of the number of mRNA vaccine patents owned and with absolute superiority, demonstrating the advanced and innovative mRNA vaccine technologies developed in the United States. Following the onset of the COVID-19 pandemic, the number of patents in various jurisdictions has increased significantly, with China leaping into second place. This change reflects how China has given much attention to mRNA vaccines when studying the use of vaccines against the COVID-19 epidemic, attaining remarkable results.
Judging from the distribution of the interval between application and public time in various countries, although Switzerland was shown to possess a relatively small total number of patents, it was found to have the smallest average interval between the time the application was filed and when it was published, thus demonstrating the country’s efficiency and emphasis on patents. According to Article 63 of the Swiss Federal Patent Ordinance (FPO), applicants may request an expedited examination procedure when applying for a patent, thereby reducing the time in which the patent application is completed. In addition, the Swiss Federal Council’s proposed partial amendment to the Swiss Patent Act in October 2020 incorporated a public consultation process, whereby applicants would also have an option to apply for a utility patent that could be granted without a substantive examination, though it would only offer protection for ten years (as opposed to the current twenty years). This served as another measure that can increase the number of patent applications. Denmark was observed to hold a similar number of patents to Switzerland; however, the average interval between application and disclosure was nearly 38 times that of Switzerland, indicating that Denmark’s work efficiency was relatively low. Evidently, China was found to have the second–largest number of mRNA vaccine patents as well as the third shortest average time interval. Accordingly, China’s mRNA vaccine field exhibited a certain degree of advantage in quantity and work efficiency. This was most likely due to the “Administrative Measures for Patent Priority Examination,” which was officially implemented by China on 1 August 2017. This priority examination policy has streamlined China’s patent system and significantly shortened the period required for a patent application to be approved.
Countries have been known to cooperate closely on mRNA vaccine research. As the country with the largest number of patents, the United States was also shown to have the highest degree of cooperation with other countries. This demonstrates that whilst its own strengths in this field are important, effective cooperation is a valuable tool to promote patent research. Moreover, the United States has established successful international cooperative relations with 15 different countries, and the diversity of cooperation was shown to be more conducive to the invention of new patents. In addition, Germany and the UK were observed to cooperate most frequently among all countries, accounting for 61% of all international cooperation in the UK.
4.3. Organizational implication
In light of the cooperative network between patent assignees, in addition to patents obtained by a single assignee, the cooperative research of multiple assignees was found to commonly occur in mRNA vaccine patent applications, suggesting that intensive collaborative efforts are important to the generation of new technologies. BioNTech AG obtained 87% of its total patents by cooperating with other assignees, of which 54% came via collaboration with TRON Translational Oncology Mainz. Notably, 99% of the patents obtained by TRON Translational Oncology Mainz were obtained through cooperation, with its BioNTech AG cooperation rate reaching 100%. The cooperation between companies and schools to take advantage of the parties’ respective advantages was shown to serve as a common and very effective form of patent research cooperation. Furthermore, the French National Institute for Health and Medical Research, BioNTech AG, and GlaxoSmithKline PLC were found to be patent assignees with the largest number of partners. By working with a more diverse range of partners, they can also enjoy additional advantages.
Although cooperation between various institutions was shown to be widespread, certain disputes and fierce competition also existed. For instance, Moderna filed an Inter–party review (IPR) with the United States Patent and Trademark Office (USPTO) on three key patents (US8058069, US9364435, and US9404127) of Arbutus’ three drug delivery Lipid Nanoparticle (LNP) technologies. The Federal Circuit upheld the Patent Trial and Appeals Board’s (PTAB) decision to confirm the validity of patent US8058069. Meanwhile, some of patent US9364435’s claims were invalidated while others were valid, whereas the US9404127 patent decision was fully invalidated. Moderna also faces legal patent challenges from Arbutus Biopharma Corporation and Genevant Sciences in relation to its lipid technology. The lawsuit relates to nucleic acid–lipid particles and lipid vesicles, their compositions, and their methods of use. Additionally, American gene therapy company Alnylam recently filed a lawsuit against Pfizer and Moderna, alleging that the two companies infringed its US patent 11246933933 on the LNP mRNA delivery technology entitled “Biodegradable lipids for delivery of active agents” when developing the mRNA COVID-19 vaccine. Alnylam’s claim is for “equitable compensation for the use of its technology” at “no less than a reasonable royalty.” Canada’s Acuitas Therapeutics filed a lawsuit against Arbutus Biopharma in an attempt to block allegations that its vaccine infringes patents related to Arbutus’s COVID-19 vaccine mRNA delivery technology.
