ABSTRACT
The high-density microneedle array patch (HD-MAP) is a novel vaccine delivery system with potential for self-administered vaccination. In this study, Vaxxas HD-MAPs were applied by both a trained user and self-administered with application sites compared to determine the response of skin and the level of engagement of the HD-MAP with human skin. Twenty healthy participants were enrolled, and the response of skin including erythema was observed at all application sites and no difference was found between trained user or self-administered applications. The majority of participants (70%) preferred the deltoid upper arm application site for applying HD-MAPs. Fluorescent dermatoscope images confirmed HD-MAPs engaged the skin surface and scanning electron microscopy (SEM) image analysis exhibited similar delivery characteristics for the upper arm and forearm sites when applied by either a trained user or self-administered. This study showed that noninvasive methods including dermatoscopy and SEM image analysis were able to estimate the engagement of HD-MAPs with human skin. HD-MAP self-vaccination technology has a unique proposition in pandemic preparedness by alleviating the need for health-care workers to administer vaccines, however greater awareness and understanding of the potential of this technology is required.
Introduction
The emergence of the coronavirus SARS-CoV-2 (COVID) pandemic has highlighted the need for continued innovation in how vaccines are delivered and administered.Citation1 The high-density microneedle array patch (HD-MAP) is a novel vaccine delivery system with potential benefits over intramuscular injection (IM), including no cold-chain requirement, reduced vaccine dose and adjuvant-sparing, convenience and no needlestick injuries.Citation2 The HD-MAP delivers the vaccine beneath the cornified layers of the skin, simplifying vaccination with the potential for self-administered applications.Citation3 A study using dissolvable microneedle patches for influenza vaccination has shown self-administration applications generated robust antibody responses,Citation4 while further work has shown that self-administration of devices with a set of application forces has repeatable performances.Citation5 HD-MAP vaccine technology could become an integral component of the global pathway out of future pandemics by streamlining vaccine storage, distribution and accessibility.Citation6
In this study, we compared the Vaxxas HD-MAP administered by a trained user with self-administered applications using noninvasive methods to determine the response of human skin and delivery characteristics of the HD-MAP.
Materials and methods
Subjects
Eligible volunteers were healthy residents of greater Brisbane, Australia (latitude 27°S) aged >18 y. Approval to perform the study was given by the Metro South Health Human Research Ethics Committee (Brisbane, Australia) and retrospectively registered with the Australian and New Zealand Clinical Trials Register (ACTRN12620000179932). The Declaration of Helsinki protocols were followed, and all participants gave their written informed consent to take part.
HD-MAP manufacture and study procedure
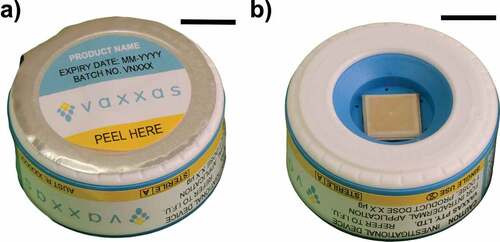
The HD-MAP is a 1 cm2 patch constituted from non-dissolvable liquid crystal polymer (Zenite®, Celanese Corporation, USA), and injection molded to contain 1584 conically shaped projections. HD-MAPs were then sputter-coated with a thin (~15 nm) layer of gold to allow for high-contrast backscatter scanning electron microscopy after HD-MAP application. An excipient formulation with additive fluorescein sodium was coated on the microprojections as previously described.Citation7 The HD-MAP is applied by an integrated applicator device and penetrates the skin at a velocity of ~20 meters per second (). The micro-projections remain intact during this process and the applicator is held in place for 30 s then removed. The applicator device is single use, disabling automatically after use.
Figure 1. The HD-MAP used in the study. (a) integrated applicator, (b) opened device with 1 cm2 MAP (scale bar = 1 cm).
