ABSTRACT
Although vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease 2019 (COVID-19) induce effective immune responses, vaccination with booster doses is necessary because of waning immunity. We conducted an open-label, non-randomized, single-arm study in adults in Japan to assess the immunogenicity and safety of a single booster dose of the KD-414 purified whole-SARS-CoV-2-virion inactivated vaccine candidate after vaccination with a primary series of BNT162b2. The primary endpoint was serum neutralizing activity at 7 days after booster injection compared with the primary series of BNT162b2. The SARS-CoV-2-structural protein-binding antibody level and T cell response against SARS-CoV-2-Spike (S) peptides were also examined as secondary endpoints, and safety profile assessments were conducted. Twenty subjects who participated in a previous study declined an injection of KD-414 (non-KD-414 group) and received a booster dose of BNT162b2 instead. The non-KD-414 group was compared to the KD-414 group as a secondary outcome. A single dose of KD-414 induced lower serum neutralizing activity against the wild-type virus within 7 days compared to after the primary series of BNT162b2 but significantly induced anti-SARS-CoV-2-S1-receptor-binding domain-binding immunoglobulin G (IgG) antibodies and SARS-CoV-2-S peptide-specific CD4+ and CD8+ T cell responses. Local or systemic symptoms were significantly lower in the participants who received KD-414 than in those who received BNT162b2 as the third COVID-19 vaccine dose. The present data indicate that a single booster dose of KD-414 induces a substantial immune response in BNT162b2-primed individuals and has a good safety profile, thereby supporting further clinical trials to identify rational targets.
Introduction
The rapid spread of the novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) disease 2019 (COVID-19) has resulted in the most serious global public health and socioeconomic disaster in this century.Citation1 To act against the pandemic, more than 160 COVID-19 vaccines are still under developmentCitation2 using multiple platforms.Citation3 Among these, whole-virion inactivated vaccines directed toward Japanese encephalitis, hepatitis A, rabies, and poliovirus,Citation4 represent one traditional approach. Unlike natural infection or live vaccines, inactivated vaccine-induced immunity is generally not long-lasting, and multiple doses are required to boost the effect over time.Citation5 On the other hand, inactivated vaccines contain the intact pathogen. Thus, the immune response is likely to target viral structural proteins such as the matrix, envelope, and nucleoprotein,Citation3 and to stimulate toll-like receptors and induce type 1 interferon (IFN).Citation6
KD-414 is a purified inactivated whole SARS-CoV-2 virion-containing vaccine adjuvanted with aluminum hydroxide and developed by KM Biologics Co., Ltd. (Kumamoto, Japan).Citation7 A double-blind, randomized, placebo-controlled phase I/II trial of KD-414 involving 210 healthy adults in Japan with no prior COVID-19 vaccination or history of COVID-19 showed good tolerability for all ages tested.Citation7 A high dose (10 ug/dose) used in the phase 1/2 study to evaluate the efficacy of three doses induced significant neutralizing antibody activity in age-dependent manner. This was especially evident in participants under 40 years of age, when the first two doses were administered intramuscularly 28 days apart.Citation7 Administration of the third dose of KD-414 after 6 or more months from the second dose was also well tolerated and induced a higher and longer-lasting neutralizing antibody response compared with after the second dose.Citation7
More than 80% of Japanese people in their 20s and 30s have already completed the primary series of vaccinations, mostly using an mRNA platform.Citation8 It is therefore important to evaluate the safety and immunogenicity of booster vaccinations. Here, we report the immunogenicity and safety of a single booster vaccination with KD-414 after primary vaccination with two doses of the BNT162b2 mRNA vaccine in Japanese volunteers. The aim was to determine whether a single booster dose of KD-414 after a primary series of BNT162b2 induces substantial SARS-CoV-2-specific humoral and cellular responses with a strong safety profile.
Materials and methods
Intervention; COVID-19 inactivated vaccine, KD-414
KD-414 is an inactivated vaccine containing purified inactivated whole SARS-CoV-2 virions cultured in Vero cells (RRID: CVCL_0059)Citation9 that provides significant immunogenicity following two-dose primary series vaccination,Citation7 especially in adults under 40 years of age. The vaccine is adjuvanted with aluminum hydroxide to boost the immune response. A SARS-CoV-2 strain isolated in Japan in 2020 was used for vaccine production.Citation7,Citation9 One dose of KD-414 (0.5 mL) contains 10 μg of inactivated whole SARS-CoV-2 virions. The concentration of aluminum hydroxide in KD-414 is 0.40 mg/mL (as aluminum). The aluminum hydroxide used in KD-414 is the same as that used in the hepatitis B (HB) vaccine (Bimmugen) licensed in Japan.
