ABSTRACT
β-N-Acetylglucosaminidases (GlcNAcases) possess many important biological functions and are used for promising applications that are often hampered by low-activity enzymes. We previously demonstrated that most GlcNAcases of the glycoside hydrolase (GH) family 20 showed higher activities than those of other GH families, and we presented two novel GH 20 GlcNAcases that showed higher activities than most GlcNAcases. A highly flexible structure, which was attributed to the presence of to a high proportion of random coils and flexible amino acid residues, was presumed to be a factor in the high activity of GH 20 GlcNAcases. In this study, we further hypothesized that two special positions might play a key role in catalytic activity. The increase in GH 20 GlcNAcase activity might correspond to the increased structural flexibility and substrate affinity of the two positions due to an increase in random coils and amino acid residues, notably acidic Asp and Glu.
Introduction
β-N-acetylglucosaminidases (GlcNAcases, EC 3.2.1.52) play a role in the hydrolysis of terminal N-acetyl-D-glucosamine residues in N-acetyl-β-D-glucosaminides. These enzymes possess many important biological functions and are involved in a wide range of industrial applications, such as dynamic balancing of cellular O-linked N-acetylglucosamine (GlcNAc) levelsCitation[1]; bacterial cell wall recyclingCitation[2]; proper assembly of bacterial flagellaCitation[3]; bioconversion of chitin waste to sialic acid[Citation4], bioethanol[Citation5], and single-cell proteinCitation[6]; and synthesis of biologically important oligosaccharides [Citation7,Citation8].
Chitin is a naturally abundant biomass and nitrogenous organic compound; 1012–1014 tons of chitin is produced annually, ranking second in worldwide production after lignocellulose[Citation9]. However, chitinous materials exhibit low natural degradation rates that can potentially become hazardous to environments [Citation10,Citation11]. Therefore, there is a growing demand for high-activity chitinases and GlcNAcases for the degradation of chitin in commercial, biotechnological, and environmental uses.
GlcNAcases are widely distributed among various natural sources including animals, plants, insects, bacteria, and fungi, and they belong to glycoside hydrolase (GH) families 3, 20, 73, 84, and 85[Citation12]. However, we previously found that most characterized GlcNAcases showed activities below 1000 μmol min−1 mg−1 toward p-nitrophenyl β-N-acetylglucosaminide (pNPGlcNAc) or 4-methylumbelliferyl β-N-acetylglucosaminide (MUGlcNAc), and approximately 70% of the characterized GlcNAcases showed activities below 100 μmol min−1 mg−1[Citation12].
Protein engineering and mining the genomic data of new organisms for novel enzymes are two methods for potentially obtaining highly active GlcNAcases. We previously revealed that most GlcNAcases of GH 20 showed higher activities than those of other GH families[Citation12], two of which were presented as exhibiting higher activity than most GlcNAcases reported in available literature: JB10Nag from Shinella sp. with a specific activity of 538.8 μmol min−1 mg−1 and a Vmax of 1030 μmol min−1 mg−1[Citation13], and HJ5Nag from Microbacterium sp. with a specific activity of 1773.1 μmol min−1 mg−1 and a Vmax of 3097 μmol min−1 mg−1[Citation14]. Furthermore, we analyzed the overall molecular characteristics of GH 20 GlcNAcases and indicated that a highly flexible structure, which was ascribed to a high proportion of random coils as well as flexible amino acid residues, was presumed to be a factor in the high activity of GH 20 GlcNAcases [Citation13,Citation14]. Improvements in the catalytic activity of GH 20 GlcNAcases can be rationally designed based on the general molecular characteristics.
According to the general molecular characteristics guide regarding the catalytic activity of GH 20 GlcNAcases, we further analyzed and compared the molecular characteristics of GH 20 GlcNAcases with various activities in the present study. The aim of this study was to reveal that which random coils and flexible amino acid residues may have a key role in the high activity of GH 20 GlcNAcases.