The trained user applied one HD-MAP to the volar forearm as well as one HD-MAP to the posterior shoulder and one to the deltoid upper arm region, all three sites were applied to the subject’s dominant hand-side before self-administration. Self-application involved one HD-MAP applied to the volar forearm, posterior shoulder and deltoid upper arm opposite the subject’s dominant hand. In this study, the trained user was an experienced user of the Vaxxas product. An information for use (IFU) paper handout was provided to all participants (Supplementary Figure S1). Participants were interviewed about their site preference following treatment and asked “Which was your preferred application site, and why?.” Inductive thematic analysis was used to group interview answers into themes by researcher (EH).
Images of the application sites were collected using both a standard dermatoscope (iC1, Heine Optotechnik GmbH & Co., Gilching, Germany) and a modified iC1 Fluorescent dermatoscope which had the integration of a light source emitting light at 490 nm with the addition of an emission filter (MF530-43, Thorlabs, New Jersey, USA). Images were collected using the dermatoscopy software (iC1, Heine Optotechnik GmbH & Co) on a smart phone (iPhone S, Apple, California, USA) and scored by a trained scorer blind to the sample’s ID using imageJ software for the presence of petechiae or fluorescein.Citation8
Skin sites were visually inspected 10 min, 7 d and 28 d post-application, and the level of erythema was categorized as none, barely perceptible, visible, moderate redness and severe redness by a trained scorer blind to the sample’s ID. Participants were given a memory aid in which they could record adverse events, and these details were confirmed by clinic personnel on Day 7. Adverse events were solicited during the Day 28 visit but only recorded if they were serious adverse events, medically attended events, or clinically significant events.
Surface engagement of HD-MAP
When applying coated HD-MAPs to the skin, the removal of the coating was determined by image analysis. Scanning electron microscopy (SEM) images were captured using the SEM system (SU3500, Hitachi High-Tech Corporation, Japan) and each image contained the entire square 1 cm2 area of projections. Images were analyzed using Biodock software.Citation9 The number of microprojections with coating removed after application to human skin was calculated and used to determine the percentage of microprojections engaged with skin (Supplementary Figure S2).
The coating removal depth for each HD-MAP was estimated by calculating the length of the microprojections without coating present across the middle (row 20th) of the HD-MAP. Microprojections were measured and averaged to give the coating removal depth for each HD-MAP (Supplementary Figure S3). All image quantification procedures were performed blind to the sample’s ID.
Statistical analysis
All statistical tests were two-sided, and a p < .05 was considered to be statistically significant. Data analysis was performed using the PRISM statistical package (version 9, GraphPad Software, 2021, California, USA).
Results
Subject characteristics and skin response
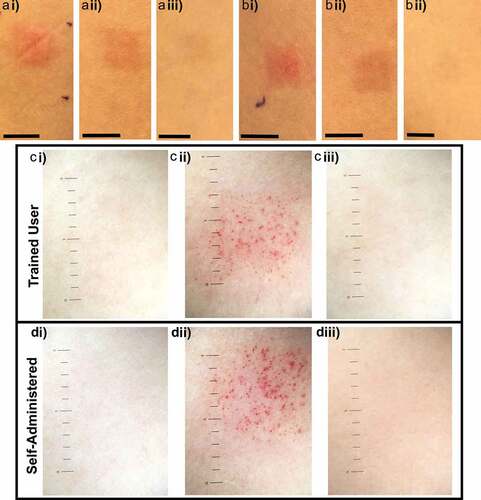
Twenty healthy participants (n = 20) were recruited from December 12, 2019, until May 6, 2020, and aged between 18 and 45 y and 65% (13/20) were female. The HD-MAPs were applied by a trained user to the volar forearm (n = 20), the deltoid upper arm (n = 20) and the posterior shoulder (n = 20) and self-administered to the forearm (n = 20), upper arm (n = 20) and shoulder (n = 20) of all participants (). Visual signs of HD-MAP engagement with the skin were observed 10 min post-application including erythema and petechiae (). Ten minutes post-application erythema was observed at all sites. Visible erythema was recorded for 90% (n = 108) of sites and 10% (n = 12) exhibited moderate erythema. Petechiae was commonly observed using white light dermatoscopy 100% (120/120) 10 min post-application. There was no observed difference between erythema and petechiae at application sites applied by either a trained user or self-administered (Supplementary Table S1). The skin response following the HD-MAP application faded between 7 and 28 d, consistent with previous studies.Citation3,Citation10 No serious adverse events were reported, and only two mild local treatment related adverse events were observed (Supplementary Table S2).
