?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
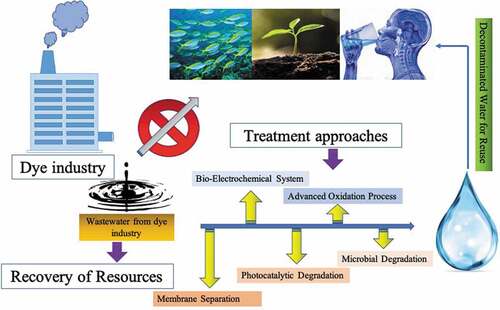
Rapid industrialization has provided comforts to mankind but has also impacted the environment harmfully. There has been severe increase in the pollution due to several industries, in particular due to dye industry, which generate huge quantities of wastewater containing hazardous chemicals. Although tremendous developments have taken place for the treatment and management of such wastewater through chemical or biological processes, there is an emerging shift in the approach, with focus shifting on resource recovery from such wastewater and also their management in sustainable manner. This review article aims to present and discuss the most advanced and state-of-art technical and scientific developments about the treatment of dye industry wastewater, which include advanced oxidation process, membrane filtration technique, microbial technologies, bio-electrochemical degradation, photocatalytic degradation, etc. Among these technologies, microbial degradation seems highly promising for resource recovery and sustainability and has been discussed in detail as a promising approach. This paper also covers the challenges and future perspectives in this field.
1. Introduction
Rapid progress in industrialization in last few years has increased discharge of pollutants into environment [Citation1,Citation2]. Discard of municipal and other industrial wastes into water bodies causes water pollution [Citation3,Citation4]. Industries release non-treated and/or incompletely treated wastewater in the environment which leads water and soil pollution. Mostly pharmaceutical, textile, food, paper & pulp and cosmetic industries uses synthetic dyes for manufacturing process. Dyeing procedure of textile industry uses a massive volume of water in fixing, dyeing, and washing procedures [Citation5]. The dyes are soluble organic compounds, specifically those classified as direct, reactive, acidic and basic [Citation6]. Dyes exhibit high solubility in water and make it more difficult to be removed by conventional procedures [Citation7].
The atmosphere is mainly polluted by release of crude and unprocessed waste in the ecosystem which mainly contains majority emissions produced by industrial activities [Citation8–10, Citation117]. Textile industries emit greenhouse gases by manufacturing processes and release hazardous gases and pollutants which are harmful for human, and plants [Citation11]. Most dyes contain mutagenic, toxic, and carcinogenic properties [Citation12,Citation13].
Industrial dyes as well as their by-products are main contaminants that are carcinogenic and toxic. Hence, they lead harmful effects on ecosystem [Citation14]. Various reports are available which shows harmful effects of azo dyes on plants (plant growth and germination) [Citation15–18].
Industrial pollutants are one of the biggest environmental problems due to their effect on groundwater and surface water along with human health [Citation2,Citation19,Citation20]. Various technologies such as advanced oxidation process, membrane filtration technique, microbial technologies, bio-electrochemical degradation, photocatalytic degradation have been reported for treatment of dye industry wastewater [Citation3,Citation21–24]. This paper presents and discusses advanced technical and scientific developments about dye industry wastewater treatments. This paper focus on microbial degradation of dyes as it has been reported as highly promising for resource recovery and sustainability. This paper also includes the bottlenecks and perspectives in this field of research.
2. Dye industry processing
The dye industrial wastewater treatment procedures are a unique key feature in effective trading of dye industry products. The dye industries produce fibers that are further converted into yarn and lastly transformed into textile materials. These materials are prepared by using steps of wet processing. In the textile coloring procedure, unsettled dye gets wash out with the water. Dry procedure produce large amount of solid waste as compared to the wet procedure.
Industrial manufacturing procedure includes basic steps for industrial processing as narrated below. Mainly, industrial procedure releases different types of pollutants like dyes, metals, crude oil and hydrocarbons, etc.
2.1. Sizing and desizing
Sizing is an important process that helps to increase strength of yarn and decrease breakage of yarn as well as scuffs its resistance. Sizing acts as a glaze or defensive material to adjust physical appearance of constituents. Selection of sizing agents is based on environmental friendliness, types of fabric, effluent treatments, cost-effective, easy removal, etc. Most sizing agents are natural elements like starch, protein-based starch, by-products of cellulose, etc., and other synthetic agents like polyacrylates, PVA (Polyvinyl Alcohol), Acrylic resins and polyesters, etc [Citation25,Citation26]. Starch is used in sizing procedure and it is degradable in H2O and CO2 by oxidation process and desizing enzymes can be converted into ethanol. Ethanol is additionally used as an energy source and also reduce BOD level in treatment process [Citation27].
Desizing is a procedure of eliminating the sizing agent from textile fabrics and blends to prepare the fabric for further processing. Starch and its derivatives are mostly used because of their easy availability, brilliant film forming capacity, and relatively low cost. Completion of this procedure can be carried out by using enzyme desizing, acid desizing, oxidative desizing, or by removing water-soluble sizes. α-amylase is used to solubilize starch by hydrolyzing starch at pH between 5.5 to 7.5 in enzymatic desizing of textile fabrics [Citation28]. Desizing process lower pH of the waste around 4–5 pH with a higher BOD (Biological Oxygen Demand) (300–450 ppm). Desizing processing step release glucose, resins, waxes, PVA, volatile organic compounds, starch and fats, etc [Citation29].
2.2. Scouring
Chemical washing procedure is known as scouring. This method is used to eliminate contaminations like surfactants, waxes, fatty acids and natural oils from fibers by bridge scour, hydrodynamic scour, ice scour and tidal scour [Citation26,Citation30]. Detergents, NaOH, oils, fats, spent solvents, wax, surfactants, alkylbenzene sulfonates, alkylphenol and ethoxylates are compounds released during scouring process [Citation31].
2.3. Bleaching
In this procedure, undesirable color or dye can be removed from fibers by using different chemicals like sodium hypochlorite, peracetic acid, chlorine, hypochlorite, and hydrogen peroxide. The natural color substance gives a creamy look to the fabrics. Peracetic acid has been reported as more beneficial because it is less destructive to yarn and elevated sheen. Bleaching processing step release hypochlorite, caustic soda, acids, chlorine, hydrogen peroxide, etc [Citation32].
2.4. Mercerization
Mercerization is a treatment process that includes use of strong alkaline (18–24% by weight) solution for yarn materials to improve shine of the material. This process involves several steps such as (i) dipping yarn fabrics into highly concentrated alkaline solution, (ii) to remove alkali, fabric is placed into the water. This process improves fabric absorbing capacity, smoothness, strength and reaction capability with various chemicals. Mercerizing step releases caustic soda, ZnCl2, cyclohexanols and it also increses pH of wastewater [Citation33,Citation34].
2.5. Dyeing and printing
The dyeing procedure involves addition of color to material and treatment with dye. The most required factors in this procedure are temperature and time. The effluent from printing and dyeing both involve presence of waste materials. Dyeing step releases reducing agents (sulfides), dyestuff, soap, mordant, acetic acids, metal salt, cationic materials, surfactants and chromophores in wastewater [Citation35,Citation36].
2.6. Finishing
Finishing process in textile manufacturing industry transforms knitted or woven material into usable material with better-quality and certain properties like waterproofing and smoothness. This final procedure mostly participates in water pollution [Citation36,Citation37]. Finishing processing step release salts, traces of starch, special finishes, tallow, etc [Citation34].
3. Characteristics of dye industry wastewater
The effluent discharged from dye industries contain a mixture of metals, dyes and other pollutants. Two types of industrial wastes (liquid and solid) are frequently formed due to manufacturing processes and they contain any material that can be reduced through product manufacturing procedure. Liquid waste (i.e. wastewaters released from industries) is dangerous to existing living organisms as well as to the environment as it also carries different types of toxic contaminants. However, the characteristics and nature of industrial effluent are based on type of manufacturing process and products [Citation38].