4.4. Technical implication
shows the number of mRNA patent documents published annually. Here, the figure released for 2021 was shown to be decreased compared to that of the previous year, which may be related to the announcement of U.S. support for a World Health Organization (WHO) proposal that would temporarily waive intellectual property rights for COVID-19 vaccines.Citation19 Katherine Tai, secretary of the U.S. Trade Department, said the government’s move was an effort to end the COVID-19 epidemic as quickly as possible through the provision of safe and effective vaccines.Citation20
Many indications were described in most cited patents, such as influenza (US20160317647, US5166057), viral, bacterial, or protozoological infectious diseases (WO1997006270, EP839912), allergy diseases (US20130142818, WO2012116811), genetic defects (EP1083232); autoimmune diseases (WO2011026641, US20130142818, US20080025944, WO2012116714, WO2012116715, US20130336998), and cancer or tumor diseases (US20050250723, US20050059624, US20080171711, EP1905844, US8217016, WO2017020026, WO2012019780). mRNA vaccines have become a promising platform for cancer immunotherapy, the treatment of allergic diseases, and autoimmunity.Citation7,Citation20,Citation21,Citation22,Citation23,Citation24
During mRNA vaccine development, LNP delivery systems serve as a key component in research. In the patent pool used in this study, a total of 115 patent families mentioned Lipid Nanoparticle, particularly patent US20160317647, which was frequently cited. The function of Lipid Nanoparticle is to deliver intact mRNA into the cell.Citation25 Due to the unstable, hydrophilic and negative charge characteristics of mRNA, it is difficult for mRNA to pass through the positively charged cell membrane to enter the cell interior as it will be rapidly degraded by the nuclease. As such, this issue became a central issue in mRNA vaccine R&D. The emergence of LNP effectively solved the above problems, however. This technology was invented by Pieter Cullis’ laboratory and several companies he founded or managed. LNP consists of four components: ionizable cationic phospholipids, cholesterol, helper phospholipids, and PEG–modified phospholipids.Citation26 More specifically, the core components are cationic phospholipids, which electrostatically bind to negatively charged mRNA and wrap it around in order to provide overall structural stability. However, they can also effectively bind to negatively charged cell membranes so as to release mRNA into the cell. At present, LNP has become one of the mainstream delivery carriers in the world due to its convenient production, readily adjustable formula, and low toxicity.Citation27,Citation28
4.5. Social implication
mRNA technologies help governments and industry stakeholders make informed decisions in order to reduce enormous economic losses due to crises. For example, the WHO listed mRNA vaccine Comirnaty for Emergency Use Listing (EUL), making the Pfizer/BioNTech vaccine the first to receive such an emergency validation in light of its low cost and high efficiency. Studies have shown that BNT162b2 (Pfizer–BioNTech) is 95% effective and mRNA-1273 (Moderna) is 94% effective against COVID-19.Citation29,Citation30,Citation31 The COVID-19 mRNA vaccine booster strategy implemented in Australia saved $1.05 billion in direct medical costs, which was both effective and cost–effective for the Omicron epidemic. The large–scale use of mRNA vaccines provides much needed headway for the already sluggish economy in Australia.Citation32 However, China’s financial hub was brought to a standstill after weeks into a COVID-19 outbreak in Shanghai. Bigger and longer lockdowns are incurring greater economic and human resource losses with the fast–spreading Omicron variant. As a result, governments who have primarily adopted Sinovac’s vaccines are considering mRNA vaccine boosters in response to the spread of Omicron.Citation33,Citation34,Citation35
Only 131 U.S. patents disclosed that their applicants or assignees were a major federal funding, including the National Institute of Allergy and Infectious Diseases (NIAID), Defense Advanced Research Projects Agency (DARPA), Operation Warp Speed (OWS), National Institutes of Health (NIH), Centers for Disease Control and the U.S. Government. This accounted for only 6.16% of the total number of U.S. mRNA vaccine patents.Citation36 In fact, vaccine–research programs have relied significantly on public investments in basic science and, at a later stage, on public support and supervision for clinical trials and regulatory approvals.Citation37,Citation38 Moderna (US20200282046, US10702600) was awarded a $1.525 billion contract by the Department of Defense and the Department of Health and Human Services to manufacture and deliver 100 million doses of its mRNA COVID-19 vaccine.Citation39 However, Moderna is at loggerheads with the NIH over the core patent application for an mRNA vaccine (mRNA-1273). Moderna only listed its own scientists on several of its patent applications (US2021228707, WO2021154763, WO2021159130, WO2021159040). The NIH scientists who worked closely with Moderna’s research and development center on the core sequence of mRNA vaccines were excluded. Moderna has been accused of insufficient disclosure as no patents or applications assigned to Moderna have disclosed United States federal government funding.Citation40 Although some U.S. patents were the products of federal funding and are thus subject to the Bayh–Dole Act, there was a lack of funding disclosure.