Figure 2. Representative images of self-administered HD-MAP applications to the (a) deltoid upper arm, (b) volar foreman and (c) the posterior shoulder (scale bar = 4 cm).
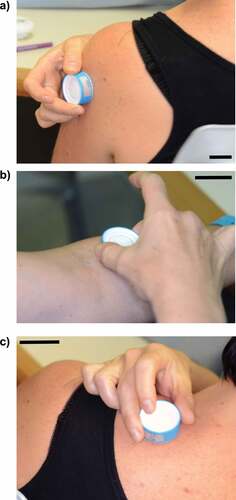
Figure 3. Representative images of skin reactions over time at HD-MAP forearm application sites. Photographs of treatment sites applied by a trained user (ai) erythema evident 10 min post-application, (aii) 7 d and (aiii) site resolved by 28 days, and self-administered application sites (bi) 10 min post-application, (bii) 7 d and (biii) 28 d (scale bar = 1 cm). Dermatoscopy images (ci) pre-application, (cii) 10 min post-application and (ciii) 28 d after application of HD-MAP by trained user and self-administered application (di) pre-application, (dii) 10 min post-application (diii) 28 d post-application (scale bar = 1 mm increments).
The majority of participants (70%) preferred the deltoid upper arm application site for applying HD-MAPs. Qualitative thematic analysis was used to classify participants’ comments into four themes: ease of use, pain and discomfort, visual appearance, and familiar site for injections. Participants commented “Less stinging than other sites and easier to access (ID-01, male)”, “prefer upper arm due to visibility of patch marks (ID-10, female)” and “Just found upper arm more natural (ID-20, female).”
Surface engagement of HD-MAP
Fluorescent dermatoscope images confirmed HD-MAPs impacted the skin surface with fluorescein observed at all application sites (), Supplementary Table S1). The SEM analysis of HD-MAPs applied to the forearm site showed that all applications were categorized as good, while 95% of the applications to the upper arm were categorized as good (, Supplementary Table S1). There was no difference between self-administered and trained user applications for the forearm and upper arm site, however only 80% of the self-administered applications to the posterior shoulder site were categorized as good compared to 95% for the trained user applications (Wilcoxon matched-pairs signed rank test, p = .03).
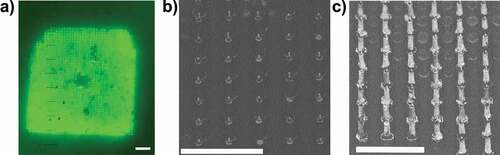
Figure 4. Surface engagement of HD-MAPs. (a) Fluorescent dermatoscope image detecting fluorescein on skin after HD-MAP application, scale bar = 1 mm. (b) SEM image of coated microprojections pre-application mounted with 45°C angle, the coating covers the microprojection construction material (scale bar = 500 um). (c) SEM image from a HD-MAP after application to human skin mounted with 45°C angle, the microprojections are no longer coated and are now visible (scale bar = 500 um).
The estimated average coating removal depth for microprojections was 156 um (SEM = 4.8, range 123–188 um) for the upper arm site applied by the trained user and 161 um (SEM = 4.9, range 129–234 um) for the self-administered site (Supplementary Table S1). While the forearm site was 143 um (SEM = 2.8, range 123–171 um) when applied by the trained user and 146 um (SEM = 6.0, range 115–215 um) for the self-administered site. The posterior shoulder site was 141 um (SEM = 4.5, range 91–179 um) when applied by the trained user and 144 um (SEM = 3.9, range 110–187 um) for the self-administered site.