The wastewater produced through the wet process from dye industries is marked by a high level of dissimilarities in many parameters like chemical oxygen demand (COD), pH, total solids (TS), biological oxygen demand, water usage, and color. Wastewater having 0.25 BOD/COD ratio determines that the industrial wastewater having vast actions of organic material which is non-biodegradable. Composition of industrial wastewater is based on various types of raw materials, chemicals, organic-based compounds and different types of dyes used in wet and dry processing phases. The industrial manufacturing procedure discards unsafe and colored dyes mostly azo dyes. These dyes are a vital base of environmental pollution that causes harmful effects on aquatic life because of its low biodegradability, strong color, high COD and BOD. Despite the pollutants, dye industrial wastewater contains adjustable ionic strength, high salt concentration, and pH variations.
Process waste is generated during different industrial manufacturing procedures. This type of waste is highly polluted and contains high COD and BOD levels, color pigments, pH, high salt concentration, etc [Citation39,Citation40].
The dyes can be classified as synthetic dyes and natural dyes. The synthetic dyes are easily produced in different colors and characterized by their fastness, so it is more widely used dye as compared to natural dyes. Synthetic dyes are classified into different groups (i) based on their mode of application like direct, reactive, basic, disperse and vat dying, etc. and (ii) based on their chemical structure like anthraquinone, azo, phthalocyanine, sulfur and triarylmethane, etc [Citation41].
The azo dyes are characterized by comparatively high recalcitrance and high polarization. When particles contain other acidic groups like carboxyl, hydroxyl or sulfonyl substituents azo dyes can be categorized by amphoteric properties. The azo dyes are cationic, anionic, or nonionic. Existence of an amino group causes high water solubility and high mobility in comparison with hydrocarbons, high boiling point and a lower Henry’s law constant [Citation42].
3.1. Effects of azo dyes on plants and human health
Textile industries release number of hazardous pollutants and which are toxic and carcinogenic effects in their nature [Citation43]. Hence, they cause environmental degradation and various diseases in humans, animals and plants [Citation44]. Red-S3B (3.19% N) azo dye is the most toxic to growth and germination of 7 days old wheat saplings. Aspergillus terreus NIAB-FM10 and Shewanella sp. NIAB-BM15 were found to be more effective for degradation of Red-S3B. The mixture of these two species give complete decolorization of Red-S3B (500 mg) in 4 hour. After treatment with industrially important consortium increased shoot length and root length, shoot biomass, root biomass of 30 day old wheat saplings were noted [Citation45].
Sudan I dye (Solvent Yellow 14) belongs to the family of azo-lipophilic complexes commonly used in industrial segments [Citation46]. When they present in the humans and animal bodies, it is enzymatically transformed into carcinogenic aromatic amines through the action of the intestinal flora [Citation47].
Basic Red 9 dye having high environmental toxicity and carcinogenicity, it breaks down under anaerobic condition into carcinogenic aromatic amines and have potential for skin irritation, cancer itself, allergic dermatitis, and mutations [Citation48–51].
4. Treatment process for dye industry wastewater
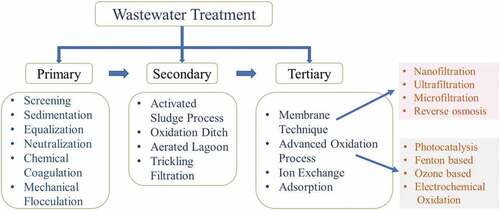
The dye industrial wastewater treatment process is mainly classified into three types: (i) biological, (ii) chemical and (iii) physical treatments. General wastewater treatment includes preliminary treatment, primary treatment, secondary treatment and tertiary treatment to treat dye industry effluents. Preliminary treatment includes neutralization and equalization [Citation34]. The primary treatment includes sedimentation, screening, chemical coagulation, flocculation and floatation. Secondary treatment includes chemical/physical separation or biological oxidation and used to reduce organic compounds. Tertiary treatment is more significant than others because it enhances effluent treatment [Citation52]. shows classification of wastewater treatment types.
4.1. Preliminary treatment
The first step in wastewater treatment is equalization and mixing wastewater streams that were discharged at different intervals from different stages during the manufacturing process. Equalization confirms that the waste has a uniform characteristics in terms of pH, pollution load and temperature [Citation52].
4.2. Primary treatment
The primary treatment involves screening, sedimentation and floatation. However, by use of steps above some suspended, fine and colloidal elements cannot be efficiently removed [Citation53]. In some cases, chemical coagulation and mechanical flocculation is employed [Citation52]. In chemical coagulation, addition of coagulants like ferrous sulfate (FeSO4), polyelectrolyte, alum, ferric chloride (FeCl3), lime [Ca(OH)2] are used for flash mixing. This procedure was carried out on flocculation and settling tank or in clariflocculator. Iron salts and aluminum are commonly used as coagulants in water and wastewater treatment [Citation54]. Chemical treatments are used to reduce suspended solids, color, COD and BOD. Chemical coagulation procedure efficiently decolorizes insoluble dyes, but it is not very much effective in reduction of soluble dyes [Citation55,Citation56].
4.3. Secondary treatment
In secondary wastewater treatment, color and dissolved or colloidal organic material present in dye wastewater is stabilized [Citation57]. This procedure is carried out with the help of fungi, bacteria, algae, yeast and other microbes. This procedure can be anaerobic or aerobic. In aerobic procedure bacteria, algae and some other microbes utilize organic compounds as food and show successive modifications: (i) Flocculation and coagulation of colloidal compounds, (ii) Oxidation of dissolved organic compounds to CO2, and (iii) Degradation of nitrogenous organic compounds to ammonia, which is then converted into nitrite and then nitrate. Anaerobic treatment is mostly used for digestion of waste. The capability of this procedure depends on temperature, pH, absence of oxygen, waste loading and presence of toxic material. Aerobic treatment for azo dyes has proven unsuccessful in maximum cases however, nowadays it is used as typical treatment methods [Citation52,Citation55,Citation56].
4.4. Tertiary treatment
The dye industrial wastewater contains various types of hazardous dyes. They require advanced treatment method or tertiary treatment to remove particular pollutants. Generally, tertiary treatment is used to remove organic color compounds by adsorption and dissolved solids by membrane filtration techniques. The wastewater can be treated with ozone (O3) or another oxidizing agent to destroy many contaminants [Citation52].
5. Treatment methods
The recent treatment approaches for dye industrial wastewater include membrane separation, adsorption, advanced oxidation procedures (AOPs), bio-electrochemical treatments and photocatalytic degradation for reduction of organic pollutants from industrial effluent [Citation58–60]. depicts treatment technologies for dye industrial wastewater.
Table 1. Treatment technologies for dye industrial wastewater
5.1. Membrane techniques
Membrane separation is an advanced technology for wastewater treatment. In this process, wastewater is allowed to pass through a porous membrane. If any solute is bigger than membrane pore size than it will be trapped and rest of the solution will pass through the membrane. The trapped solutes from filter cake or layer, are removed constantly during the filtration procedure. The membrane separation procedures are classified based on size of porous membranes.
Pressure-driven membrane procedures can be divided into four main classes, (i) RO (Reverse osmosis), (ii) UF (Ultrafiltration), (iii) MF (Microfiltration) and (iv) NF (Nanofiltration) [Citation61]. NF membranes contain 0.1 to 10 mm pore size with the lowest applied pressure. UF membranes contain 2 to 100 nm pore size with high applied pressure and low water permeability. MF and UF involve the same sieving mechanism, which is innovative and sustainable technology [Citation62]. UF is used for the recycling and separation of water-insoluble dyes such as disperse dye and indigo dye, whereas NF and RO procedures are used to hydrolyze reactive dyes from dye wastewater. MF is generally not used for wastewater treatment because of their large pore size [Citation63,Citation64]. Type of membrane filter used for separation depends on numerous factors like nature of dye, dyeing process and chemical composition of pollutants, etc. Membrane used for reverse osmosis and ultra-filtration are generally prepared from different polymers like polyacrylonitrile, polysulphonates, polycarbonate, polyamides, fluorocarbon-based polymers and polypropylene etc [Citation32,Citation56].