Materials and methods
GlcNAcases and strains
Yunnan Province, China is a source of diverse and novel genetic samples [Citation15,Citation16]. We previously isolated Shinella sp. JB10 from the slag collected from a phosphate rock-stacking site and Microbacterium sp. HJ5 from the saline soil of an old salt mine located in Yunnan Province [Citation13,Citation14]. Strains JB10 and HJ5 were deposited in the Strains Collection of the Yunnan Institute of Microbiology under registration nos. YMF 3.00678 and YMF 4.00007, respectively. Amino acid sequences of GH 20 GlcNAcases JB10Nag and HJ5Nag can be retrieved from GenBank with accession numbers AQM74372 and ARJ33352, respectively.
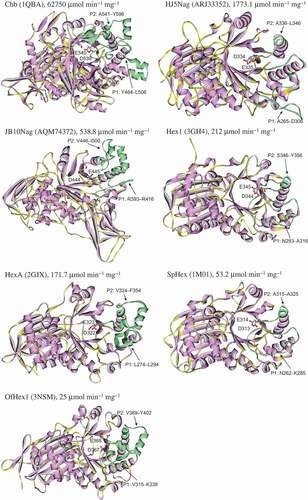
Besides JB10Nag and HJ5Nag, five GH 20 GlcNAcases exhibiting different specific activities and Vmax values were selected for bioinformatics analysis in this study. Crystal structures of the five GH 20 GlcNAcases have been resolved and published at the Protein Data Bank (PDB; https://www.rcsb.org/). The GlcNAcase Chb (PDB ID: 1QBA) from Serratia marcescens shows the highest activity among reported GlcNAcases to date, with a specific activity of 62 750 μmol min−1 mg−1 toward pNPGlcNAc [Citation17,Citation18]. The Vmax values of the other GlcNAcases are as follows: Hex1 (PDB ID: 3GH4) from Paenibacillus sp., 212 μmol min−1 mg−1 toward pNPGlcNAcCitation[19]; HexA (PDB ID: 2GJX) from Homo sapiens, 171.7 μmol min−1 mg−1 toward MUGlcNAc [Citation20,Citation21]; SpHex (PDB ID: 1M01) from Streptomyces plicatus, 53.2 μmol min−1 mg−1 toward MUGlcNAcCitation[22]; and OfHex1 (PDB ID: 3NSM) from Ostrinia furnacalis shows a specific activity of 53.2 μmol min−1 mg−1 toward pNPGlcNAc [Citation23,Citation24].
Bioinformatics analysis
Amino acid sequences of GH 20 GlcNAcases were aligned using Vector NTI 10.3 software (InforMax, Gaithersburg, MD, USA). The structure of HJ5Nag was previously modelled using the homology modelling approach with the SwissModel platform (http://swissmodel.expasy.org/) using SpHex as template, a GMQE score of 0.72, and a sequence identity of 48.9%[Citation14]. The structure of JB10Nag was previously modelled using the threading approach with the I-TASSER platform (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) with a determined C-score of 0.39 and a TM-score of 0.77[Citation13]. Tertiary structures of GH 20 GlcNAcases were visualized using Discovery Studio v2.5 software (Accelrys, San Diego, CA, USA).
Results
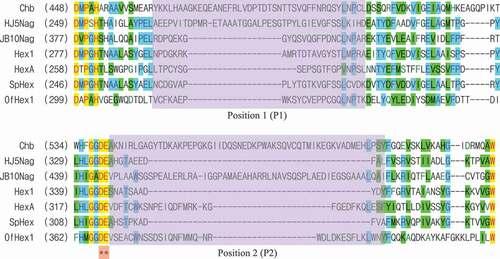
As shown in , there were two positions that showed great diversity among the examined GH 20 GlcNAcases. These two positions were located near the catalytic aspartic acid (Asp) residue that functions as a nucleophile/base and the glutamic acid (Glu) residue that functions as a proton donor/acceptor.