Discussion
This study found that good delivery characteristics of HD-MAPs to the upper arm and forearm sites were achieved when administered by either a trained user or self-administered. Participants reported a preference for the deltoid upper arm site and the coating removal depth measurement for microprojections at this site also showed it would provide suitable vaccine delivery to the abundant immune cells in the skin.Citation11
Developing noninvasive methods to determine the delivery efficiency of a vaccine from topical HD-MAP applications will be an important element in building confidence and uptake of this technology. This study showed dermatoscopy images were able to highlight the response of the skin following HD-MAP applications including detection of petechiae. Fluorescent dermatoscopy detected the transfer of coated substrates from HD-MAPs onto human skin. SEM analysis of HD-MAP following application to human skin was able to estimate the proportion of microprojections with coating removed. The self-administered and trained-user HD-MAP applications showed similar delivery characteristics for the upper arm and forearm site, while self-application to the posterior shoulder site showed a reduction in the proportion of microprojections with coating removed and may not be a suitable treatment site for self-administration of HD-MAP vaccines. Potential difficulties reaching the posterior shoulder may be a contributing factor to the poor applications observed in the self-administration group. Further investigation is warranted to determine the preferred application site, which will likely be dictated by perceived pain and patient or user preference for ease of use. Forster et al. (2022) found no difference in immune response to a monovalent influenza vaccine when delivered to the upper arm or forearm.
The estimated average coating removal depth suggests that both self-administered applications and those applied by a trained user may reach the cellular epidermis and dermis, with an estimated thickness of the stratum corneum at the dorsal forearm of 18.3 um and at the posterior shoulder of 11.0 um and 56.6 um for the cellular epidermis at the forearm and 70.3 um at the shoulder.Citation12,Citation13 Our findings are similar to previously published work showing microprojections deposit antigen into both the epidermis and dermis.Citation13 The coating removal depth method was not confirmed by the histology of the skin, and future studies comparing SEM imaging post treatment would benefit from including skin morphology assessments.
This study explored the impact of HD-MAP self-administration and the response of human skin. The dermatoscope image captured unraised round red spots under the skin illustrating petechiae caused by capillary bleeding. There was no difference observed in petechiae formation between the trained-user and self-administered treatment sites. Engineering controls have been integrated into the applicator device including the audible and tactile click of the device, which provides feedback to the user that the device has worked as intended. These design features may have mitigated any potential harm caused by the administration of the HD-MAP by untrained end-users. HD-MAP material should be carefully selected to ensure biocompatibility and potential allergens labeled such as aluminum which can cause contact dermatitis in 1% of adults.Citation14
Participants raised key themes when discussing HD-MAP application site preferences with ease of use, pain and discomfort common considerations, while the deltoid upper arm site was the preferred site. This attitude may be shaped by the current clinical care and the acceptance for IM injections at this site. Several female participants raised the topic of visual appearance of marks on the skin and the upper arm site might be preferable as it can be easily covered by clothing.
HD-MAPs have a range of potential advantages over IM vaccine delivery during a pandemic emergency, and previous studies have shown that self-administration would be acceptable in a pandemic response setting.Citation2 However, concerns have been raised over self-administered HD-MAP vaccines and the risk of anaphylaxis outside a clinical care environment. The attributable risk of anaphylaxis after commonly administered vaccines is estimated at 1/100,000 to 1/1,000,000 and the rate of anaphylaxis for the HD-MAP will not be known, until it is used in a population-based vaccination program.Citation2 Mitigation strategies to reduce the risk of anaphylactic shock should be undertaken and include restricting access to people with a history of anaphylaxis or other life-threatening allergic reactions, along with using the HD-MAP to self-administer only subsequent doses following tolerance of the initial vaccine. Remote patient monitoring services have rapidly expanded during COVID and continued to demonstrate suitable patient safety as well as expansion to integrate pulse oximeter monitoring and forward triage of patients to emergency departments.Citation15 Leveraging these remote patient monitoring capabilities during a pandemic could also be extended to monitoring side effects from vaccinations.