5.1.1. Ultrafiltration
Ultrafiltration technique requires lower pressure than reverse osmosis and nanofiltration, thus make it more efficient. Polyether sulfone (PES) membrane is used for removal of dyes from wastewater. PES contains 1kDa and 10kDa porous membranes used for dyes removal. 1kDa polyether sulfone (PES) membrane gives 80% to 100% dye removal whereas 10kDa polyether sulfone (PES) membrane is not useful for the removal of dyes. The ultrafiltration technique is more suitable to be used as a pre-treatment procedure [Citation65].
5.1.2. Nanofiltration
NF shows higher permeability and low transmembrane pressure. The energy consumption in NF is lower than MF [Citation66]. Hence, NF is one type of competitive technique to treat dye industrial wastewater. In the past decade many researchers have focused on removal of reactive dyes from dye industrial wastewater. Eg. [Citation67], found the rejection of Reactive Black-5 up to 98% by Nanofiltration membranes. [Citation68], reported 99.5% removal of reactive brilliant blue (KN-R) from dye industrial wastewater using ES404 polyether-sulfone (PCI, UK) membrane.
5.1.3. Microfiltration
This method is used as a pre-treatment procedure for nanofiltration or reverses osmosis. Microfiltration with 0.1–1 μm porous membrane was used for removal of dye pigments from the dye industrial effluent [Citation35,Citation61].
5.1.4. Reverse osmosis
Reverse osmosis procedure is used to eliminate chemical compounds as well as decolorization of different dyes from dye wastewater. Decolorization and removal of chemical complexes from dyehouse wastewater can be passed out in a single phase of reverse osmosis procedure. RO membrane has 90% retention rate for most types of ionic compounds. Reverse osmosis supports elimination of chemical compounds, hydrolyzed reactive dyes and minimal salts [Citation35,Citation61].
5.2. Advanced oxidation processes
The advanced oxidation process (AOP) has been reported as one of the efficient procedure to reduce organic pollutants from dye industry effluent from the environment and improving availability of organic contaminants free water for humanity. AOP is a most suitable technique for degradation of organic dyes by radiation of visible light due to its eco-friendly nature, complete degradation, low cost, increase reusability of water and decrease in the pollutant load [Citation69–71].
5.2.1. Combined bio-advanced oxidation process
Citation72, reported that combined biological treatment with AOPs treatment (Bio-AOP) gives 100% decolorization of Remazol Red (RR), Reactive Black 5 and Reactive Red 180 (RR 180) by Aeromonas hydrophila SK16. However for individual treatment 72% decolorization rate was reported. Combination of AOPs treatment with biological treatment is more effective than single wastewater treatment.
5.2.2. Photocatalytic degradation
Photocatalytic degradation has been reported for treatment of dye containing wastewater. Heterogeneous photocatalytic oxidation is one of the most important technique among the AOPs which is commonly known as photo-catalysis [Citation73,Citation74]. Semiconductor nanoparticles are used for photocatalytic degradation of organic contaminants. Many oxides contain suitable bandgap for photocatalytic reactions. TiO2 (Titanium Dioxide) is used in photocatalytic degradation due to its nontoxic nature, chemical stability and environmental compatibility [Citation24]. The use of TiO2 has been recognized as a proficient AOP and versatile because of its ability to produce reactive oxidative species (like O2- and •OH, ROS) which is highly desired for photocatalytic degradation of organic pollutants and dyes [Citation75]. The combination of coagulation and flocculation procedure with TiO2- modified UF membrane gives best results for degradation of reactive black 5 dye [Citation76].
[Citation77], have reported photocatalytic experiments and noted that Zeo-TiO2 mixture gives best result in photocatalytic performance on degradation of Rhodamine B dye than Zeo-ZnO composites under UV radiation. Zeo-TiO2 catalyst has excellent recycling stability.
Reactions responsible for photocatalytic degradation of dye can be written as below [Citation78].
In photocatalytic degradation technique, UV radiation is required for photocatalytic activation. Thus, TiO2 photocatalysts show low absorption in visible light and because of that efficiency of TiO2 interrupts in natural sunlight. Light absorbing properties of TiO2 can be spread by doping TiO2 with metals. Correct doping can improve photo-reactivity of TiO2 in both visible and UV light [Citation79–81].
Factors that are responsible for different photocatalytic action of manufactured materials are the nature of cations (Ni, Cu), irradiation source, light power and photo-catalyst synthesis methods [Citation82]. Nickle and copper metal ions are generally used as bimetallic substance and have been described as the effective process to improve effectivity of many reactions, such as (i) Cu and Ni for photocatalytic water splitting [Citation83], (ii) Cu TiO2/ZnO for Methyl Orange (MO) [Citation14,Citation23,Citation84], (iii) doping TiO2 with low concentrations of Co and Ni or Fe and Cu recovers its photocatalytic effectiveness [Citation85]. Literature is available which shows use of Cu or Ni-based TiO2 photocatalysts for degradation of dye, like methylene blue and methyl orange under UV irradiation [Citation14,Citation23,Citation84].
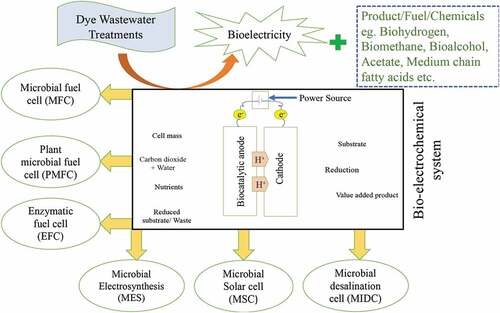
5.3. Bio-electrochemical system
BES is a developing technology to improve energy and environment relevant problems by making wastewater treatment procedures more sustainable and more economical. The bio-electrocatalytic reaction combined with extracellular electron transfer can drive several procedures such as synthesizing chemicals, producing electricity from wastewater, removing pollutants and desalinating seawater [Citation86,Citation87]. Removal of untreated wastewater from different industries such as textile, dyestuff, paper, etc., involves 70% identified commercial dyes which includes common chromophores in reactive dyes. Bio-electrochemical systems (BESs) contain great potential for azo dye removal [Citation88]. [Citation88], used bio-electrochemical systems to decrease the concentration of Reactive Black 5 (RB5) from 0.503 ± 0.002 mM to 0.124 ± 0.007 mM after 10 h of operating. shows schematics of different types of bio-electrochemical systems (BES) and their applications.
5.4. Microbial degradation
Various microorganisms like bacteria, algae, fungi and yeast can be used for dye degradation [Citation89].
5.4.1. Bacterial biodegradation
Many bacterial strains are used to degrade dyes in aerobic or anaerobic conditions [Citation90]. Pseudomonas luteola, Xanthophilus azovorans, Klebsiella pneumonia, Clostridium perfringens, are used in azo dye degradation. The genetically engineered E. coli strain gives increased azo reductase activity [Citation91].
[Citation92], has found that the Aeromonas hydrophila LZ-MG14 was able to degrade 96.8% (200 mg L−1) malachite green (MG) within 12 h from dye industry wastewater. Bioaugmentation by Aeromonas hydrophila LZ-MG14 in a membrane bioreactor [MBR) improved the efficiency of malachite green degradation. [Citation93], have reported that Pseudomonas aeruginosa and Bacillus subtilis can reduce 92.13% and 88.21% Allura Red (R-40] dye respectively, under microaerophilic conditions. Halomonas sp. strain was isolated from coastal sediments which was contaminated by chemical wastewater and was found to give 90% azo dye degradation in 24 hours using yeast extract as a carbon source at temperature 30° C. The result showed that bacterial strain decolorizes different azo dyes in higher saline conditions [Citation94].