Position 1 (P1) of Chb contained 43 amino acid residues, of which 5 were Asp and Glu. P1 of HJ5Nag contained 42 amino acid residues, of which 7 were Asp and Glu. The other GlcNAcases contained fewer than 24 amino acid residues and 3 Asp and Glu (). Position 2 (P2) of Chb contained 56 amino acid residues, of which 8 were Asp and Glu. P2 of JB10Nag contained 55 amino acid residues, of which 6 were Asp and Glu. The other GlcNAcases contained fewer than 34 amino acid residues and 6 Asp and Glu (). Thus, Chb, HJ5Nag, and JB10Nag contained many more amino acid residues, including Asp and Glu, than other GlcNAcases in P1 and/or P2.
Table 1. Molecular characteristics of GH 20 GlcNAcases.
To investigate whether random coils were composed of amino acid residues at P1 and P2, structures of the GH 20 GlcNAcases were compared and shown in . Among the GH 20 GlcNAcases, HJ5Nag had the longest coil at P1, and JB10Nag had the longest coil at P2 (; ).
Discussion
Some studies have reported that enzyme activity can be enhanced by increasing flexibility [Citation25–Citation27] or reduced by decreasing flexibility, because the formation of the transition state is affected by the enzyme conformation for sampling higher-energy conformational substates[Citation28].
A high proportion of flexible residues, such as Asp and Glu that usually have high B-factor values, are believed to cause high structural flexibility. Three mutants of Candida antarctica lipase B, V139E, A151D, and I255E, showed enhanced specific activity relative to that of the wild type [Citation25,Citation27]. Chen et al. reported that both the I368E mutation in LXYL-P1-1 and the T368E mutation in LXYL-P1-2 could increase the catalytic activity toward 7–xylosyl-10-deacetyltaxol [Citation29,Citation30]. Therefore, the increase in the number of amino acid residues in P1 and P2, notably Asp and Glu, may lead to an increase in catalytic activity.
Higher protein flexibility is usually accompanied by a higher percentage of random coils at the expense of the α-helix structure. Increasing the loop flexibility can also enhance the catalytic activity of the enzyme[Citation31]. We previously calculated the ratios of random coils to α-helix structures in the seven GH 20 GlcNAcases [Citation13,Citation14]. The results showed that a high ratio of random coils to α-helix structures was related to a high activity in GH 20 GlcNAcases [Citation13,Citation14]. Therefore, the P1 structure of HJ5Nag and P2 structure of JB10Nag might be more flexible than those of most GH 20 GlcNAcases, and this increased flexibility might contribute to the higher activity of HJ5Nag and JB10Nag compared to most GH 20 GlcNAcases.
Considering that the number of amino acid residues in P2 of HJ5Nag was not greater than that of other GlcNAcases, P1 may play more important role than P2 in catalytic activity. Furthermore, structural analysis of bacterial and fungal GH 20 GlcNAcases revealed that P1 played a role in the specific binding of chitooligosaccharides[Citation32]. Notably, E328 in P1 of OfHex1 was confirmed to be necessary for substrate binding[Citation33].
Moreover, the charge of an amino acid residue can play a significant role in enzyme-substrate affinity, thus affecting catalytic activity. Tu et al. reported that the catalytic efficiency of endo-polygalacturonase PG8fn was improved by introducing basic Lys or Arg at position 113, as its positively charged side chain might provide a desirable environment for binding to the highly negatively charged substrate, polygalacturonic acid[Citation34]. It is well-known that chitin and chitooligosaccharides are positively charged while other polysaccharides and oligosaccharides are negatively charged. Thus, acidic Asp and Glu with negatively charged side chains in P1 and P2 might contribute to substrate affinity, therefore improving the catalytic performance of GH 20 GlcNAcases.