Workforce shortages were experienced in the health-care sector during the pandemic, and HD-MAP technology could potentially assist by reducing staffing requirements at vaccination hubs with lower skill requirements to administer HD-MAPs compared with needle and syringe.Citation16 HD-MAP may also provide an avenue to assist boasting uptake of multi-dose vaccines by providing self-administration options for subsequent doses.Citation5 However, new approaches would need to be developed to verify and record successful administration of the vaccine to the right person in community settings.Citation17 Considerations for the safe disposal of HD-MAPs in community settings would also need to be explored, and the possibility of returning the product for recycling would warrant further investigation. Regulatory bodies may determine certain vaccines, such as live attenuated virus vaccines, are biohazardous and disposal processes may need to follow medical waste incineration. While HD-MAP vaccines adhere to strict aseptic manufacturing standards and sterility requirements, further guidance from regulatory bodies on required testing and evidence for self-administered vaccine products is needed.Citation18 Targeted investment to enhance capabilities in the rapid scale-up of manufacturing HD-MAP vaccination products would be essential for accelerating vaccination and improving pandemic preparedness.Citation19 The lessons from the COVID pandemic have shown vaccination of a population can result in fewer cases and deaths, reducing potential for emerging variants, as well as reducing the duration of lockdowns and other pandemic restrictions that negatively affect the community.Citation16 Faster rollout of vaccines in a pandemic could also reduce economic losses, and HD-MAP is potentially a new tool to contribute to future pandemic planning.Citation6
Limitations of this study include the lack of immunogenicity data confirming the delivery of HD-MAP content. The SEM image analysis method calculated the proportion of microprojections with coating removed and categorized applications, however this was not confirmed by an ELISA comparing the residual amount able to be eluted from the patch following application. The HD-MAP used in this study had a shorter duration of incubation on the skin and fewer microprojections compared with previously tested HD-MAPs, which have shown immunogenicity responses.Citation18 The excipient formulation coated on microprojections contained fluorescein and as such delivery characteristics may not be representative of antigen material coated on HD-MAP in vaccine trials. The sample size was also small (n = 20), and participants ranged in age from 18 to 45 y, as such the results may not be generalizable to other subgroups of the population.
Conclusion
This study demonstrated the suitability of noninvasive methods including analyzing SEM images to determine how well microprojections engaged human skin. Dermatoscopy images were able to highlight the response of human skin following HD-MAP applications and fluorescent dermatoscopy detected the transfer of coated substrate. These findings may assist to develop standardized noninvasive methodology for estimating the surface engagement of HD-MAPs with human skin.
Comparisons of the surface engagement of the HD-MAP when applied by different users give confidence that in the scenario of self-administration a user would be able to use the device and the delivered dose would be comparable with administration by a trained user. Our results showed that the HD-MAP exhibited similar delivery characteristics for the upper arm and forearm sites when applied by either a trained user or self-administered, while the posterior shoulder site was not suitable for self-administration.
Supplemental Material
Download PDF (858.4 KB)Acknowledgments
The authors would like to thank all participants for their time and generosity. The authors would also like to thank Thomas Pitney and Todd Gumbleton for their assistance with the clinical procedures, Jodie Robinson for assistance with ethics and governance processes, the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, the University of Queensland for scientific and technical assistance and Peter Goddard for data analysis and Sandra Depelsenaire and Hwee Ng for technical assistance and Charles Ross and Sophie Greensill for helpful discussions. EH is supported by a REDI Fellowship from the Medical Research Future Fund.