[Citation95], studied the complete degradation of Methyl Orange (sulfonated azo dye) by Bacillus stratospheric SCA1007. Bacillus stratospheric SCA1007 gave comprehensive degradation of Methyl Orange (150 mg/L) in a different range of dye concentration, at 7 pH and 35°C temperature under static condition. The degradation of azo dyes was studied by Fourier Transform Infrared Spectroscopy (FTIR) and Ultra-violet Visible spectroscopy (UV-vis). Toxicity studies were done on Vigna radiata and E. coli to check the nontoxic nature of degraded products. shows degradation of different dyes using bacteria.
Table 2. Degradation of different dyes using bacteria
5.4.2. Fungal biodegradation
Filamentous fungi can grow on range of ecological niches like living plants, soil and organic waste because of their speedy adaptation and metabolism on varying nitrogen and carbon sources. Fungi produce a huge quantity of extracellular and intracellular enzymes with degrading capability of many types of organic contaminants, like dye effluents, organic waste, steroid compounds and polyaromatic hydrocarbons. Various studies have reported on biodegradation of azo dyes by using white-rot fungi [Citation32]. Mycoremediation has been reported as a safe, low-cost and natural procedure for dye removal [Citation96].
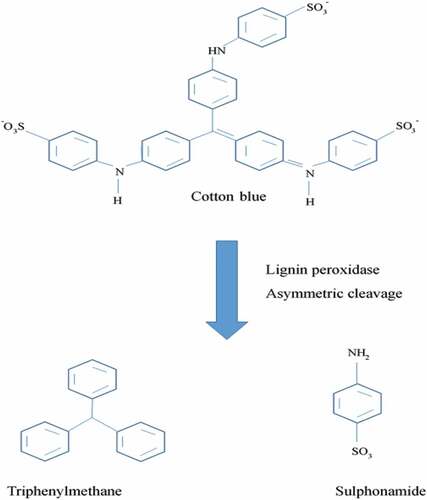
Enzymes used for textile dye degradation: The use of enzymes for degradation of textile dye is the most popular method. Triphenylmethane dyes come from the most significant group of synthetic dyes and are used extensively in textile dye industries. They are usually included as xenobiotic compounds. Penicillium ochrochloron decolorizes cotton blue dye within 2 hrs under static condition at temperature 25 °C and 6.5 pH.
shows pathway for the degradation of cotton blue as model dye. HPLC, FTIR and TLC analysis confirms biodegradation of cotton blue. GC-MS and FTIR spectroscopy analysis showed triphenylmethane and sulfonamide as the final products of cotton blue degradation. Temperature, pH and biomass affected rate of decolorization. Presence of tyrosinase, lignin peroxidase and aminopyrine N-demethylase activities in the cell as well as increase in extracellular action of lignin peroxidase proposes role of these enzymes in the decolorization procedure [Citation97].
[Citation98], have reported that A. flavus strain F10 was able to reduce up to 98–99% (150 mg/L MG) malachite green after six days of incubation under optimum experimental conditions. Malachite green decolorization mechanisms approved by A. flavus include enzymatic reactions like hydroxylation, demethylation and ring cleavage. The immobilized growing cells are more stable and reusable than free cells for decolorization and dye degradation. shows degradation of different dyes using fungi.
Table 3. Degradation of different dyes using fungi
5.4.3. Algal biodegradation
Algae are photosynthetic microbes and they are universally spread in a wide range of surroundings. Studies reported that azo dye degradation by algae can be induced by azoreductase. Some algal species like Oscillatoria and Chlorella were able to transform toxic aromatic amines into simple metabolic intermediates like water and CO2 [Citation99]. [Citation100], have reported 98.20% and 94.19% removal of methylene blue using C. pyrenoidosa and Spirulina maxima, respectively.
5.4.4. Yeast biodegradation
Numerous yeasts have been reported having ability to degrade dyes by metabolic activity such as enzymatic, biosorption, or a mixture of both [Citation101]. Several yeasts species like Candida tropicali, Debaryomyces polymorphous [Citation102], Candida albicans [Citation103], and Issatchenkia occidentalis [Citation104] were reported for decolorization and enzymatic biodegradation of different azo dyes. shows degradation of different dyes using algae and yeast.
Table 4. Degradation of different dyes using algae and yeast
6. Dye industry waste and resource recovery strategy
Industrial waste can be used in resource recovery process, as input material for recovering value-added products. The main aim of these processes is to reduce the amount of waste generated from industries [Citation105]. [Citation106], have investigated that hybrid loose nanofilter bipolar membrane electro dialysis (BMED) procedure system can be used for removal of dye, and salt and can recycle water from dye effluent. Movable nanofiltration membrane (Ultra, Sepro NF 6) contains outstanding diafiltration performance for fractionation of (reactive and direct) dye/salt mixtures, high retention of dyes (> 99.93%) and permit the free passage of salt (R ≤ 2.2%). The addition of BMED can appreciate the making of acid or base and pure water from the salt-containing nanofillers.
Microbial fuel cell (MFC) technology is mostly used for wastewater treatment as well as energy generation. MFC technology is also used to remove odor and salinity from sea water [Citation107]. [Citation108], have reported use of hybrid BMED and tight ultrafiltration (TUF) procedure for resource recovery (i.e. dye extraction, pure water regeneration and, acid/base conversion) from extremely salted wastewater. They have reported that TUF membrane of 5000 Da MW can attain enough rejection rate [about >99.6%) of both direct dye and reactive dye, because of the dye collection. They have concluded that the reported procedures can be applied to sustainable management of textile wastewater.
[Citation109], have found that commercial and sustainable chemicals can be used to recover cotton from dye wastewater. The technology involves three progressive methods: (i] leaching of textile dyes by using Nitric Acid as a pre-treatment of original waste, (ii) Dimethyl Sulfoxide (DMSO) used in dissolution procedure and (iii) bleaching procedure used diluted hydrochloric acid and sodium hypochlorite for final recovered cotton.
[Citation118], have studied bioenergy generation and decolorization of dye by using microbial fuel cell [MFC) from dye wastewater which has shown 940.61 ± 5 mW/m2 power density with 790 ± 5 mV voltage output generation and 83% decolorization. [Citation110], have reported that the methylene blue (91.9%] and CR(VI) [90.3%) recovered by using combined technical procedure with multifunctional adsorbents. [Citation111], have reported that 66.7 ± 4.7% phosphate and 66 ± 5.3% nitrogen recovery by using multi-ion exchange membrane (IEM] stack microbial electrolysis desalination cell [MEDC). [Citation112], have found that the maximum volumetric power density (123.2 ± 27.5 mW/m3] achieved by using a single-chamber microbial fuel cell (SCMFC). Other technologies like hydrothermal gasification, microbial fuel cell, constructed wetland and microbial fuel cell, etc. have been reported for degradation of dyes [Acid Orange 7, Congo Red, etc) with recovery of value-added products like bio-energy, bio-fuel etc. [Citation113], have reported electricity generated by phytoremediation technology using microalgal strains viz. Anabaena ambigua, Chlorella pyrenoidosa, and Scenedesmus abundans.
7. Challenges and perspectives
Dye industrial wastewater can be used as a promising source for recovery of value-added products and treated wastewater can alos be used for agriculture applications. Overall, while dye(s] degradation using microbial system is quite promising, use of single culture is time consuming whereas; use of microbial consortium or mixed culture decreases degradation time and leads improved removal of pollutants. In this regard, membrane based processes offer potential performance benefits, especially when they are coupled with electrochemical advanced oxidation procedures such as photoelectron-catalysis, electro-Fenton and electro-catalysis, etc. However, membrane blockage and fouling still remain as major challenge when it comes to the use of pressure driven membrane process. Bio-electrochemical systems play an important role in the resource recovery process by producing clean energy, low sludge and microbial biomass.
