ABSTRACT
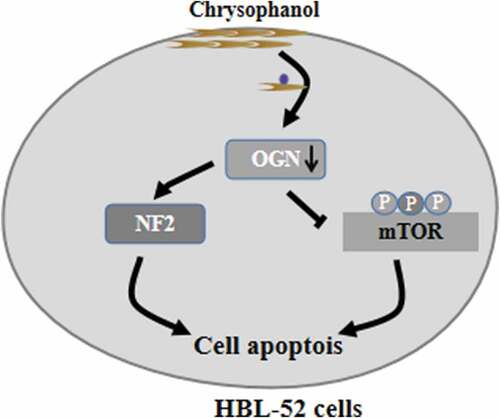
Chrysophanol shows promising antitumor activity, but how it may work against malignant meningioma is poorly understood. In addition, osteoglycin (OGN) may help mediate the antitumor effects of chrysophanol; thus, this study investigated the potential antitumor mechanism of chrysophanol in malignant meningioma cultures. Meningioma cell line HBL-52 were incubated with varying doses of chrysophanol (0–90 μM) for different time points, and osteoglycin (OGN) was overexpressed or inhibited in some cell cultures to assess its roles. Cell viability was quantified using the CCK8 assay and colony formation assays, while effects on cell cycle distribution and apoptotic rates were examined by flow cytometry and enzyme-linked immunosorbent assays (ELISA) to detect histone DNA levels. Caspase-3 and −9 activities were detected by related commercial kits. Protein expression was assessed using Western blotting. Chrysophanol significantly reduced HBL-52 cell viability, based on reduced colony formation, and proliferation, based on low levels of bromodeoxyuridine incorporation. Annexin V/propidium iodide staining revealed a 30% increase in apoptotic cells at 90 μM chrysophanol (33.7% vs 3.3% in control cultures). Chrysophanol treatment greatly decreased the Bcl-2/Bax expression ratio and increased the expressions of cleaved caspase-3 and −9, and the activities of caspase-3 and −9. Chrysophanol blocked cells in G1 phase and inhibited the OGN/mTOR signaling cascade, but activated neurofibromatosis 2 (NF2) cascade. OGN overexpression activated mTOR, down-regulated NF2, and partially reversed growth inhibition by chrysophanol. Chrysophanol may be useful as a treatment against malignant meningioma by inhibiting OGN/mTOR signaling and activating NF2 signaling.
Introduction
Meningiomas are the most common primary brain cancers in over the world [Citation1]. Disease severity is classified into benign, atypical, and anaplastic/malignant, and 15 histologic subtypes have been identified [Citation2]. Although most meningiomas are benign and grow slowly, malignant tumor frequently recurs and leads to the poor prognosis [Citation3,Citation4]. Therefore, more effective pharmacotherapeutic strategies are needed against meningiomas.
Osteoglycin (OGN) has important physiological roles in maintaining bone formation and normal vasculature [Citation5–7], while its expression is associated with several diseases [Citation7]. For example, OGN promotes meningioma cell proliferation by interacting with other drivers of tumor development, including neurofibromatosis 2 (NF2) and mammalian target of rapamycin (mTOR) [Citation8]. Indeed, NF2 overexpression is associated with neurofibromatosis type 2, an inherited genetic disease involving meningioma [Citation9]. High expression of mTOR is closely related to the tumor deterioration and poor survival time in meningioma [Citation10]. Thus, investigating the expression and activation of OGN, mTOR, and NF2 signaling in meningioma cells will not only uncover key molecular mechanisms, but may also provide promising targets for the treatment of meningioma [Citation8].
Chrysophanol is an anthraquinone with purported potent antitumor effects [Citation11,Citation12]. How chrysophanol exerts its anticancer effects in meningioma is unknown. To address this, we exposed cancer cells with increasing concentrations of chrysophanol in different periods. We supposed that chrysophanol could inhibit the growth of HBL-52 cells via decreasing cell proliferation and inducing apoptosis, and regulate the signaling transduction of OGN/mTOR and NF2 cascades. Therefore, this study assessed its effects on cell proliferation, cell viability, apoptosis, and signaling via OGN/mTOR and NF2 pathways.