Disclosure statement
BB, EH and AF are paid employees of Vaxxas Pty Ltd. GS received consulting fees from Vaxxas Pty Ltd. ML and NM are paid employees of Biodock Inc, which carried out work for the study on a contract basis paid by Vaxxas Pty Ltd.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2189409
Additional information
Funding
References
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–6. doi:10.1016/S0140-6736(21)00306-8.
- Davies C, Taba M, Deng L, Karatas C, Bag S, Ross C, Forster A, Booy R, Skinner SR. Usability, acceptability, and feasibility of a High-Density Microarray Patch (HD-MAP) applicator as a delivery method for vaccination in clinical settings. Hum Vaccin Immunother. 2022;18(4):2018863. doi:10.1080/21645515.2021.2018863.
- Forster AH, Witham K, Depelsenaire ACI, Veitch M, Wells JW, Wheatley A, Pryor M, Lickliter JD, Francis B, Rockman S, et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: results from a randomized, controlled phase I clinical trial. PLoS Med. 2020;17(3):e1003024. doi:10.1371/journal.pmed.1003024.
- Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390(10095):649–58. doi:10.1016/S0140-6736(17)30575-5.
- Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62. doi:10.1016/j.vaccine.2014.01.076.
- Becker L, Whorley M, Hess T, Payne H, Snow B, Von Eisebrg M. Estimating the economic and public health impact of microarray patch (MAP)-administered vaccines in pandemics. 2022.
- Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, Chang D, Ruutu MP, Jenkins DWK, Pyke A, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–84. doi:10.1002/smll.201000331.
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017;18(1):529. doi:10.1186/s12859-017-1934-z.
- Biodock. AI Software Platform. 2022. www.biodock.ai.
- Fernando GJP, Hickling J, Jayashi Flores CM, Griffin P, Anderson CD, Skinner SR, Davies C, Witham K, Pryor M, Bodle J, et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine. 2018;36(26):3779–88. doi:10.1016/j.vaccine.2018.05.053.
- Badizadegan K, Goodson JL, Rota PA, Thompson KM. The potential role of using vaccine patches to induce immunity: platform and pathways to innovation and commercialization. Expert Rev Vaccines. 2020;19(2):175–94. doi:10.1080/14760584.2020.1732215.
- Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 2003;83(6):410–3. doi:10.1080/00015550310015419.
- Depelsenaire ACI, Witham K, Veitch M, Wells JW, Anderson CD, Lickliter JD, Rockman S, Bodle J, Treasure P, Hickling J, et al. Cellular responses at the application site of a high-density microarray patch delivering an influenza vaccine in a randomized, controlled phase I clinical trial. PLoS One. 2021;16(7):e0255282. doi:10.1371/journal.pone.0255282.
- Siemund I, Dahlin J, Hindsen M, Zimerson E, Antelmi A, Hamnerius N, Hauksson I, Isaksson M, Pontén A, Mowitz M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33(1):31–35. doi:10.1097/DER.0000000000000787.
- Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–81. doi:10.1056/NEJMp2003539.
- O’Shea J, Prausnitz MR, Rouphael N. Dissolvable microneedle patches to enable increased access to vaccines against SARS-CoV-2 and future pandemic outbreaks. Vaccines (Basel). 2021;9(4):320. doi:10.3390/vaccines9040320.
- Nguyen TT, Oh Y, Kim Y, Shin Y, Baek SK, Park JH. Progress in microneedle array patch (MAP) for vaccine delivery. Hum Vaccin Immunother. 2021;17(1):316–27. doi:10.1080/21645515.2020.1767997.
- Forster A, Junger M. Opportunities and challenges for commercializing microarray patches for vaccination from a MAP developer’s perspective. Hum Vaccin Immunother. 2022;18(4):2050123. doi:10.1080/21645515.2022.2050123.
- Creelman B, Frivold C, Jessup S, Saxon G, Jarrahian C. Manufacturing readiness assessment for evaluation of the microneedle array patch industry: an exploration of barriers to full-scale manufacturing. Drug Deliv Transl Res. 2022;12(2):368–75. doi:10.1007/s13346-021-01076-4.