Future studies should focus on developing pure culture as well mixed cultures-based bioremediation processes in which microbial strains should be improved for developing desirable characteristics employing tools and approaches of genetic engineering, proteomics and metabolic engineering. To fix membrane blocking and fouling issues, the research focus should be continued to develop new membranes with anti-fouling properties. Also, studies should be carried out to analyze and evaluate toxicity of degradation products due to microbial action and determine their application(s) as value-added products. Technical and economic feasibility of the treatment methods should be critically studied i.e. it is yet another way to perform in depth studies on dye degradation. Amount of the chemical used with particular textile process should be studied in detail. Scale-up of the processes with focus on resource recovery and sustainability would open up the way for integration of various technologies for treatment of dye industrial waste with simultaneous recovery of resources from it.
8. Conclusions
Dyes are generally released by textile, paper, pulp, tannery, cosmetic and leather industries. These types of dyes are mostly toxic when released in the environment. Substantial advancement has been made on the treatment and management of dye wastewater, which could be treated employing biological, physical and chemical methods among which biological or advanced eco-friendly methods seem most appropriate as they offer potential opportunities for resource recovery (in form of energy or chemicals) and are sustainable in nature. This review paper summarized state-of-art information and also provided scientific data that would be used for generating new opportunities in research and scientific innovations in this area.
Highlights
Treatment technologies for dye industrial wastewater have been summarised.
Recovery of resources from dye industry wastewater have been narrated.
In depth studies are necessary to close knowledge gaps in field of bioremediation of pollutants released from dye industry
Integration of technologies is necessary for speedy and higher remediation of pollutants.
Supplemental Material
Download ()Acknowledgements
SV, PR and TS are grateful to the management of the Gujarat Pollution Control Board, Gandhinagar, Gujarat, India for providing necessary facilities to perform the literature review presented in this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
-
ta for this article can be accessed here
References
- Das A, Dey A. P-Nitrophenol-Bioremediation using potent Pseudomonas strain from the textile dye industry effluent. J Environ Chem Eng. 2020;8(4):103830.
- Sivasubramaniam D, Franks AE. Bioengineering microbial communities: their potential to help, hinder and disgust. Bioengineered. 2016;7:137–144.
- Lakshmi S, Suvedha K, Sruthi R, et al. Hexavalent chromium sequestration from electronic waste by biomass of Aspergillus carbonarius. Bioengineered. 2020;11(1):708–717.
- Varjani SJ, Upasani VN. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol. 2017;232:389–397.
- Rajkumar D, Kim J. Oxidation of various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewater treatment. J Hazard Mater. 2006;136(2):203–212.
- Mahapatra NN. Textile dyes. New Delhi, India: CRC Press, WPI; 2016.
- Hassan MM, Carr CM. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere. 2018;209:201–219.
- Bhatia SC. Pollution control in textile industry. Delhi, India: WPI; 2017. ( Survesh Devraj, (ed.)).
- Orts F, Del Río AI, Molina J, et al. Electrochemical treatment of real textile wastewater: Trichromy Procion HEXL@. J Electroanal Chem. 2018;808:387–394.
- Wang DM. Environmental protection in clothing industry. In: Zhu L, Ouadha A, editors. sustainable development: proceedings of the 2015 International Conference on sustainable development (ICSD2015). Singapore: World Scientific. 2016. p. 729–735. DOI:10.1142/9789814749916_0076.
- Akhtar S, Baig SF, Saif S, et al. Five year carbon footprint of a textile industry: A podium to incorporate sustainability. Nat Environ Pollut Technol. 2017;16:125–132.
- Aquino JM, Rocha-Filho RC, Ruotolo LAM, et al. Electrochemical degradation of a real textile wastewater using β-PbO2 and DSA® anodes. Chem Eng J. 2014;251:138–145.
- Khatri J, Nidheesh PV, Singh ATS, et al. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem Eng J. 2018;348:67–73.
- Saravanan R, Gupta VK, Mosquera E, et al. Visible light induced degradation of methyl orange using βAg0.333V2O5 nanorod catalysts by facile thermal decomposition method. J Saudi Chem Soc. 2015;19:521–527.
- Imran M, Crowley DE, Khalid A, et al. Microbial biotechnology for decolorization of textile wastewaters. Rev Environ Sci Biotechnol. 2014;4:73–92.
- Mahmood F, Shahid M, Hussain S, et al. Potential plant growth-promoting strain Bacillus sp. SR-2-1/1 decolorized azo dyes through NADH-ubiquinone: oxidoreductase activity. Bioresour Technol. 2017;235:176–184.
- Rehman K, Shahzad T, Sahar A, et al. Effect of reactive black 5 azo dye on soil processes related to C and N cycling. PeerJ. 2018. DOI:10.7717/peerj.4802.
- Shafqat M, Khalid A, Mahmood T, et al. Evaluation of bacteria isolated from textile wastewater and rhizosphere to simultaneously degrade azo dyes and promote plant growth. J Chem Technol Biotechnol. 2017;92(10):2760–2768.
- Anas M, Han-Dong S, Mahmoud K, et al. Photocatalytic degradation of organic dye using titanium dioxide modified with metal and non-metal deposition. Mater Sci Semicond Process. 2016;41:209–218.
- Vidales MJM, Nieto-Márquez A, Morcuende D, et al. 3D printed floating photocatalysts for wastewater treatment. Catal Today. 2019;328:157–163.
- Bharathiraja B, Selvakumari IAE, Iyyappan J, et al. Itaconic acid: an effective sorbent for removal of pollutants from Dye Industry effluent. Curr Opinion Environ Sci Health. 2019;12:6–17.
- Foteinis S, Monteagudo JM, Durán A, et al. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci Total Environ. 2018;612:605–612.
- Khaki MRD, Shafeeyan MS, Raman AAA, et al. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J Mol Liq. 2018;258:354–365.
- Yu A, Wang Q, Wang J, et al. Rapid synthesis of colloidal silver triangular nanoprisms and their promotion of TiO2 photocatalysis on methylene blue under visible light. Catal Commun. 2017;90:75–78.
- Choo KH, Choi SJ, Hwang ED. Effect of coagulant types on textile wastewater reclamation in a combined coagulation/ultrafiltration system. Desalination. 2007;202(1–3):262–270.
- Madhav S, Ahamad A, Singh P, et al. A review of textile industry: wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manag. 2018;27(3):31–41.
- Sarayu K, Sandhya S. Current technologies for biological treatment of textile wastewater-a review. Appl Biochem Biotechnol. 2012;167(3):645–661.
- Maiti S. Biotechnology in textile wet processing. Glo J Biomed Sci. 2018;2:7–13.
- Harane RS, Adivarekar RV. Sustainable processes for pre-treatment of cotton fabric. Text Cloth Sustain. 2017;2:2.
- Chatha SA, Asgher M, Iqbal HM. Enzyme-based solutions for textile processing and dye contaminant biodegradation-a review. Environ Sci Pollut Res. 2017;24(16):14005–14018.
- Sarayu K, Sandhya S. Aerobic biodegradation pathway for Remazol Orange by Pseudomonas aeruginosa. Appl Biochem Biotechnol. 2010;160(4):1241–1253.
- Shah M, Banerjee A. Combined application of physico-chemical microbiological processes for industrial effluent treatment plant. Singapore: Springer; 2020. DOI:10.1007/978-981-15-0497-6.
- Correia VM, Stephenson T, Judd JS. Characterisation of textile wastewaters - a review. Environ Technol. 1994;15(10):917–929.
- Ghaly AE, Ananthashankar R, Alhattab MVVR, et al. Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol. 2014;5:1–18.
- Babu BR, Parande AK, Raghu S, et al. Cotton textile processing: waste generation and effluent treatment. Text Technol. 2007;11:141–153.
- Holkar CR, Jadhav AJ, Pinjari DV, et al. A critical review on textile wastewater treatments: possible approaches. J Environ Manag. 2016;182:351–366.
- Kant R. Textile dyeing industry an environmental hazard. Nat Sci. 2012;4:22–26.
- Saxena G, Bharagava RN. Organic and inorganic pollutants in industrial wastes: ecotoxicological effects, health hazards and bioremediation approaches. In: Bharagava RN, editor. Environmental pollutants and their bioremediation approaches. Uttar Pradesh, India: CRC Press; 2017. p. 23–56. DOI:10.1201/9781315173351-3.