Study design and participants
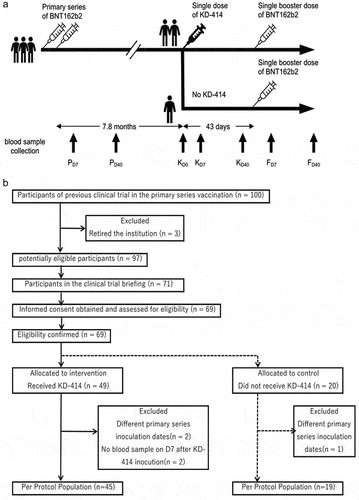
This open-label, non-randomized, single-arm study was conducted using 100 volunteer staff members of the National Center for Global Health and Medicine (NCGM) in Tokyo, Japan, from November 2021 to January 2022 (Certified Review Board of NCGM approval No. NCGM-C-004374, Japan Clinical Trial Registry No.: jRCTs031210388), as previously described.Citation10
In brief, 100 individuals who participated in a clinical trial to evaluate the antigenicity and safety of the primary series of BNT162b2 (Ethics review committee of NCGM approval No. NCGM-A-004175)Citation11 and followed from March to June 2021 were selected as potential candidates (). Blood samples were collected at 7 (PD7) and 40 (PD40) days after the primary series of BNT162b2. This study intervention was performed 7.8 months after the second inoculation of BNT162b2 (). Blood samples were collected from individuals confirmed to be eligible and who agreed to participate in the present clinical study. All participants were monitored for participant assessed adverse reactions. Of these, those who requested KD-414 vaccination were vaccinated (KD-414 group) and those who did not request KD-414 vaccination had blood samples drawn and their antibody titers evaluated (non-KD-414 group). Subsequently, it was decided by the Japanese government that a third (single booster) dose of BNT162B2 would be administered to healthcare professionals in Japan, and thus all subjects in the KD-414 and non-KD-414 groups received a booster vaccination with BNT162b2 ().
Blood samples were obtained at 0 (immediately before the KD-414 shot, KD0), 7 (KD7), and 40 (KD40) days after vaccination with the first dose of KD-414 (). Blood samples were subsequently obtained from the participants who had received a booster dose of BNT162b2 at 50 (7 days after the booster dose of BNT162b2, FD7) and 83 (40 days after the booster dose of BNT162b2, FD40) days after a single dose of KD-414 (). Whole blood specimens were subjected to T cell profiling analysis immediately after sample collection, and sera were stored at −80 °C until use.
All participants were also asked to record any solicited local (e.g., tenderness or spontaneous pain, erythema/redness, induration, swelling of the injection site) and constitutional symptoms (e.g., nausea/vomiting, headache, fatigue/malaise, myalgia, chills, diarrhea, arthralgia), body temperature, and antipyretic use every day for 7 days after each inoculation.
The kinetics of SARS-CoV-2-specific humoral and cellular responses and safety profiles were evaluated. This study was conducted in accordance with the Declaration of HelsinkiCitation12 and the Clinical Research Act.Citation13
Endpoints of the study
The primary endpoint of the study was the in vitro serum viral neutralizing titer at 7 days after vaccination compared with that after the primary series of BNT162b2. The secondary endpoints were (1) the serum neutralizing titer at 40 days after each vaccine injection, (2) the amount of anti-SARS-CoV-2-Spike (S) or -Nucleocapsid (NC)-binding antibodies, (3) CD4-positive (CD4+) and CD8-positive (CD8+) T cell responses against the SARS-CoV-2-S protein, (4) CD4+ T cell profiling, and (5) participant-assessed solicited symptoms and adverse effects for 7 days after each injection.
Statistical analysis
The main purpose of the present study was to assess the non-inferiority of serum-neutralizing activity after a single booster dose of KD-414 compared with that after the primary series of BNT162b2 at 7 days after the vaccination. Serum neutralizing activity after the primary series of BNT162b2 was used as a historical control. The lower bound (LB) of the 95% confidence interval (CI) for a geometric mean ratio (GMR) ratio>0.67 is often used as the non-inferiority limit for new COVID-19 vaccine candidates seeking approval based on the immunobridging approach in non-inferiority studies.Citation14 As for the historical control, the population mean and standard deviation of the geometric mean of the 50% neutralizing titer (GM-NT50) was set to 4.25 and 0.4, respectively, on a common logarithm scale. Regarding 4.25, the geometric mean of the 50% neutralizing titer (GM-NT50) on the common logarithm scale using the historical control as a population mean, and assuming a standard deviation (SD) 0.4 at KD7 and a significance level of 2.5% for one side, 58 participants were required for 90% power and 44 for 80% power to assess with the LB of a 95% CI for a GMR>0.67. To assess the LB of a 95% CI for a GMR>0.67, 58 participants were required for 90% power and 44 for 80% power with a significant level of 2.5% for one side. Sixty of the participants (n = 100) enrolled in a previous study (NCGM approval No. NCGM-A-004175) were enrolled in the KD-414 group. The primary analysis population included members of the per-protocol population who received a booster KD-414 dose.