Furthermore, vaccines developed with substantial public contributions have been shown to generate hundreds of billions of dollars in sales for pharmaceutical companies, while the coronavirus continues to be rampant in poorer nations that cannot afford a vaccination program. Correcting such irregularities would require simultaneously rethinking the patent system as well as the role of the state. Accordingly, governments should more explicitly steer research and innovation, rather than merely co–funding and supervising it. In regard to infectious diseases, the government should have strong control over vaccines in order to ensure that manufacturers are unable to block production or access to raw materials and finished products worldwide. Moreover, doing so would also prevent companies from charging unaffordable prices while being insulated from competition.Citation19,Citation41
The original intention of the patent system is to support innovation to encourage more people to apply for patents.Citation42,Citation43 Without mRNA technology patented by pioneer scientists from University of Pennsylvania, this field would not have achieved such critical progress.Citation43 However, patent thickets may stand in the way of this progress. Of the 9 types of COVID-19 vaccines issued by WHO EUL, two are mRNA vaccines, both of which are held by American companies of BioNTech and Moderna.Citation44 This may be because most of mRNA vaccine patents belong to developed countries. In context, among the valid mRNA vaccines in our sample, 73.87% were held by developed countries while 26.13% were held by developing countries. The main developing countries of patent authorization were China, Mexico and India (Supplementary Figure S4). Due to patent protection and restriction, low and middle income countries are unable to produce complex mRNA vaccines without support from the technology holders.Citation19 With the commercial development of the mRNA vaccine, Moderna has stated that it would not share its COVID-19 vaccine recipe, despite pressures to increase the global vaccine supply.Citation45,Citation46
Patent sharing may be essential for global vaccine equity, and sharing knowledge would save time as well as lives. To some extent, voluntary product patent pools should be created in order to collect patent rights with non–exclusive voluntary licenses or intellectual property waivers. Waiving patents is a critical first step, though it would not solve the problem overnight.Citation47 In addition, it would be less voluntary for companies, which would be without any benefit. Meanwhile, the free sharing of waiving Agreement on Trade–Related Aspects of Intellectual Property Rights (TRIPS) in the mRNA vaccine “technology transfer hub” can also reduce the motivation to innovate for enterprises.Citation48,Citation49 Unlike patent waivers, impairing private incentives for further innovation is unnecessary in buyouts. Patent buyouts picked by the COVID-19 Vaccines Global Access initiative have the potential to facilitate the global production of vaccines while reducing prices to lower manufacturing costs in poorer countries.Citation50,Citation51,Citation52
In regard to study limitations, although we have tried to adopt a search strategy to be as comprehensive as possible, this study was unable to cover all patents related to mRNA vaccines.Citation53 In case of unavoidable omissions, they are also controllable and traceable.
5. Conclusion
The findings of this study showed that the United States is the global leader in mRNA vaccine patents, whose numbers have been rapidly increasing in recent years. National cooperation was also shown to be widespread, which was accompanied by the United States establishing cooperative relations with most countries, and Germany building a solid relationship with the UK. Although these results highlight the US’s current monopoly, it is also clear that China’s rise cannot be ignored. Specifically, the collaboration network of mRNA vaccine patent grantees is close and interconnected, which is comprised of public organizations, research institutes, and universities. COVID-19 is a field of application for mRNA vaccines, which has already shown to have great potential. Meanwhile, mRNA vaccines for autoimmunity, infectious diseases, cancer immunity, and allergic diseases are being developed. In recent years, platform technologies for delivery, such as LNP, have been a popular research area in this field. However, patent disputes and humanitarianism should be balanced. According to the flow of knowledge, this study may serve as a reference for governments, academia and industry stakeholders to make relevant decisions and provides directions for technological development and industry transformation.
Supplemental Material
Download MS Word (3 MB)Supplemental Material
Download MS Excel (299.4 KB)Acknowledgement
The authors gratefully acknowledge Sijun Qin and Ziyao Jiang for valuable comments that helped in improving this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2095837.