In conclusion, this study proposed that these two positions, especially P1, might affect the catalytic performance of GH 20 GlcNAcases. The increase in the activity of GH 20 GlcNAcases might correspond to the enhanced structural flexibility and substrate affinity exhibited by P1 and P2. These enhancements are likely caused by increases in random coils and the number of amino acid residues, notably acidic Asp and Glu. These results are useful for guiding protein engineering to improve the catalytic activity of GH 20 GlcNAcases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Li BB, Li H, Lu L, et al. Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat Struct Mol Biol. 2017;24:362–370. PMID:28319083.
- Litzinger S, Duckworth A, Nitzsche K, et al. Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by β-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase. J Bacteriol. 2010;192:3132–3143. PMID:20400549.
- Herlihey FA, Moynihan PJ, Clarke AJ. The essential protein for bacterial flagella formation FlgJ functions as a β-N-acetylglucosaminidase. J Biol Chem. 2014;289:31029–31042. PMID:25248745.
- Lee JO, Yi JK, Lee SG, et al. Production of N-acetylneuraminic acid from N-acetylglucosamine and pyruvate using recombinant human renin binding protein and sialic acid aldolase in one pot. Enzyme Microb Tech. 2004;35:121–125.
- Inokuma K, Hasunuma T, Kondo A. Ethanol production from N-acetyl-D-glucosamine by Scheffersomyces stipitis strains. AMB Express. 2016;6:83. PMID:27699702.
- Vyas P, Deshpande M. Enzymatic hydrolysis of chitin by Myrothecium verrucaria chitinase complex and its utilization to produce SCP. J Gen Appl Microbiol. 1991;37:267–275.
- Matsuo I, Kim S, Yamamoto Y, et al. Cloning and overexpression of β-N-acetylglucosaminidase encoding gene nagA from Aspergillus oryzae and enzyme-catalyzed synthesis of human milk oligosaccharide. Biosci Biotech Bioch. 2003;67:646–650. PMID:12723619.
- Rajnochova E, Dvorakova J, Hunkova Z, et al. Reverse hydrolysis catalysed by β-N-acetylhexosaminidase from Aspergillus oryzae. Biotechnol Lett. 1997;19:869–872.
- Kaur S, Dhillon GS. Recent trends in biological extraction of chitin from marine shell wastes: a review. Crit Rev Biotechnol. 2015;35:44–61. PMID:24083454.
- Da Silva AF, Garcia-Fraga B, Lopez-Seijas J, et al. Optimizing the expression of a heterologous chitinase: a study of different promoters. Bioengineered. 2017;8:428–432. PMID:27893301.
- Hamed I, Ozogul F, Regenstein JM. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Tech. 2016;48:40–50.
- Zhang R, Zhou JP, Song ZF, et al. Enzymatic properties of β-N-acetylglucosaminidases. Appl Microbiol Biot. 2018;102:93–103. PMID:29143882.
- Zhou JP, Song ZF, Zhang R, et al. A Shinella β-N-acetylglucosaminidase of glycoside hydrolase family 20 displays novel biochemical and molecular characteristics. Extremophiles. 2017;21:699–709. PMID:28432475.
- Zhou JP, Song ZF, Zhang R, et al. Distinctive molecular and biochemical characteristics of a glycoside hydrolase family 20 β-N-acetylglucosaminidase and salt tolerance. BMC Biotechnol. 2017;17:37. PMID:28399848.
- Shen JD, Zhang R, Li JJ, et al. Characterization of an exo-inulinase from Arthrobacter: a novel NaCl-tolerant exo-inulinase with high molecular mass. Bioengineered. 2015;6:99–105. PMID:25695343.
- Zhou JP, Liu Y, Shen JD, et al. Kinetic and thermodynamic characterization of a novel low-temperature-active xylanase from Arthrobacter sp. GN16 isolated from the feces of Grus nigricollis. Bioengineered. 2015;6:111–114. PMID:25587940.
- Tews I, Perrakis A, Oppenheim A, et al. Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay–sachs disease. Nat Struct Mol Biol. 1996;3:638–648. PMID:8673609.