The proportion of T helper (Th) cells and the results of interferon-gamma (IFN-γ) release assay (IGRA) and other obtained data were log-transformed before analysis. NT50 values under the detection limit (≤40-fold dilution) were regarded as 20. GMR values were calculated based on the GM-NT50 ratio between the groups or between time points, and the corresponding 95% CIs were determined. Immunogenicity data (e.g., serum neutralizing activity, SARS-CoV-2-S1-receptor-binding domain [RBD]-binding immunoglobulin G (IgG) level, IGRA results, and rate of Th cell production) after KD-414 vaccination were compared with the values obtained from the non-KD-414 group and at different time points as the secondary endpoint analysis. The difference between groups or time points in the IGRA results was estimated by the difference in the medians, and the corresponding CIs were calculated using the bootstrap method. CIs for proportions of Th cells were computed using the exact binomial (Clopper – Pearson) method. The correlation between neutralizing activity and the amount of SARS-CoV-2-S1-RBD-binding IgG was computed using Spearman’s correlation coefficient. Demographic characteristics were presented using the median and range for continuous variables or the number and proportion for categorical variables, and p values were calculated using Fisher’s exact test or Student’s t-test. Missing data were not imputed. A safety assessment was conducted for all participants who received a booster dose of KD-414. Descriptive summary data (numbers and percentages) were assessed for participants with any subjective adverse reaction up to 7 days after injection with KD-414. The incidence of self-reported solicited adverse events was compared with that after the third injection of BNT162b2 in the non-KD-414 group. The statistical significance threshold was p < 0.05. All analyses were performed using SASⓇ software, version 9.4 (Cary, NC, USA) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Cells, viruses, and in vitro SARS-CoV-2 neutralizing assay
Transmembrane protease serine 2 (TMPRSS2)-overexpressing VeroE6 (VeroE6TMPRSS2) cells (RRID: CVCL_YQ49) were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). Two SARS-CoV-2 strains, wild-type (PANGO lineage B) and Delta (hCoV-19/Japan/TKYK01734/2021, PANGO lineage B.1.617.2, GISAID Accession ID: EPI_ISL_2080609), were isolated in March 2020 and April 2021, respectively, in Tokyo, Japan, as previously described.Citation15 The SARS-CoV-2 neutralizing activity of sera samples was determined as previously described.Citation10,Citation15,Citation16 All samples used in the present study were evaluated in parallel. High neutralizing activity-confirmed convalescent plasma sample D43Citation16 was used as a reference control to assess the intra-assay deviation.
Quantification of anti-SARS-CoV-2-S- and -NC-binding antibodies
Serum anti-SARS-CoV-2-S- and -NC-binding antibodies were examined as previously reported.Citation10 In brief, the amount of anti-SARS-CoV-2-S1-RBD-binding IgG (IgG-S) was quantified using AdviceDx SARS-CoV-2 IgG II, while the presence of anti-SARS-CoV-2-S-binding immunoglobulin M (IgM-S) and anti-SARS-CoV-2-NC-binding IgG (IgG-N) was interpreted using AdviceDx SARS-CoV-2 IgM and SARS-CoV-2 IgG (Abbott, Chicago, IL), respectively, with the Architect apparatus (Abbott) based on a chemiluminescent microparticle immunoassay.
Peripheral T cell profiling using SARS-CoV-2-S peptides and flow cytometry analysis
Peripheral T cell responses against the SARS-CoV-2-S peptides were evaluated with the IGRA using a QuantiFERON SARS-CoV-2 RUO (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions, as previously described.Citation10,Citation17 An IFN-γ concentration of 0.20 IU/mL or higher (≥0.20 IU/mL) was interpreted as a positive T cell response, as previously reported.Citation17
To characterize peripheral CD4+ Th cells, phorbol 12-myristate 13-acetate and ionomycin (PMA-I)-stimulated CD4+ cells in the presence of brefeldin-A were stained with fluorescent-conjugated antibodies for human IFN-γ and interleukin-4 (IL-4), then subjected to flow cytometric analysis (SRL, Tokyo, Japan). Th1, Th2, and Th0 (naïve T) cells were defined as IFN-γ+/IL-4–, IFN-γ–/IL-4+, and IFN-γ+/IL-4+ CD4+ cells, respectively. The amount of each phenotype cell among all CD4+ cells and the ratio between Th1 and Th2 cells (Th1/Th2) were also calculated.
Results
Clinical characteristics of the participants
A CONSORT diagram of the present study is shown in . Of the 100 candidates who received a primary series of BNT162b2 injections,Citation10 71 joined the clinical trial briefing and 69 freely and voluntarily agreed to participate in the present interventional study. All 69 of these participants were confirmed to meet the eligibility criteria; 49 (71.0%) agreed to receive a single dose of KD-414 (KD-414 group) and the remaining 20 (29.0%) did not (non-KD-414 group) (). Several individuals were excluded due to deviation from the study protocol. Forty-five individuals were injected with KD-414 and included in the final analysis as a per-protocol population, and 19 members of the non-KD-414 group were included in the comparison for reference. Most of the participants received an additional booster dose of BNT162b2 at 43 days after the KD-414 injection (, S1). Blood samples, except for KD0 for the non-KD-414 group, were collected intermittently for 83 days after a single dose of KD-414 (40 days after the booster dose of BNT162b2) ().
The demographic characteristics of the participants are shown in . No statistically significant differences in characteristics were found between the KD-414 and non-KD-414 groups, except for nine individuals in the KD-414 group who had certain medical histories such as hypertension (n = 3), asthma (n = 2), or atopic dermatitis (n = 1) (p = .0481) ().
Table 1. Characteristics of the participants.