Additional information
Funding
References
- Belongia EA, Naleway AL. Smallpox vaccine: the good, the bad, and the ugly. Clin Med Res. 2003;1(2):1–10. doi:10.3121/cmr.1.2.87.
- Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020;65:14–20. doi:10.1016/j.coi.2020.01.008.
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949):1465–1468. doi:10.1126/science.1690918.
- Lamb YN. Bnt162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–501. doi:10.1007/s40265-021-01480-7.
- Abramson A, Kirtane AR, Shi Y, Zhong G, Collins JE, Tamang S, Ishida K, Hayward A, Wainer J, Rajesh NU, et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter. 2022;5(3):975–987. doi:10.1016/j.matt.2021.12.022.
- Abbasi J. First mRNA HIV vaccine clinical trial launches. JAMA. 2022;327:909.
- Martin C, Lowery D. mRNA vaccines: intellectual property landscape. Nat Rev Drug Discov. 2020;19(9):578–579. doi:10.1038/d41573-020-00119-8.
- Gaviria M, Kilic B. A network analysis of COVID-19 mRNA vaccine patents. Nat Biotechnol. 2021;39(5):546–548. doi:10.1038/s41587-021-00912-9.
- Shores D. mRNA patent and competitive landscape: 2021 year in review and 2022 outlook. 2022 Jan 3. https://www.jdsupra.com/legalnews/mrna-patent-and-competitive-landscape-8092666/
- Smith JA. Improving transparency and reproducibility of patent landscapes: the Reporting Items for Patent Landscapes (RIPL) statement and other considerations. World Patent Inf. 2020;62:101985. doi:10.1016/j.wpi.2020.101985.
- Smith JA, Arshad Z, Trippe A, Collins GS, Brindley DA, Carr AJ. The reporting items for patent landscapes statement. Nat Biotechnol. 2018;36(11):1043–1047. doi:10.1038/nbt.4291.
- Scherngell T, Rohde C, Neuländtner M, Lozano S. The dynamics of global R&D collaboration networks in ICT: Does China catch up with the US? Plos One. 2020;15(9):e0237864. doi:10.1371/journal.pone.0237864.
- Chen Z, Guan J. The core‐peripheral structure of international knowledge flows: Evidence from patent citation data. R&D Manage. 2016;46(1):62–79. doi:10.1111/radm.12119.
- Brenner S, Jacob F, Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190(4776):576–581. doi:10.1038/190576a0.
- Xu S, Yang K, Li R, Zhang L. mRNA vaccine era—mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21(18):6582. doi:10.3390/ijms21186582.
- Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi:10.1016/j.nantod.2019.100766.
- May M. After COVID-19 successes, researchers push to develop mRNA vaccines for other diseases. Nat Med. 2021;27(6):930–932. doi:10.1038/s41591-021-01393-8.
- Zhang Y-Z, Holmes E. Novel 2019 coronavirus genome. 2020. https://virological.org/t/novel-2019-coronavirus-genome/319
- Krishtel P, Malpani R. Suspend intellectual property rights for covid-19 vaccines. BMJ. 2021;373: n1344. doi:10.1136/bmj.n1344.
- Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–622. doi:10.1038/s41586-021-03324-6.
- Flemming A. mRNA vaccine shows promise in autoimmunity. Nat Rev Immunol. 2021;21(2):72. doi:10.1038/s41577-021-00504-3.
- Weiss R, Scheiblhofer S, Roesler E, Weinberger E, Thalhamer J. mRNA vaccination as a safe approach for specific protection from type I allergy. Expert Rev Vaccines. 2012;11(1):55–67. doi:10.1586/erv.11.168.
- Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):1–23. doi:10.1186/s12943-021-01335-5.
- Weng Y, Li C, Yang T, Hu B, Zhang M, Guo S, Xiao H, Liang X-J, Huang Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv. 2020;40:107534. doi:10.1016/j.biotechadv.2020.107534.
- Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20(11):817–838. doi:10.1038/s41573-021-00283-5.
- Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-Based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4):359. doi:10.3390/vaccines9040359.
- Granados-Riveron JT, Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother. 2021;142:111953. doi:10.1016/j.biopha.2021.111953.
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Nat Acad Sci. 1989;86(16):6077–6081. doi:10.1073/pnas.86.16.6077.