- Yaseen DA, Scholz M. Shallow pond systems planted with Lemna minor treating azo dyes. Ecol Eng. 2016;94:295–305.
- Yaseen DA, Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol. 2018;16:1193–1226.
- Popli S, Patel UD. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol. 2014;12(1):405–420.
- Carmen Z, Daniela S. Textile organic dyes-characteristics, polluting effects and separation/elimination procedures from industrial effluents-a critical overview. In: Organic pollutants ten years after the stockholm convention environmental and analytical update. Vol. 10. 2012. p. 32373. london, UK: Intech Open.
- Sharma B, Dangi AK, Shukla P. Contemporary enzyme based technologies for bioremediation: A review. J Environ Manage. 2018;210:10–22.
- Khan S, Malik A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ Sci Pollut Res Int. 2018;25(5):4446–4458.
- Shakeel M, Imran M, Ashraf M, et al. Biodegradation by co‐inoculated bacteria and fungi alleviates adverse effects of red‐S3B on growth and nitrogen uptake of wheat. Clean–Soil, Air, Water. 2020;48(3):1900305.
- Petrakis EA, Cagliani LR, Tarantilis PA, et al. Sudan dyes in adulterated saffron (Crocus sativus L.): identification and quantification by 1H NMR. Food Chem. 2017;217:418–424.
- Piatkowska M, Jedziniak P, Olejnik M, et al. Absence of evidence or evidence of absence? A transfer and depletion study of Sudan I in eggs. Food Chem. 2018;239:598–602.
- Duman O, Tunc S, Polat TG. Adsorptive removal of triarylmethane dye (Basic Red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Micropor Mesopor Mat. 2015;210:176–184.
- Foguel MV, Ton XA, Zanoni MV, et al. A molecularly imprinted polymer-based evanescent wave fiber optic sensor for the detection of basic red 9 dye. Sensor Actuat B-Chem. 2015;218:222–228.
- Lacasse K, Baumann W. Textile chemicals: environmental data and facts. Dortmund: Springer; 2012.
- Sivarajasekar N, Baskar R. Adsorption of basic red 9 on activated waste Gossypium hirsutum seeds: process modeling, analysis and optimization using statistical design. J Ind Eng Chem. 2014;20(5):2699–2709.
- Atul K, Pratibha C, Poonam V. A ccomparative study on the treatment methods of textile dye effluents. J Chem Pharm Res. 2012;4(1):763–771.
- Mandal B, Purkayastha A, Prabhu AA, et al. Development in wastewater treatment plant design. Emerging Technol Environ Bioremediation. 2020;311–321. DOI:10.1016/B978-0-12-819860-5.00013-4.
- Khouni I, Marrot B, Moulin P, et al. Decolourization of the reconstituted textile effluent by different process treatments: enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination. 2011;268(1–3):27–37.
- Ghaly AE, Ananthashankar R, Alhattab M, et al. Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Technol. 2013;5:182.
- Thamaraiselvan C, Noel M. Membrane processes for dye wastewater treatment: recent progress in fouling control in critical reviews. Environ Sci Technol. 2015;45(10):1007–1040.
- Xue F, Tang B, Bin L, et al. Residual micro organic pollutants and their biotoxicity of the effluent from the typical textile wastewater treatment plants at Pearl River Delta. Sci Total Environ. 2019;657:696–703.
- Ahmad A, Mohd-Setapar SH, Chuong CS, et al. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015;5(39):30801–30818.
- Rott U, Minke R. Overview of wastewater treatment and recycling in the textile processing industry. Water Sci Technol. 1999;40:137–144.
- Varjani S, Joshi R, Srivastava VK, et al. Treatment of wastewater from petroleum industry: current practices and perspectives. Env Sci Pollut Res. 2019;1–9. DOI:10.1007/s11356-019-04725-x.
- Mani S, Chowdhary P, Bharagava RN. Textile wastewater dyes: toxicity profile and treatment approaches. In: Bharagava R, Chowdhary P, editors. Emerging and eco-friendly approaches for waste management. Singapore: Springer; 2018. p. 219–244. DOI:10.1007/978-981-10-8669-4_11.
- Goh PS, Wong TW, Lim JW, et al. Innovative and sustainable membrane technology for wastewater treatment and desalination application. In: Galanakis CM, editor. Innovation strategy in environmental science. Netherlands: Elsevier; 2020. p. 291–319. DOI:10.1016/B978-0-12-817382-4.00009-5.
- Singh K, Arora S. Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. critical reviews. Environ Sci Technol. 2011;41(9):807–878.
- Wu B. Membrane-based technology in greywater reclamation: A review. Sci Total Environ. 2019;656:184–200.
- Aouni A, Fersi C, Cuartas-Uribe B, et al. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination. 2012;297:87–96.
- Cao XL, Yan YN, Zhou FY, et al. Tailoring nanofiltration membranes for effective removing dye intermediates in complex dye-wastewater. J Membr Sci. 2019;595:117476.
- Tang C, Chen V. Nanofiltration of textile wastewater for water reuse. Desalination. 2002;143(1):11–20.
- He Y, Li G, Wang H, et al. Diafiltration and water recovery of Reactive Brilliant Blue KN-R solution by two-stage membrane separation process. Chem Eng Process. 2010;49(5):476–483.
- Bahramian A, Rezaeivala M, He K, et al. Enhanced visible-light photoelectrochemical hydrogen evolution through degradation of methyl orange in a cell based on coral-like Pt-deposited TiO2 thin film with sub-2 nm pores. Catal Today. 2018;335:333–344.
- Huang D, Hu C, Zeng G, et al. Combination of Fenton processes and biotreatment for wastewater treatment and soil remediation. Sci Total Environ. 2017;574:1599–1610.
- Upadhyay GK, Rajput JK, Pathak TK, et al. Tailoring and optimization of hybrid ZnO:TiO2: cdOnanomaterials for advance oxidation process under visible light. Appl Surf Sci. 2020;509:145326.
- Thanavel M, Kadam SK, Biradar SP, et al. Combined biological and advanced oxidation process for decolorization of textile dyes. SN Appl Sci. 2018;1:97.
- Pandey S, Mandari KK, Kim J, et al. Recent advancement in visible‐light‐responsive photocatalysts in heterogeneous photocatalytic water treatment technology. In: Fosso‐Kankeu E, Pandey S, Ray SS, editors. Photocatalysts in advanced oxidation processes for wastewater treatment. 2020. p. 167–196. Hoboken, USA: Wiley Online Library. DOI:10.1002/9781119631422.ch6.
- Rauf MA, Ashraf SS. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem Eng J. 2009;151:1–3.
- Covei M, Perniu D, Bogatu C, et al. CZTS-TiO2 thin film heterostructures for advanced photocatalytic wastewater treatment. Catal Today. 2019;321-322:172–177.
- Beluci NDCL, Mateus GAP, Miyashiro CS, et al. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TIO2-modified membranes to improve the removal of reactive black 5 dye. Sci Total Environ. 2019;664:222–229.
- Alakhras F, Alhajri E, Haounati R, et al. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites. Surf Interfaces. 2020;20:100611.
- Hassan A, Asad M, Jechan L, et al. Photocatalysts for degradation of dyes in industrial effluents: opportunities and challenges. Nano Res. 2019;2(5):955–972.
- Riaz N, Chong F, Man Z, et al. Preparation, characterization and application of Cu-Ni/TiO2 in Orange II photodegradation under visible light: effect of different reaction parameters and optimization. RSC Adv. 2016;6:55650–55665.
- Riaz N, Chong FK, Dutta BK, et al. Photodegradation of Orange II under visible light using Cu–Ni/TiO2: effect of calcination temperature. Chem Eng J. 2012;185-186:108–119.