A booster dose of KD-414 induced substantial serum neutralizing antibodies
Two or three doses of KD-414 as a primary series of vaccine injections demonstrate a favorable safety profile with substantial neutralizing antibody responses, especially in adults under 40 years of age.Citation7 In the present study, we administered a single dose of KD-414 as a booster dose at 7.8 months after the primary series of BNT162b2 in 49 participants (). We first evaluated the kinetics of BNT162b2-induced serum neutralizing activity against wild-type SARS-CoV-2 (PANGO lineage B) during the observation period and found it gradually decreased after the primary series of vaccine injections (KD0) (). In contrast, the single booster dose of KD-414 induced significant serum neutralizing activity against the wild-type virus within 7 days after the injection (, S2(a)) but activity was significantly lower than that after the primary series of BNT162b2 (which did not exceed the non-inferiority GMR value) (). Activity was further increased 40 days after the KD-414 booster injection but continuously decreased in the non-KD-414 group, resulting in a much larger GMR value between groups (, S2(b)). The elevated neutralizing activity at KD40 did not exceed that at 40 days after the primary series of BNT162b2 (PD40) but the GMR value was slightly lower than the non-inferiority GMR value (). The same trend was observed against the Delta variant (PANGO lineage B.1.617.2), which was the most dominant variant in Japan at the time of the intervention (). It is noteworthy that the serum neutralizing activity against the Delta variant was below the detection limit in all participants in the non-KD-414 group at KD40, whereas KD-414 induced significant neutralizing activity against the variant (Figure S2(b), lower column).
Figure 2. Kinetics of serum SARS-CoV-2-neutralizing activity.
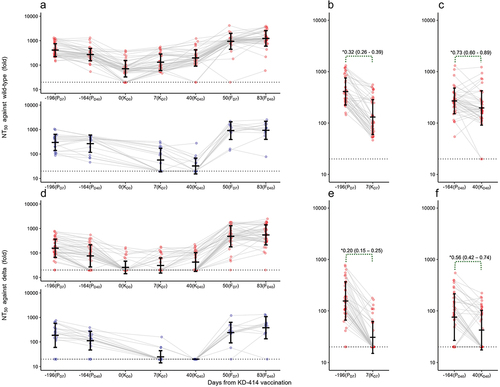
These results also confirmed, based on the kinetics of the IgG-S level (Figure S3(a)), the strong positive correlation observed between the NT50 values against wild-type SARS-CoV-2 and the amount of IgG-S at all time points (Figure S3(e)). This suggests that the neutralizing activity after vaccination with KD-414 is largely due to the amount of IgG-S in the sera of booster-vaccinated participants.Citation18 IgM-S was detected in many participants after the primary series of BNT162b2 (PD7) (Supp. Table). On the other hand, a single dose of KD-414 had limited effects on IgM-S induction at 7 days after the booster dose of BNT162b2 (FD7), with prevalence being significantly lower compared with after the primary series of BNT162b2. No IgG-N was detected throughout the study, including in the samples collected after a single dose of KD-414, suggesting that a single dose of KD-414 does not produce a detectable amount of IgG-N and that no study participants were infected with SARS-CoV-2 during the 40-day observation period (Supp Table).
A single booster dose of KD-414 as the third dose of COVID-19 vaccination enhanced the induction of neutralizing antibodies after injection of BNT162b2 as the fourth COVID-19 dose
The observation period was extended, and at 43 days after the single dose of KD-414, 47 participants (95.9%) in the KD-414 group and 14 (70%) in the non-KD-414 group received an additional booster dose of BNT162b2 (). The serum neutralizing activity at 7 (FD7) and 40 (FD40) days after the additional booster dose of BNT162b2, or 50 and 83 days after the single dose of KD-414, respectively, was evaluated. The additional single booster dose of BNT162b2 (fourth injection for the KD-414 group and third injection for the non-KD-414 group) induced drastically higher neutralizing activity compared with that after the primary series of BNT162b2 or the KD-414 booster injection (). It is noteworthy that the participants who received KD-414 showed higher neutralizing activity against both the wild-type and Delta variant (Figure S2(c,d)) than did the non-KD-414 group after the booster dose of BNT162b2, although no statistically significant differences were observed other than the activity against the Delta variant at FD7 (Figure S2(c)).
KD-414 recalled SARS-CoV-2-S protein-specific CD4+ and CD8+ T cell responses
We next attempted to evaluate the kinetics of SARS-CoV-2-S peptide-specific CD4+ and/or CD8+ T cell responses across the observation period (). In the 64 participants immunized with the primary series of BNT162b2, a substantial T cell response was observed within 7 days (at PD7) in all the individuals with RBD-stimulated CD4+ (Ag1, ) and S1/S2 subunit-stimulated CD4+ and CD8+ (Ag2, ) T cell responses with positive IFN-γ production (≥0.20 IU/mL).Citation10,Citation17 The responses gradually waned by 7.8 months after the primary series (). The single dose of KD-414 induced a substantial Ag1 response at KD7 and was further significantly elevated by KD40 (). The Ag2 response showed similar but relatively modest trends to that of Ag1 (). An additional booster dose of BNT162b2 re-stimulated both the Ag1 and Ag2 responses within 7 days after vaccination (FD7), then decayed in a time-dependent manner within 40 days (FD40), with no statistically significant differences seen between groups ().
Figure 3. CD4+ and/or CD8+ T cell responses after vaccination.