Materials and methods
Cell culture
HBL-52 cells were obtained from Bafeier Biotechnology (Wuhan, China). Cells were cultured in DMEM medium (Sigma-Aldrich, MA, USA) supplemented with 10% fetal bovine serum and 1% streptomycin (100 μg/mL) and penicillin G (100 U/mL). Cells were maintained in an incubator at 37 °C with 5% CO2. The fourth to sixth generation of HBL-52 cells was used in the follow-up experiments, and each test repeats for three times.
OGN overexpression and inhibition
HBL-52 cells were transfected with control siRNA (Lot#09212-212, Bafeier Biotechnology, Wuhan), OGN mimic (Sequence: 5’-GAAGGUGUAUGAGUA-3’, Lot#HSS210987, Bafeier Biotechnology, Wuhan), or OGN inhibitor (Sequence: 5’-GCGGCAUGUAUUGAG-3’, Lot#HSS123089, Bafeier Biotechnology, Wuhan) plasmids per the manufacturer’s instructions. Briefly, cells were cultured in a density of 4.0 × 105 cells/well in 12-well plates for 12 h. Then 5 μL of plasmids and 500 μL of DMEM medium were mixed for 5 min; 5 μL of lipofectamineTM2000 and 500 μL of DMEM medium were also mixed for 5 min. The two mixtures were mixed for 20 min, then added and incubated cells. Cells transfecting with control siRNA, OGN mimic, and OGN inhibitor were grown for 24 h, rinsed with phosphate-buffered saline (PBS) and treated with chrysophanol.
Cell viability assay
HBL-52 cells were grown in 96-well plates for overnight and cultured with varying doses of chrysophanol (0, 15, 30, 60, or 90 μM) for 12, 24, 48, and 72 h. At these time points, cell supernatant were removed, cultures were rinsed twice with PBS, and the CCK8 assay (Invitrogen Life Technologies, MA, USA) was performed based on the manufacturer’s instructions.
Colony formation assay
HBL-52 cells were grown in 6-well plates for 24 h and incubated with increasing doses of chrysophanol (0, 15, 30, 60, and 90 μM) for 48 h. Cells were counted using a inverted microscope (OLYMPUS, IX73-A12FL/PH). Cell colonies were assessed by selecting 10 views and calculating the average number of cells.
Histone DNA enzyme-linked immunosorbent assay (ELISA)
Cancer cells were grown in 6-well plates to a confluence of 60% and incubated for 48 h with different concentrations of chrysophanol (0, 15, 30, 60, and 90 μM). Then cells were lyzed, and recovered the supernatant after centrifugation. Bromodeoxyuridine (BrdU) and histone DNA levels were quantified using the respective ELISA kits per the manufacturers’ protocols. Optical density (OD) at 507 nm was determined by a microplate reader (Potenov, Beijing, China).
Caspase-3 and −9 activity assays
Cells were grown in 6-well plates as above and incubated for 48 h with increasing doses of chrysophanol (0, 15, 30, 60, and 90 μM). Caspase activity was assayed using commercial kits (Abcam, UK).
Annexin V/propidium iodide (PI) staining experiment
Treated cells were collected and adjusted to a density of 1 × 106 cells/mL. Cell suspensions (0.5 mL) were stained with 1.25 μL Annexin V-FITC (Potenov, Beijing, China) at 37 °C in the dark for 10 min, and 10 μL of PI (Potenov, Beijing, China) was added for 10 min. Proportions of cells in different stages of cell cycle and apoptotic rates were estimated using flow cytometry (Beckman, USA).
Western blotting
Protein from HBL-52 cells was extracted using RIPA buffer (Beyotime Biotechnology). Protein was quantified and equal amounts (50 μg) from each sample were resolved in 12% SDS-PAGE. Protein was transferred onto a nitrocellulose membrane (Invitrogen Life Technologies, MA, USA) before overnight incubation at 4 °C with primary antibodies (diluted 1:1000; Abcam, Cambridge, USA) against OGN, phosphorylated mTOR (p-mTOR, ab1098763), mTOR (ab2098461), NF2 (ab1128974), cleaved caspase-3 (ab1198329), cleaved caspase-9 (ab1232458), Bcl-2 (ab1123894), and Bax (ab1126478). Strips were then incubated for 2 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3000; Cell Signaling, Danvers, USA). Then strips were detected and analyzed to assess the relative expression levels of target proteins.