- Riaz N, Chong FK, Man ZB, et al. Photodegradation of Orange II under visible light using Cu–Ni/TiO 2: influence of Cu:Ni mass composition, preparation, and calcination temperature. Ind Eng Chem Res. 2013;52(12):4491–4503.
- Soto-Arreola A, Huerta-Flores AM, Mora-Hernández JM, et al. Comparative study of the photocatalytic activity for hydrogen evolution of MFe2O4 (M = Cu, Ni) prepared by three different methods. J Photochem Photobiol A Chem. 2018;357:20–29.
- Ibrahim S, Majeed I, Qian Y, et al. Novel hetero-bimetallic coordination polymer as a single source of highly dispersed Cu/Ni nanoparticles for efficient photocatalytic water splitting. Inorg Chem Front. 2018;5:1816–1827.
- Saravanan R, Manoj. D, Jiaqian Q, et al. Mechanothermal synthesis of Ag/TiO2 for photocatalytic methyl orange degradation and hydrogen production. Process Saf Environ Prot. 2018;120:339–347.
- Roselin LS, Alyoubi MM, Mousa SM, et al. Transformation of commercial TiO2 into anatase with improved activity of Fe, Cu and Cu-Feoxides loaded TiO2. J Nano Sci Nanotechnol. 2019;19:1098–1104.
- Sayed ET, Shehata N, Abdelkareem MA, et al. . Recent progress in environmentally friendly bio-electrochemical devices for simultaneous water desalination and wastewater treatment. Sci Total Environ. 2020;748:141046.
- Yuan Y, Zhang J, Xing L. Effective electrochemical decolorization of azo dye on titanium suboxide cathode in bioelectrochemical system. Int J Environ Sci Technol. 2019;16:8363–8374.
- Yang HY, Liu J, Wang YX, et al. Bioelectrochemical decolorization of a reactive diazo dye: kinetics, optimization with a response surface methodology, and proposed degradation pathway. Bioelectrochemistry. 2019;128:9–16.
- Lellis B, Fávaro-Polonio CZ, Pamphile JA, et al. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3(2):275–290.
- Varjani S, Rakholiya P, Ng HY, et al. Microbial degradation of dyes: an overview. Bioresour Technol. 2020;314:123728.
- Kumar A, Kumar A, Singh R, et al. Genetically engineered bacteria for the degradation of dye and other organic compounds. In: Singh P, Kumar A, Borthakur A, editors. Abatement of environmental pollutants, trends and strategies. 2020. p. 331–350. DOI:10.1016/B978-0-12-818095-2.00016-3.
- Jing J, Kulshreshtha S, Kakade A, et al. Bioaugmentation of membrane bioreactor with Aeromonas hydrophila LZ-MG14 for enhanced malachite green and hexavalent chromium removal in textile wastewater. Int Biodeter Biodeg. 2020;150:104939.
- Herrera-García S, Aguirre-Ramírez M, Torres-Pérez J. Comparison between Allura Red dye discoloration by activated carbon and azo bacteria strain. Environ Sci Pollut Res. 2020;27:29688–29696.
- Guo J, Zhou J, Wang D, et al. A novel moderately halophilic bacterium for decolorizing azo dye under high salt condition. Biodegradation. 2007;19:15–19.
- Akansha K, Chakraborty D, Sachan SG. Decolorization and degradation of methyl orange by Bacillus stratosphericus SCA1007. Biocatal Agric Biotechnol. 2019;18:101044.
- Jain A, Yadav S, Nigam VK, et al. Fungal-mediated solid waste management: a review. In: Prasad R, editor. Mycoremediation and environmental sustainability in fungal biology. Springer; 2017. p. 153–170. DOI:10.1007/978-3-319-68957-9_9.
- Shedbalkar U, Dhanve R, Jadhav J. Biodegradation of triphenylmethane dye cotton blue by Penicillium ochrochloron MTCC 517. J Hazard Mater. 2008;157(2–3):472–479.
- Barapatre A, Aadil KR, Jha H. Biodegradation of malachite green by the ligninolytic fungus Aspergillus flavus. CLEAN - Soil, Air, Water. 2017;45(4):1600045.
- Acuner E, Dilek F. Treatment of tectilon yellow 2G by Chlorella vulgaris. Process Biochem. 2004;39(5):623–631.
- Lebron YAR, Moreira VR, Santos LVS, et al. Remediation of methylene blue from aqueous solution by Chlorella pyrenoidosa and Spirulina maxima biosorption: equilibrium, kinetics, thermodynamics and optimization studies. J Environ Chem Eng. 2018;6(5):6680–6690.
- Yu Z, Wen X. Screening and identification of yeasts for decolorizing synthetic dyes in industrial wastewater. Int Biodeterior Biodegradation. 2005;56(2):109–114.
- Yang Q. Decolorization of synthetic dyes and production of manganese-dependent peroxidase by new fungal isolates. Biotechnol Lett. 2003;25(9):709–713.
- Vitor V, Corso CR. Decolorization of textile dye by Candida albicans isolated from industrial effluents. J Ind Microbiol Biot. 2008;35(11):1353–1357.
- Ramalho PA, Cardoso MH, Cavaco-Paulo A, et al. Characterization of azo reduction activity in a novel ascomycete yeast strain. Appl Environ Microbiol. 2004;70(4):2279–2288.
- Wang H, Luo H, Fallgren PH, et al. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol Adv. 2015;33(3–4):317–334.
- Lin J, Ye W, Huang J, et al. Toward resource recovery from textile wastewater: dye extraction, water and base/acid regeneration using a hybrid NF-BMED process. ACS Sustain Chem Eng. 2015;3(9):1993–2001.
- Do MH, Ngo HH, Guo WS, et al. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci Total Environ. 2018;639:910–920.
- Lin J, Lin F, Chen X, et al. Sustainable management of textile wastewater: A hybrid tight ultrafiltration/bipolar-membrane electrodialysis process for resource recovery and zero liquid discharge. Ind Eng Chem Res. 2019;58(25):11003–11012.
- Yousef S, Tatariants M, Tichonovas M, et al. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour Conserv Recycl. 2019;145:359–369.
- Ding J, Pan Y, Li L, et al. Synergetic adsorption and electrochemical classified recycling of Cr(VI) and dyes in synthetic dyeing wastewater. Chem Eng J. 2019;384:123232.
- Li J, Liu R, Zhao S, et al. Simultaneous desalination and nutrient recovery during municipal wastewater treatment using microbial electrolysis desalination cell. J Clean Prod. 2020;261:121248.
- Logrono W, Pérez M, Urquizo G, et al. Single chamber microbial fuel cell (SCMFC) with a cathodic microalgal biofilm: A preliminary assessment of the generation of bioelectricity and biodegradation of real dye textile wastewater. Chemosphere. 2017;176:378–388.
- Brar A, Kumar M, Vivekanand V, et al. Phycoremediation of textile effluent-contaminated water bodies employing microalgae: nutrient sequestration and biomass production studies. Int J Environ Sci Technol. 2018;16:7757–7768.
- Saxena G, Kishor R, Bharagava RN. Application of microbial enzymes in degradation and detoxification of organic and inorganic pollutants. In: Bioremediation of industrial waste for environmental safety. 2019. p. 41–51. Singapore;.Springer. DOI:10.1007/978-981-13-1891-7_3.
- Ngang HP, Ooi BS, Ahmad AL, et al. Preparation of PVDF–TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties. Chem Eng J. 2012;197:359–367.
- Yu M, Wang J, Tang L, et al. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: mechanisms, recent advances and environmental applications. Water Res. 2020;175:115673.
- Mishra, varjani, kumar, Awasthi, Awasthi, sindhu, binod, rene, zhang. Microbial approaches for remediation of pollutants: innovations, future outlook, and challenges. Energy Environ. 2020;1–30. DOI:10.1177/0958305X19896781.
- Mishra S, Nayak JK, Maiti A. Bacteria-mediated bio-degradation of reactive azo dyes coupled with bio-energy generation from model wastewater. Clean Technol Environ Policy. 2020;22:651–667.