- Tews I, Vincentelli R, Vorgias CE. N-Acetylglucosaminidase (chitobiase) from Serratia marcescens: gene sequence, and protein production and purification in Escherichia coli. Gene. 1996;170:63–67. PMID:8621090.
- Sumida T, Ishii R, Yanagisawa T, et al. Molecular cloning and crystal structural analysis of a novel β-N-acetylhexosaminidase from Paenibacillus sp. TS12 capable of degrading glycosphingolipids. J Mol Biol. 2009;392:87–99. PMID:19524595.
- Lemieux MJ, Mark BL, Cherney MM, et al. Crystallographic structure of human β-hexosaminidase A: interpretation of Tay–sachs mutations and loss of GM2 ganglioside hydrolysis. J Mol Biol. 2006;359:913–929. PMID:16698036.
- Hou Y, Tse R, Mahuran DJ. Direct determination of the substrate specificity of the α-active site in heterodimeric β-hexosaminidase A. Biochemistry. 1996;35:3963–3969. PMID:8672428.
- Mark BL, Wasney GA, Salo TJS, et al. Structural and functional characterization of Streptomyces plicatus β-N-acetylhexosaminidase by comparative molecular modeling and site-directed mutagenesis. J Biol Chem. 1998;273:19618–19624. PMID:9677388.
- Liu TA, Zhang HT, Liu FY, et al. Structural determinants of an insect β-N-acetyl-D-hexosaminidase specialized as a chitinolytic enzyme. J Biol Chem. 2011;286:4049–4058. PMID:21106526.
- Yang Q, Liu T, Liu FY, et al. A novel β-N-acetyl-D-hexosaminidase from the insect Ostrinia furnacalis (Guenee). FEBS J. 2008;275:5690–5702. PMID:18959754.
- Yagonia CFJ, Park HJ, Hong SY, et al. Simultaneous improvements in the activity and stability of Candida antarctica lipase B through multiple-site mutagenesis. Biotechnol Bioproc E. 2015;20:218–224.
- Hong SY, Park HJ, Yoo YJ. Flexibility analysis of activity-enhanced mutants of bacteriophage T4 lysozyme. J Mol Catal B-Enzym. 2014;106:95–99.
- Hong SY, Yoo YJ. Activity enhancement of Candida antarctica lipase B by flexibility modulation in helix region surrounding the active site. Appl Biochem Biotech. 2013;170:925–933. PMID:23625607.
- Bhabha G, Lee J, Ekiert DC, et al. A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science. 2011;332:234–238. PMID:21474759.
- Chen JJ, Liang X, Chen TJ, et al. Site-directed mutagenesis of a β-glycoside hydrolase from Lentinula edodes. Molecules. 2019;24:59. PMID:30586935.
- Chen JJ, Liang X, Li HX, et al. Improving the catalytic property of the glycoside hydrolase LXYL-P1-2 by directed evolution. Molecules. 2017;22:2133. PMID:29207529.
- Zheng F, Tu T, Wang XY, et al. Enhancing the catalytic activity of a novel GH5 cellulase GtCel5 from Gloeophyllum trabeum CBS 900.73 by site-directed mutagenesis on loop 6. Biotechnol Biofuels. 2018;11:76. PMID:29588661.
- Liu T, Duan YW, Yang Q. Revisiting glycoside hydrolase family 20 β-N-acetyl-D-hexosaminidases: crystal structures, physiological substrates and specific inhibitors. Biotechnol Adv. 2018;36:1127–1138. PMID:29597028.
- Liu T, Zhou Y, Chen L, et al. Structural insights into cellulolytic and chitinolytic enzymes revealing crucial residues of insect β-N-acetyl-D-hexosaminidase. PLoS One. 2012;7:e52225. PMID:23300622.
- Tu T, Pan X, Meng K, et al. Substitution of a non-active-site residue located on the T3 loop increased the catalytic efficiency of endo-polygalacturonases. Process Biochem. 2016;51:1230–1238.