Statistical analysis
All data were shown as average value± standard deviation. Student’s t-test was carried out to assess statistical differences in different groups, whereas ANOVA was performed to analyze the differences of multiple groups. Differences associated with P< 0.05 were regarded as significant.
Results
Chrysophanol suppresses growth of HBL-52 cells
Based on prior knowledge, chrysophanol may affect the proliferation and apoptosis of HBL-52 cells through the inhibition of OGN/mTOR pathway and the activation of NF2. Thus, we further explore the impacts of chrysophanol on the growth of malignant meningioma cultures, and observe its potential antitumor mechanism.
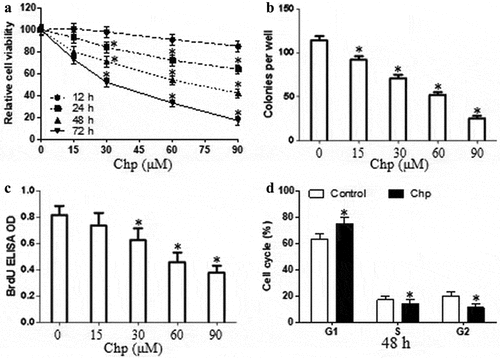
We determined whether chrysophanol affected HBL-52 cell proliferation using the CCK8 assay. Cultures were exposed to concentrations ranging from 2 μM (data not shown) to 90 μM for different periods. Chrysophanol inhibited proliferation of HBL-52 cells in a time- and concentration-dependence (p< 0.05, ). These findings based on viability were confirmed in an assay based on colony formation (p< 0.05, ). Treated cells also incorporated lower amounts of BrdU into the genome, indicating reduced proliferation (p< 0.05, ). We then exposed cultures to 60 μM of chrysophanol for 48 h to assess the compound’s effects on the cell cycle (p< 0.05). We chose this concentration and time point because the relative cell viability is 53%. Chrysophanol incubation elevated the proportion of cells in G1 phase and reduced the proportion in S or G2 phases ().
Figure 1. Chrysophanol (Chp) inhibits the growth of HBL-52 cells in vitro. Cells were treated with different concentrations of chrysophanol for the indicated periods, and (a) CCK8, (b) cell colony formation, and (c) bromodeoxyuridine (BrdU) assays were conducted. (d) The distribution of cells in different phases of the cell cycle was determined by flow cytometry after treatment with 60 μM chrysophanol for 48 h. *P< 0.05, compared with 0 μM chrysophanol group or control group
Chrysophanol induces apoptosis in HBL-52 cells
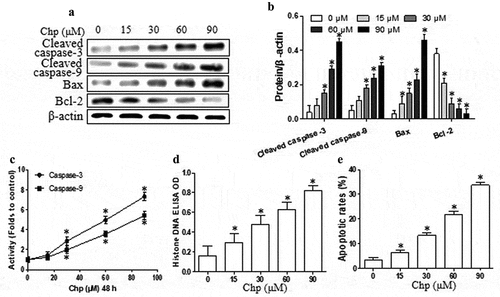
Next, we investigated whether and how chrysophanol induces apoptosis in HBL-52 cells. Treating HBL-52 cells with chrysophanol for 48 h significantly up-regulated cleaved caspase-3, cleaved caspase-9, and Bax protein, while dramatically down-regulating Bcl-2 (p< 0.05, and b). Interestingly, chrysophanol at concentrations as low as 15 µM appreciably affected Bax and Bcl-2 expression. Furthermore, chrysophanol increased the activity of caspase-3 and −9, as reflected in higher levels of histone DNA, in a dose-dependence (p< 0.05, and d). Treating cells with 90 μM chrysophanol for 48 h led to a 10-fold higher proportion of apoptotic cells than in untreated cultures (33.7% vs 3.3%, p< 0.05, ).