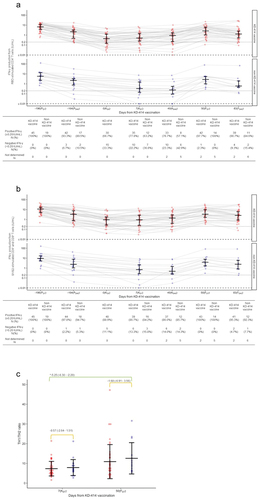
We also characterized peripheral CD4+ Th cell responses stimulated with PMA-I on KD7 and FD7 using intracellular human IFN-γ and IL-4 flow cytometric analysis. No statistically significant differences in the percentage of Th1 (IFN-γ+/IL-4–) or Th0 (IFN-γ+/IL-4+, naïve T) cells were found between the groups at KD7 or FD7 (Figure S4(a)). The population of Th2 (IFN-γ–/IL-4+) cells was slightly higher in the KD-414 group than in the non-KD-414 group at both KD7 and FD7 (Figure S4b), indicating a bias toward Th2 cell responses at both KD7 and FD7 (); although, no statistically significant differences were observed between groups. The rate of naïve T cells production was comparable at both KD7 and FD7 (Figure S4(c)).
A booster dose of KD-414 had minimal systemic side effects compared with a booster dose of BNT162b2
Finally, we evaluated participant-assessed adverse effects and symptoms after each single booster dose of KD-414 and BNT162b2 for 40 days. The presence of local adverse events and subjective symptoms and whether body temperature exceeded 37.5 °C during the monitoring period after the third dose (KD-414 for the KD-414 group and BNT162b2 for the non-KD-414 group) were compared ().
Table 2. Self-reported adverse events.
For the 49 participants in the KD-414 group, pain at the injection site was the most common participant-assessed solicited local adverse event (), but the incidence was significantly lower than that after BNT162b2 in the non-KD-414 group (p = .0026). No induration or swelling at the injection site was reported after KD-414 administration (). None of the participants in the KD-414 group reported an elevated body temperature above 37.5 °C during the 7-day observation period after vaccination, in contrast with that after BNT162b2 administration (p = .0003) (). Further, the use of antipyretic agents (e.g., acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), including routine or prophylactic use, was significantly lower in the KD-414 group (p = .0100) (). The incidence of systemic symptoms was significantly lower in the KD-414 group, with less than a half of the participants reporting at least one of the systemic symptoms described above after KD-414 injection (19 of 49, 38.8%), whereas all the individuals in the non-KD-414 group reported at least one of these symptoms after BNT162b2 (p < .0001) ().
From 8 through 40 days after KD-414 injection, three unspecified adverse events – urticaria, irregular menstruation, and elevated carcinoembryonic antigen (CEA)—were observed as adverse events with a causal or undeniable causal relationship to KD-414. The participants with elevated CEA levels had a medical history of lung cancer, and there was no suspicion of recurrence on periodic imaging diagnosis, leading the investigator to conclude that these elevated levels had an undeniable causal relationship to KD-414. All these adverse events were non-serious, and no serious adverse events were reported during the 40-day observation period after KD-414 injection.
Discussion
Increasing evidence suggests that booster vaccines do can be heterologous, especially mRNA-booster vaccines, because of their robust immunogenicity response elicited.Citation19–23 In contrast, boosting with adenovirus-vector vaccines (e.g., Ad26.COV2.S, AZD1222),Citation19,Citation20,Citation24 protein subunit vaccines (e.g., NVX-CoV2373),Citation25,Citation26 or inactivated vaccine (e.g., CoronaVac)Citation27,Citation28 results in relatively lower efficacy compared with mRNA-booster vaccines in individuals who have not received an mRNA vaccine as their primary series COVID-19 vaccine, especially with Omicron as the predominant circulating variant.Citation24,Citation29
To our knowledge, no study has reported the immunogenicity or safety of heterologous immunization involving initial immunization with an mRNA vaccine followed by inactivated vaccine booster. We conducted the present open-label, non-randomized, single-arm study in Japan to evaluate the immune responses to and the safety of KD-414, an investigational new COVID-19 inactivated vaccine,Citation7 after primary immunization with the BNT162b2 COVID-19 mRNA vaccine. We found that a single dose of KD-414 booster vaccine induced significant neutralizing antibodies against both the wild-type and Delta variant, especially at 40 days after the vaccination (), eliciting a SARS-CoV-2-S-specific T cell response (). When the KD-414-boosted participants received an additional booster dose of BNT162b2 as the fourth COVID-19 vaccine dose (two doses of BNT162b2 in the primary series, a single booster dose of KD-414, and a single booster dose of BNT162b2) at 43 days after KD-414 vaccination, the KD-414 group exhibited higher neutralizing activity against both the wild-type and Delta variant at 40 days after the booster dose of BNT162b2.