- Vitiello A, Ferrara F. Commentary of the mRNA vaccines COVID-19. Asian J Pharm Sci. 2021;16(5):531. doi:10.1016/j.ajps.2021.05.004.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Li R, Liu H, Fairley CK, Ong JJ, Guo Y, Zou Z, et al. mRNA-Based COVID-19 booster vaccination is highly effective and cost-effective in Australia. medRxiv 2022:2022.05.08.22274797.
- China’s biggest Covid failure is not deploying an mRNA vaccine. 2022 Apr 26. https://www.bloomberg.com/news/articles/2022-04-26/china-covid-situation-worsened-by-lack-of-local-mrna-vaccine
- Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, Chen C, Yiu K, Lam BHS, Lau EHY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28(3):486–489. doi:10.1038/s41591-022-01704-7.
- Novak D. China’s hope for homegrown mRNA vaccine holds back nation. 2022 May 26. https://learningenglish.voanews.com/a/china-s-hope-for-homegrown-mrna-vaccine-holds-back-nation/6589400.html
- Lalani HS, Avorn J, Kesselheim AS. US taxpayers heavily funded the discovery of COVID‐19 caccines. Clin Pharmacol Ther. 2021;111(3):542–544. doi:10.1002/cpt.2344.
- Andreoni A. COVID-19 pandemic’s innovation lessons for the climate crisis. 2022 May 30
- Ga L. Foundational mRNA patents are subject to the Bayh-Dole Act provisions. 2020 Nov 30. https://www.keionline.org/34733.
- Neumann PJ, Cohen JT, Kim DD, Ollendorf DA. Consideration of value-based pricing for treatments and vaccines is important, even in the COVID-19 pandemic: study reviews alternative pricing strategies (cost-recovery models, monetary prizes, advanced market commitments) for COVID-19 drugs, vaccines, and diagnostics. Health Aff. 2021;40(1):53–61. doi:10.1377/hlthaff.2020.01548.
- Shen AK, Hughes Iv R, DeWald E, Rosenbaum S, Pisani A, Orenstein W. Ensuring equitable access to COVID-19 vaccines in the US: current system challenges and opportunities: analysis examines ensuring equitable access to COVID-19 vaccines. Health Aff. 2021;40(1):62–69. doi:10.1377/hlthaff.2020.01554.
- Lamb YN. Remdesivir: first approval. Drugs. 2020;80(13):1355–1363. doi:10.1007/s40265-020-01378-w.
- Eccleston-Turner M. The economic theory of patent protection and pandemic influenza vaccines: do patents really incentivize innovation in the field? Am J Law Med. 2016;42(2–3):572–597. doi:10.1177/0098858816658280.
- Uhthoff GV, Uhthoff SC. Patent protection of mRNA vaccines and regulatory authorization. 2021 July 5. https://www.lexology.com/library/detail.aspx?g=96be730d-80e6-461c-9a40-cd678a5c1496
- WHO. COVID-19 vaccines WHO EUL issued. 2022. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued
- Fortunato P. Fighting Covid-19 requires fewer patents and more state. 2021 July 20. https://socialeurope.eu/fighting-covid-19-requires-fewer-patents-and-more-state
- Ardizzone K. Moderna claims compulsory license from U.S. government to use third party patents in its Covid-19 vaccine. 2022 May 10. https://www.keionline.org/37751
- Tayag Y. The stalemate over patents is fueling vaccine inequity—and the pandemic. 2021 Oct 15. https://fortune.com/2021/10/14/covid-vaccine-global-distribution-inequity/
- Agreement on Trade-Related Aspects of Intellectual Property Rights (unamended). 1994 Apr 15. https://www.wto.org/english/docs_e/legal_e/27-trips_01_e.htm
- Jerving S. Moderna’s patents stand in way of mRNA vaccine hub’s grand vision. 2022 Apr 21. https://www.devex.com/news/moderna-s-patents-stand-in-way-of-mrna-vaccine-hub-s-grand-vision-103055
- Kremer M. Patent buyouts: a mechanism for encouraging innovation. Q J Econ. 1998;113(4):1137–1167. doi:10.1162/003355398555865.
- WHO. No one is safe, until everyone is safe. 2022. https://www.who.int/initiatives/act-accelerator/covax
- Towse A, Chalkidou K, Firth I, Kettler H, Silverman R. How should the world pay for a coronavirus disease (COVID-19) vaccine? Value in Health. 2021;24(5):625–631. doi:10.1016/j.jval.2020.12.008.
- Arinas I. How vague can your patent be? Vagueness strategies in US patents. Vagueness Strategies in US Patents (July 26, 2012) HERMES-Journal of Language and Communication in Business 2012:55–74.