Figure 2. Chrysophanol (Chp) induces apoptosis of HBL-52 cells in vitro. Cells were treated with different concentrations of chrysophanol for 48 h. (a) Expression levels of caspase-3, caspase-9, Bax, and Bcl-2 were determined using western blot. (b) Densitometry of proteins from the experiments in panel A. (c) Activity of caspase-3 and −9. (d) Histone DNA levels. (e) Annexin V and propidium iodide staining, as captured by flow cytometry. *P< 0.05, compared with 0 μM chrysophanol
Chrysophanol reduces OGN/mTOR signaling but activates NF2 signaling in HBL-52 cells
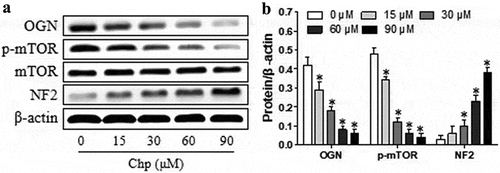
OGN is regarded as a novel oncogene in meningioma development.8 The OGN protein induces meningioma proliferation by down-regulating NF2 and activating mTOR signaling.8 Therefore, we used a western blot assay to evaluate the expressions of OGN, p-mTOR, and NF2 in cells after 48-h exposure to different concentrations of chrysophanol (p< 0.05, ). Treatment reduced levels of OGN and p-mTOR but increased NF2 levels in a concentration-dependence (p< 0.05). Our findings indicated that chrysophanol inhibits OGN/mTOR signaling but induces NF2 signaling in meningioma cells.
Figure 3. Chrysophanol (Chp) reduces OGN/mTOR signaling and activates NF2 signaling in HBL-52 cells in vitro. Cells were treated with different concentrations (0, 15, 30, 60, and 90 μM) of chrysophanol for 48 h. (a) Western blot of OGN, p-mTOR, total mTOR, and NF2. (b) Densitometry quantifying the relative levels in the experiments in panel A. *P< 0.05, compared with 0 μM chrysophanol
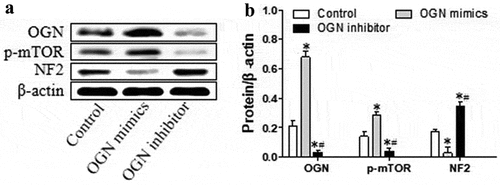
OGN activates mTOR and down-regulates NF2
OGN is known to interact with p-mTOR and NF2 during meningioma development, but how OGN affects these other proteins is not clear [Citation8]. Therefore, we modified OGN expression and examined the effects on p-mTOR and NF2. HBL-52 meningioma cells overexpressing OGN showed increased levels of activated mTOR and down-regulated NF2 protein at 48 h (p< 0.05, ). Conversely, silence of OGN reduced levels of p-mTOR and up-regulated NF2 (p< 0.05, ). These findings suggest that OGN is an upstream factor to regulate mTOR and NF2 signaling.
Figure 4. OGN induces activation of mTOR and down-regulates NF2 in HBL-52 cells in vitro. Cells were transfected for 48 h with plasmids (100 nM) overexpressing OGN mimics or inhibitors, then assayed. (a) Western blot of OGN, p-mTOR, and NF2. (b) Quantitation of the experiments in panel A. *P< 0.05, compared with 0 μM chrysophanol
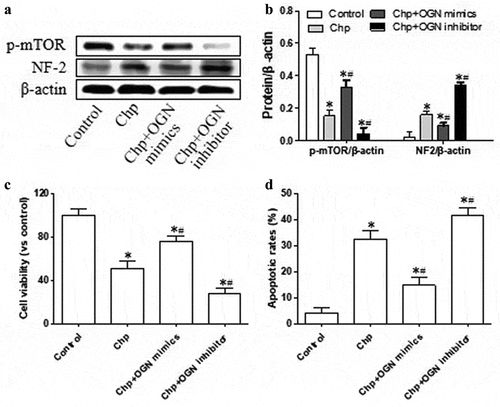
Chrysophanol regulates OGN, which in turn regulates signaling by mTOR and NF2
Next we investigated whether the effects of chrysophanol on OGN may mediate its effects on levels of p-mTOR, expression of NF2, viability, and apoptosis in HBL-52 cells. Cells overexpressing OGN and treated with chrysophanol produced significantly higher levels of activated mTOR and significantly lower levels of NF2 than cells only treated with chrysophanol (p< 0.05, ). OGN overexpression also partially reversed the impacts of chrysophanol on cell viability and apoptosis (p< 0.05). Loss of OGN led to the opposite results. These experiments suggest that chrysophanol alters levels of p-mTOR and NF2 and cell growth by altering OGN expression.