- Rahmani AR, Navidjouy N, Rahimnejad M, et al. Application of the eco-friendly bio-anode for ammonium removal and power generation from wastewater in bio-electrochemical systems. J Clean Prod. 2020;243:118589.
- Eslami H, Shariatifar A, Rafiee E, et al. Decolorization and biodegradation of reactive Red 198 Azo dye by a new Enterococcus faecalis - Klebsiella variicola bacterial consortium isolated from textile wastewater sludge. World J Microbiol Biotechnol. 2019;35:38.
- Ayed L, Bekir K, Achour S, et al. Exploring bioaugmentation strategies for azo dye CI Reactive Violet 5 decolourization using bacterial mixture: dye response surface methodology. Water Environ J. 2016;31(1):80–89.
- Guadie A, Tizazu S, Melese M, et al. Biodecolorization of textile azo dye using Bacillus sp. strain CH12 isolated from alkaline lake. Biotechnol Rep. 2017;15:92–100.
- Talha MA, Goswami M, Giri BS, et al. Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour Technol. 2018;252:37–43.
- Mehta R, Singhal P, Singh H, et al. Insight into thermophiles and their wide-spectrum applications. 3 Biotech. 2016;6(1):81.
- Li H, Zhang R, Tang L, et al. Evaluation of Bacillus sp. MZS10 for decolorizing Azure B dye and its decolorization mechanism. J Environ Sci. 2014;26(5):1125–1134.
- Maiti S, Sinha SS, Singh M. Microbial decolorization and detoxification of emerging environmental pollutant: cosmetic hair dyes. J Hazard Mater. 2017;338:356–363.
- Chanwala J, Kaushik G, Dar MA, et al. Process optimization and enhanced decolorization of textile effluent by Planococcus sp. isolated from textile sludge. Environ Technol Innov. 2018;13:122–129.
- Siddique R, Alif FA. Isolation and identification of orange M2R and green GS dye decolourizing Bacteria from textile sludge (soil) samples and determination of their optimum decolourization conditions. Ann Res Rev Biol. 2018;22:1–12.
- Anjaneya O, Souche SY, Santoshkumar M, et al. Decolorization of sulfonated azo dye Metanil Yellow by newly isolated bacterial strains: bacillus sp. strain AK1 and Lysinibacillus sp. strain AK2. J Hazard Mater. 2011;190(1–3):351–358.
- Carolin CF, Kumar PS, Joshiba GJ. Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technol Environ. 2020;1–9. DOI:10.1007/s10098-020-01934-8.
- Amin S, Rastogi RP, Chaubey MG, et al. Degradation and toxicity analysis of a reactive textile diazo dye-direct Red 81 by newly isolated Bacillus sp. DMS2. Front Microbiol. 2020;11:576680.
- Mani A, Hameed SAS. Improved bacterial-fungal consortium as an alternative approach for enhanced decolourisation and degradation of azo dyes: a review. Net Environ Pollut Technol. 2019;18:49–64.
- Manai I, Miladi B, El Mselmi A, et al. Industrial textile effluent decolourization in stirred and static batch cultures of a new fungal strain Chaetomium globosum IMA1 KJ472923. J Environ Manag. 2016;170:8–14.
- Sinha A, Osborne WJ. Biodegradation of reactive green dye (RGD) by indigenous fungal strain VITAF-1. Int Biodeter Biodegr. 2016;114:176–183.
- Munck C, Thierry E, Gräßle S, et al. Biofilm formation of filamentous fungi Coriolopsis sp. on simple muslin cloth to enhance removal of triphenylmethane dyes. J Environ Manag. 2018;214:261–266.
- Krishnamoorthy R, Jose PA, Ranjith M, et al. Decolourisation and degradation of azo dyes by mixed fungal culture consisted of Dichotomomyces cejpii MRCH 1-2 and Phoma tropica MRCH 1-3. J Environ Chem Eng. 2018;6:588–595.
- Shanmugam S, Ulaganathan P, Sivasubramanian S, et al. Trichoderma asperellum laccase mediated crystal violet degradation - Optimization of experimental conditions and characterization. J Environ Chem Eng. 2017;5:222–231.
- Khan R, Fulekar MH. Mineralization of a sulfonated textile dye Reactive Red 31 from simulated wastewater using pellets of Aspergillus bombycids. Bioresour. Bioprocess. 2017;4:23.
- Sayahi E, Ladhari N, Mechichi T, et al. Azo dyes decolourization by the laccase from Trametes trogii. J Text I. 2016;107(11):1478–1482.
- Bankole PO, Adekunle AA, Obidi OF, et al. Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungus, Peyronellaea prosopidis. Sustain Enviro Res. 2018;28(5):214–222.
- Barathikannan K, Ramasamy KP, Manohar CS, et al. Diversity and decolorization potential of fungi isolated from the coral reef regions off Kavaratti. India Indian J Geo-Mar Sci. 2017;46(3):497–503.
- Tochhawng L, Mishra VK, Passari AK, et al. Endophytic fungi: role in dye decolorization. In: Singh B, editor. Advances in endophytic fungal research. In fungal biology. Cham: Springer; 2019. p. 1–15. DOI:10.1007/978-3-030-03589-1_1.
- Rodríguez-Couto S. Fungal laccase: a versatile enzyme for biotechnological applications. In: Yadav A, Mishra S, Singh S, et al, editors. Recent advancement in white biotechnology through fungi. Fungal biology. Cham: Springer; 2019. p. 429–457. DOI:10.1007/978-3-030-10480-1_13.
- Rekik H, Jaouadi NZ, Bouacem K, et al. Physical and enzymatic properties of a new manganese peroxidase from the white-rot fungus Trametes pubescens strain i8 for lignin biodegradation and textile-dyes biodecolorization. Int J Biol Macromol. 2018;125:514–525.
- Patel VR, Bhatt NS, Bhatt H. Involvement of ligninolytic enzymes of Myceliophthora vellerea HQ871747 in decolorization and complete mineralization of Reactive Blue 220. Chem Eng J. 2013;233:98–108.
- Ortiz-Monsalve S, Valente P, Poll E, et al. Biodecolourization and biodetoxification of dye-containing wastewaters from leather dyeing by the native fungal strain Trametes villosa SCS-10. Biochem Eng J. 2018;141:19–28.
- Vantamuri AB, Kaliwal BB. Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech. 2016;6:189.
- Singh G, Dwivedi SK. Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ Technol Innov. 2020;18:100751.
- Riegas-Villalobos A, Martínez-Morales F, Tinoco-Valencia R, et al. Efficient removal of azo-dye Orange II by fungal biomass absorption and laccase enzymatic treatment. 3 Biotech. 2020;10:146.
- Sinha S, Singh R, Chaurasia AK, et al. Self-sustainable Chlorella pyrenoidosa strain NCIM 2738 based photobioreactor for removal of Direct Red-31 dye along with other industrial pollutants to improve the water-quality. J Hazard Mater. 2016;306:386–394.
- López-Miranda JL, Silva R, Molina GA, et al. Evaluation of a dynamic bioremediation system for the removal of metal ions and toxic dyes using Sargassum Sp. J Mar Sci Eng. 2020;8(11):899.
- Al-Fawwaz AT, Abdullah M. Decolorization of methylene blue and malachite green by immobilized Desmodesmus sp. isolated from North Jordan. Int J Environ Sci Dev. 2016;7(2):95–99.
- Sinha S, Nigam S, Singh R. Potential of Nostoc muscorum for the decolorisation of textiles dye RGB-red. Int J Pharm Bio Sci. 2015;6:1092–1100.
- Samir Ali S, Al-Tohamy R, Xie R, et al. Construction of a new lipase and xylanase producing oleaginous yeast consortium capable of reactive azo dye degradation and detoxification. Bioresour Technol. 2020;313:123631.
- Al-Tohamy R, Sun J, Fareed MF, et al. Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmatomyces halophilus SSA1575, valued for textile azo dye wastewater processing and detoxification. Sci Rep. 2020;10:12370.