T cells play an important role in COVID-19 outcomesCitation30 and the maintenance of SARS-CoV-2 immunity, distinct from humoral immune kinetics.Citation31–33 Most T cell responses, whether induced by vaccination or natural infection, cross-recognize multiple amino acid mutations in variants, including the Omicron variant.Citation34 Therefore, the induction of T cell immunity against SARS-CoV-2 is an important goal for vaccine development,Citation35 especially in patients with B cell deficiencies due to, for example, hematopoietic malignancy or chemotherapyCitation36 and are thus more susceptible to a severe case of COVID-19. We observed the dynamic kinetics of the T cell response to SARS-CoV-2-S peptides during the study period using IGRA (). A significantly higher CD4+ T cell response to SARS-CoV-2-S1-RBD (Ag1) was induced at 40 days after KD-414 injection (KD40) compared with the non-KD-414 group (). Importantly both BNT162b2 and KD-414 induced SARS-CoV-2-S1/S2 subunit-specific CD4+ and CD8+ responses (), and these T cell responses varied greatly between the participants. Individuals with a stronger response to the primary vaccination series tended to have a stronger T cell response after the booster dose, regardless of whether it was KD-414 or BNT162b2. The mechanisms underlying such high responses should be investigated further.
Previous studies of adverse effects have indicated more robust and broad immunity responses with certain heterologous vaccine combinations (e.g., AZD1222 priming followed by BNT162b2 or mRNA-1273 boosting), as higher systemic reactions may occur in such heterologous vaccine combinations.Citation27,Citation37 Adenovirus-vector or inactivated vaccines are generally well tolerated.Citation38 In the present study, no local or systemic symptoms were reported more frequently by the participants who received a booster dose of KD-414 compared with those who received a booster dose of BNT162b2 (), which is a critical benefit for older adults, infants, and those who are reluctant for further vaccination because of adverse effects.Citation39
This study had some limitations. First, the sample size was smaller than initially planned (60 participants were planned, but only 49 participated). Second, this study was conducted as an open-label, non-randomized study; although the characteristics of the groups were mostly equivalent (). Third, since all the participants received an additional booster dose of BNT162b2 during the observation period, no long-term evaluation could be conducted. Fourth, we did not assess the viral neutralizing activity against multiple variants of concern, especially the Omicron variant, which has reduced susceptibility to neutralizing antibodies.Citation40–43 Further, no elevation of cytokines or chemokines such as IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, or tumor necrosis factor-α (TNF-α), was observed in the phase I/II clinical trial, even after administration of a primary series of KD-414;Citation7 therefore, a further detailed analysis should be conducted to assess CD4+ and CD8+ T cell responses in booster dosing.
In conclusion, the safety and immunogenicity evaluation results obtained in the present study indicate that KD-414 is a promising inactivated vaccine candidate for heterologous immunization after mRNA vaccine priming. KM Biologics initiated several clinical trials at the end of April 2022: i) a phase III trial to compare the immunogenicity, efficacy, and safety of three doses of KD-414 as a primary series with that of the primary series of ChAdOx1 nCoV-19 (AZD1222) in individuals aged 18–40 years (jRCT2031210679); and ii) a phase II/III trial to determine the appropriate dosing of the primary series of KD-414 in children and teens aged 6 months to 17 years (jRCT2031220032). These studies should also provide further evidence of the efficacy, dosage, and rational target of the KD-414 vaccine for clinical application.
Contributor
Conceptualization; M.U., W.S., Data curation; N.T., Y.T., J.S.-T., Formal analysis; Y.S. and Y.U., Funding acquisition; M.U., K.S., W.S., Investigation; M.U., Y.T., J.S.-T., Ko.M., Ke.M., Methodology; Y.U., J.-T.H., A.M., S.Y., A.F., Project administration; J.T.-H., A.M., Supervision; H.M., N.O., Writing – original draft; Y.T., J.S.-T., Y.S., Y.U., J.T.-H., N.T.; Writing – review & editing; Y.T., J.T.-H., and W.S.
Supplemental Material
Download PDF (483.7 KB)Acknowledgments
We thank all the individuals who participated in this study. This work was supported in part by KM Biologics Co., Ltd. and the Japan Agency for Medical Research and Development (AMED) (grant No. JP20fk0108417 to W.S.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Sho Saito, Katsue Watanabe, Yuriko Horikawa, and Shinsuke Kojima for managing the participants and administering the injections; Moto Kimura for collaboration with the company; Tetsuya Mizoue for providing information on previous studies; Akiko Kimura for coordinating the clinical research; Hiroko Aoshika and Keiko Hosokawa for assistance with the research administration; Yusuke Ohshiro for analyzing the specimens; Yasuharu Sasaki of the Japan Clinical Research Assist Center (JCRAC) Data Center for data management; Rina Yano for monitoring; Mariko Kato, Azusa Kamikawa, and Yumiko Kito for the technical assistance; and the Global Initiative for Infectious Diseases (GLIDE) support team for protocol development and administrative assistance.
Disclosure statement
Kengo Sonoda is an employee of KM Biologics Co., Ltd. All rights, titles, and interests to the patent of KD-414 have been assigned to KM Biologics Co., Ltd., Kumamoto, Japan. Mugen Ujiie received research funding from KM Biologics, Co., Ltd. for this and one other study. All other authors declare that they have no competing interests related to this study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2193074.
Additional information
Funding
References
- Mitsuya H. Fight against COVID-19 but avoid disruption of services for other communicable diseases (CDs) and noncommunicable diseases (NCDs). Glob Health Med. 2020;2(6):343–11. doi:10.35772/ghm.2020.01111. PMID: 33409412.