Figure 5. Chrysophanol acts via OGN to alter mTOR and NF2 signaling and growth of HBL-52 in vitro. Cells were transfected with plasmids encoding OGN mimetic or inhibitor, then treated with 60 µM chrysophanol for 48 h. (a) Western blot of p-mTOR and NF2. (b) Densitometry of the experiments in panel A. (c) Cell viability was analyzed by CCK8 assay. (d) Proportions of cells in apoptosis based on flow cytometry. *P< 0.05, compared with control group; #P< 0.05, compared with chrysophanol group
Discussion
Despite extensive observations about genomic and epigenomic maps have explored the pathogenesis and disease progression in meningioma, little is understood about the signaling pathways and key molecules involved [Citation13–15]. OGN has been implicated in meningioma through effects on mTOR and NF2 signaling, but whether it may be a therapeutic target is unclear [Citation16,Citation17]. Here we provide evidence that chrysophanol suppresses the growth of HBL-52 cells by regulating the OGN cascade, implying its potential value as a drug target.
Chrysophanol belongs to a family of organic compounds with antitumor effects [Citation18], and it suppresses the growth of multiple tumor cells through regulation of different signaling pathways. It has reported that chrysophanol has an anti-tumor role in the cell proliferation of lung cancer via mediating ROS/HIF-1a/VEGF signaling cascade [Citation19]; additionally, it also suppresses cell growth, migration, and reactive oxygen species generation in oral cancer cell lines [Citation20]. Moreover, it induces apoptosis of malignant optic nerve meningioma cells via inducing the caspase activation and targeting the MAPKs signaling cascade [Citation21]. Similarly, our results in a menangioma cell type suggest that chrysophanol suppresses the proliferation of tumor cells while promoting their apoptosis. Our study goes further by suggesting that these antitumor effects begin with downregulation of OGN.
In colon cancer cells, chrysophanol blocks proliferation by inhibiting the mTOR pathway [Citation22]. We also found that chrysophanol dose-dependently reduced proliferation of a meningioma cell line in vitro. This finding was based on the observation of decreases in cell viability, colony formation and BrdU+ incorporation. It is well known that Bcl-2/Bax ratio is a vital determinant of apoptosis induction [Citation23–25]; our research demonstrated that chrysophanol induced Bax expression and inhibited Bcl-2 level, thus biasing the ratio toward cell death. This was further supported by increased expression and activity of pro-apoptotic caspase-3 and −9 in our experiments [Citation26–30].
There is a obvious limitation in this study. We could not clearly demonstrate the interact of chrysophanol with OGN protein in malignant meningioma cells. Therefore, further studies should examine how chrysophanol affects the expression of OGN in malignant meningioma cells. Additionally, in our study, the potential of chrysophanol for treating malignant meningioma and other types of cancer can be verified and fully understood, improving its chances for clinical use.
Conclusions
In summary, this present observation demonstrates the inhibitory effect of chrysophanol on malignant meningioma cells and its regulatory roles in the signaling transduction of OGN/mTOR and NF2 cascades. Our results should be verified and extended in other malignant meningioma cell lines and in preclinical models. OGN may be a useful target for treating this disease.
Highlights
• Chrysophanol suppresses the growth of meningioma cells.
• Chrysophanol inhibites OGN/mTOR signaling pathway.
• Chrysophanol activates NF2 signaling.
Supplemental Material
Download ()Disclosure statement
No conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Notes on contributors
Jiapeng Wang
Jiapeng Wang, MD, works in Department of Intensive Medicine, Suizhou Hospital, Hubei University of Medicine, Suizhou ,Hubei, China.
Peng Lv
Peng Lv, MD, works in Department of Neurosurgery, Suizhou Hospital, Hubei University of Medicine, Suizhou , Hubei,China
References
- Mawrin C. Molecular biology, diagnosis, and therapy of meningiomas. Pathologe. 2019;40(5):514–518.
- Louis DN, Perry A, Reifenberger G. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820.
- Buttrick S, Shah AH, Komotar RJ. Management of atypical and anaplastic meningiomas. Neurosurg Clin N Am. 2016;27(2):239–247.
- Johnson MD, Abu-Farsakh S. Clinicopathologic features of incidental meningiomas: A review of the literature and the University of Rochester autopsy experience. Clin Neuropathol. 2019;38(3):118–121.
- Tanaka K, Matsumoto E, Higashimaki Y. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287:11616–11628.
- Fernandez B, Kampmann A, Pipp F. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem. 2003;246:3–11.
- Van Aelst LN, Voss S, Carai P. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res. 2015;116(3):425–436.
- Mei Y, Du Z, Hu CC. Osteoglycin promotes meningioma development through downregulation of NF2 and activation of mTOR signaling[J]. Cell Commun Signal. 2017;15(1):34–43.
- van Hinsbergh VW. Osteoglycin’s embracement of VEGF receptor-2 limits angiogenesis and collateralization. Cardiovasc Res. 2017;113(1):10–12.
- Barresi V, Lionti S, La Rocca L. High p-mTOR expression is associated with recurrence and shorter disease-free survival in atypical meningiomas. Neuropathology. 2019;39(1):22–29.
- Lim W, An Y, Yang C. Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. J Cell Biochem. 2018;119(12):10216–10227.
- Ren L, Li Z, Dai C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol Med Rep. 2018;17(3):4376–4382.
- Abedalthagafi M, Bi WL, Aizer AA. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016;18:649–655.
- Clark VE, Erson-Omay EZ, Serin A. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080.
- Shankar GM, Abedalthagafi M, Vaubel RA. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19:535–545.
- Orr B, Riddick AC, Stewart GD. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene. 2012;31:1130–1142.
- Zheng CX, Zhao SX, Wang P. Different expression of mimecan as a marker for differential diagnosis between NSCLC and SCLC. Oncol Rep. 2009;22:1057–1061.
- Huang Q, Lu G, Shen HM, et al. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27(5):609–630..
- Zhang J, Wang Q, Wang Q. Chrysophanol exhibits anti-cancer activities in lung cancer cell through regulating ROS/HIF-1a/VEGF signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(3):469–480.
- Hsu PC, Cheng CF, Hsieh PC. Chrysophanol regulates cell death, metastasis, and reactive oxygen species production in oral cancer cell lines. Evid Based Complement Alternat Med. 2020;2020. DOI:10.1155/2020/5867064.
- Zeng CH, Guo B, Chen J. Antitumor effects of chrysophanol in malignant optic nerve meningioma cell lines are mediated via caspase activation, induction of mitochondrial mediated apoptosis, mitochondrial membrane depolarization and targeting the mitogen-Activated protein kinase signaling pathway. Pharmacology. 2019;104(1–2):28–35.
- Lee MS, Cha EY, Sul JY. Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway. Phytother Res. 2011;25(6):833–837.
- Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27–36.
- Toscano ECB, Vieira ÉLM, Portela ACDC. Bcl-2/Bax ratio increase does not prevent apoptosis of glia and granular neurons in patients with temporal lobe epilepsy. Neuropathology. 2019;39(5):348–357.
- Wang R, Song F, Li S. Salvianolic acid A attenuates CCl(4)-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther. 2019;13:1889–1900.
- Gowda Saralamma VV, Lee HJ, Raha S. Inhibition of IAP’s and activation of p53 leads to caspase-dependent apoptosis in gastric cancer cells treated with Scutellarein. Oncotarget. 2017;9(5):5993–6006.
- Lossi L, Castagna C, Merighi A. Caspase-3 mediated cell death in the normal development of the mammalian cerebellum. Int J Mol Sci. 2018;19(12):3999.
- Crowley LC, Waterhouse NJ. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb Protoc. 2016;16(11):201.
- Choudhary GS, Al-Harbi S, Almasan A. Caspase- 3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015;1219:1–9.
- Zhou M, Liu X, Li Z. Caspase- 3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143(4):921–930.