- R&D Blue Print Team, World Health Organization. COVID-19 vaccine tracker and landscape. Website Operator; 2022 Aug 23 [accessed 2022 Aug 25]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–27. doi:10.1038/s41586-020-2798-3. Epub 2020 Sep 23. PMID: 32967006.
- Epidemiology and prevention of vaccine-preventable diseases. The pink book: course textbook 14th edition. 2021 Washington, DC: Public Health Foundation, Centers for Disease Control and Prevention; [accessed 2022 Nov 10]. https://www.cdc.gov/vaccines/pubs/pinkbook/index.html.
- Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS-CoV-2 induced by infection or vaccination. J Intern Med. 2022;291(1):32–50. doi:10.1111/joim.13372. Epub 2021 Aug 5. PMID: 34352148.
- García-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162(1–2):12–18. doi:10.1016/j.virusres.2011.10.017. Epub 2011 Oct 21. PMID: 22027189.
- Tanishima M, Ibaraki K, Kido K, Nakayama S, Ata K, Nakamura H, Shinmura Y, Endo M, Sonoda K, Ueda K, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, KD-414, in healthy adult and elderly subjects: a randomized, double-blind, placebo-controlled, phase 1/2 clinical study in Japan. medRxiv. 2022. doi:10.1101/2022.06.28.22276794.
- Prime Minister’s Office of Japan. COVID-19 vaccines. [accessed 2023 Sep 4]. https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html.
- Sonoda K. Development of an inactivated COVID-19 vaccine. Translat Regulat Sci. 2021;3(3):120–1. doi:10.33611/trs.2021-022.
- Terayama Y, Tomita N, Terada-Hirashima J, Uemura Y, Shimizu Y, Takeuchi JS, Takamatsu Y, Maeda K, Mikami A, Ujiie M, et al. Protocol of an exploratory single-arm study to evaluate the safety and immunogenicity of KD-414 as a booster vaccine for SARS-CoV-2 in healthy adults (KAPIVARA). Life (Basel). 2022;12(7):966. doi:10.3390/life12070966. PMID: 35888056.
- Takeuchi JS, Fukunaga A, Yamamoto S, Tanaka A, Matsuda K, Kimura M, Kamikawa A, Kito Y, Maeda K, Ueda G, et al. SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2: a 9 months longitudinal study. Sci Rep. 2022;12(1):15447. doi:10.1038/s41598-022-19581-y. PMID: 36104370.
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373–4. Epub 2003 Jul 2. PMID: 11357217.
- Clinical Research Act. [accessed 2023 Nov 25]. https://www.mhlw.go.jp/file/06-Seisakujouhou-10800000-Iseikyoku/0000213334.pdf.
- World Health Organization. Considerations for the evaluation of COVID-19 vaccines. https://extranet.who.int/pqweb/sites/default/files/documents/Considerations_Assessment_Covid-19_Vaccines_v30March2022.pdf.
- Maeda K, Amano M, Uemura Y, Tsuchiya K, Matsushima T, Noda K, Shimizu Y, Fujiwara A, Takamatsu Y, Ichikawa Y, et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci Rep. 2021;11(1):22848. doi:10.1038/s41598-021-01930-y. PMID: 34819514.
- Takamatsu Y, Imai M, Maeda K, Nakajima N, Higashi-Kuwata N, Iwatsuki-Horimoto K, Ito M, Kiso M, Maemura T, Takeda Y, et al. Highly neutralizing COVID-19 convalescent plasmas potently block SARS-CoV-2 replication and pneumonia in Syrian hamsters. J Virol. 2022;96(4):e0155121. doi:10.1128/JVI.01551-21. Epub 2021 Nov 24. PMID: 34818068.
- Jaganathan S, Stieber F, Rao SN, Nikolayevskyy V, Manissero D, Allen N, Boyle J, Howard J. Preliminary evaluation of QuantiFERON SARS-CoV-2 and QIAreach anti-SARS-CoV-2 total test in recently vaccinated individuals. Infect Dis Ther. 2021;10(4):2765–76. doi:10.1007/s40121-021-00521-8. Epub 2021 Aug 25. PMID: 34435336.
- Takamatsu Y, Omata K, Shimizu Y, Kinoshita-Iwamoto N, Terada M, Suzuki T, Morioka S, Uemura Y, Ohmagari N, Maeda K, et al. SARS-CoV-2-neutralizing humoral IgA response occurs earlier but is modest and diminishes faster than IgG response. Microbiol Spectr. 2022 Dec 21; 10(6):e0271622. doi:10.1128/spectrum.02716-22. Epub 2022 Oct 11. PMID: 36219096.
- Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, Dernstedt A, Christ W, Tevell S, Evander M, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385(11):1049–51. doi:10.1056/NEJMc2110716. Epub 2021 Jul 14. PMID: 34260850.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. doi:10.1056/NEJMoa2116414. Epub 2022 Jan 26. PMID: 35081293.
- Tan SHX, Pung R, Wang LF, Lye DC, Ong B, Cook AR, Tan KB. Association of homologous and heterologous vaccine boosters with COVID-19 incidence and severity in Singapore. JAMA. 2022;327(12):1181–2. doi:10.1001/jama.2022.1922. PMID: 35147657.
- Mayr FB, Talisa VB, Shaikh O, Yende S, Butt AA. Effectiveness of homologous or heterologous COVID-19 boosters in veterans. N Engl J Med. 2022;386(14):1375–7. doi:10.1056/NEJMc2200415. Epub 2022 Feb 9. PMID: 35139265.
- Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, Wan H, Schubert M, Cassaniti I, Wang Y, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13(1):2670. doi:10.1038/s41467-022-30340-5. PMID: 35562366.
- Accorsi EK, Britton A, Shang N, Fleming-Dutra KE, Link-Gelles R, Smith ZR, Derado G, Miller J, Schrag SJ, Verani JR. Effectiveness of homologous and heterologous COVID-19 boosters against Omicron. N Engl J Med. 2022;386(25):2433–5. doi:10.1056/NEJMc2203165. Epub 2022 May 25. PMID: 35613039.
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–76. doi:10.1016/S0140-6736(21)02717-3. Epub 2021 Dec 2. Erratum in: Lancet. 2021;398(10318):2246. PMID: 34863358.
- Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36–49. doi:10.1016/S0140-6736(21)02718-5. Epub 2021 Dec 6. Erratum in: Lancet. 2022;399(10327):802. PMID: 34883053.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–9. doi:10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. PMID: 35074136.
- Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–95. doi:10.1016/S1473-3099(21)00681-2. Epub 2021 Dec 8. PMID: 34890537.
- Chen LL, Lu L, Choi CY, Cai JP, Tsoi HW, Chu AW, Ip JD, Chan WM, Zhang RR, Zhang X, et al. Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant-associated receptor binding domain (RBD) mutations on the susceptibility to serum antibodies elicited by coronavirus disease 2019 (COVID-19) infection or vaccination. Clin Infect Dis. 2022;74(9):1623–30. doi:10.1093/cid/ciab656. PMID: 34309648.
- Kedzierska K, Thomas PG. Count on us: t cells in SARS-CoV-2 infection and vaccination. Cell Rep Med. 2022;3(3):100562. doi:10.1016/j.xcrm.2022.100562. PMID: 35474748.
- Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, Takehara KK, Eggenberger J, Hemann EA, Waterman HR, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184(1):169–83.e17. doi:10.1016/j.cell.2020.11.029. Epub 2020 Nov 23. PMID: 33296701.
- Rodda LB, Morawski PA, Pruner KB, Fahning ML, Howard CA, Franko N, Logue J, Eggenberger J, Stokes C, Golez I, et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell. 2022;185(9):1588–601.e14. doi:10.1016/j.cell.2022.03.018. Epub 2022 Mar 17. PMID: 35413241.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi:10.1126/science.abf4063. Epub 2021 Jan 6. PMID: 33408181.
- Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, Khan K, Cele S, Bernstein M, Karim F, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–92. doi:10.1038/s41586-022-04460-3. Epub 2022 Jan 31. Erratum in: Nature. 2022;604(7907):E25. PMID: 35102311.
- Heitmann JS, Bilich T, Tandler C, Nelde A, Maringer Y, Marconato M, Reusch J, Jäger S, Denk M, Richter M, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature. 2022;601(7894):617–22. doi:10.1038/s41586-021-04232-5. Epub 2021 Nov 23. PMID: 34814158.
- Okamoto A, Fujigaki H, Iriyama C, Goto N, Yamamoto H, Mihara K, Inaguma Y, Miura Y, Furukawa K, Yamamoto Y, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6(11):3230–3. doi:10.1182/bloodadvances.2021006302. PMID: 35026843.
- Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, Campins M, Portolés A, González-Pérez M, García Morales MT, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacs): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–30. doi:10.1016/S0140-6736(21)01420-3. Epub 2021 Jun 25. Erratum in: Lancet. 2021;398(10300):582. PMID: 34181880.
- Kouhpayeh H, Ansari H. Adverse events following COVID-19 vaccination: a systematic review and meta-analysis. Int Immunopharmacol. 2022;109:108906. doi:10.1016/j.intimp.2022.108906. Epub 2022 May 30. PMID: 35671640.
- Stefanizzi P, Bianchi FP, Brescia N, Ferorelli D, Tafuri S. Vaccination strategies between compulsion and incentives. The Italian Green Pass experience. Expert Rev Vaccines. 2022;21(4):423–5. doi:10.1080/14760584.2022.2023012. Epub 2022 Jan 13. PMID: 34962214.
- Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–56.e11. doi:10.1016/j.cell.2021.12.032. Epub 2021 Dec 24. PMID: 35026151.
- Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, Fukushi S, Suzuki T, Maeda K, Halfmann P, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387(5):468–70. doi:10.1056/NEJMc2207519. Epub 2022 Jul 20. PMID: 35857646.
- Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, Iwatsuki-Horimoto K, Halfmann P, Watanabe S, Maeda K, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022;386(15):1475–7. doi:10.1056/NEJMc2201933. Epub 2022 Mar 9. PMID: 35263535.
- Amano M, Maeda K, Tsuchiya K, Shimada S, Mitsuya H. Third-dose BNT162b2 vaccination elicits markedly high-level SARS-CoV-2–neutralizing antibodies in vaccinees who responded poorly to a second dose in Japan. J Infect Dis. 2022;226:jiac209. doi:10.1093/infdis/jiac209. Epub ahead of print. PMID: 35580786.