Article title: A halotolerant thermostable lipase from the marine bacterium Oceanobacillus sp. PUMB02 with an ability to disrupt bacterial biofilms
Authors: George Seghal Kiran, Anuj Nishanth Lipton, Jonathan Kennedy, Alan DW Dobson, and Joseph Selvin
Journal: Bioengineered
DOI: https://doi.org/10.4161/bioe.29898
When this article was published online, there were minor errors introduced in the . The following figures were corrected with appropriate changes. These changes do not affect the main conclusion of this manuscript.
Figure 4. Purification of PUMB02 lipase. The dialyzed solution was purified with a fast-performance liquid chromatography (FPLC) system (BioRad) equipped with an anionic exchange (DEAESepharose) which was previously equilibrated with 50mM tris-HCl buffer (pH 7.4). The column was washed with 50 ml of the buffer and then eluted with a linear gradient of 0 to 1.0 M sodium chloride (NaCl) in the same buffer. Linear flow rate was 0.5 ml/min and fractions were collected every 2.0 min. The lipase activity and protein concentration (A280) in each fraction were measured. (A) Butyl-sepharose anion exchange chromatography. (B) DEAEsephadex gel
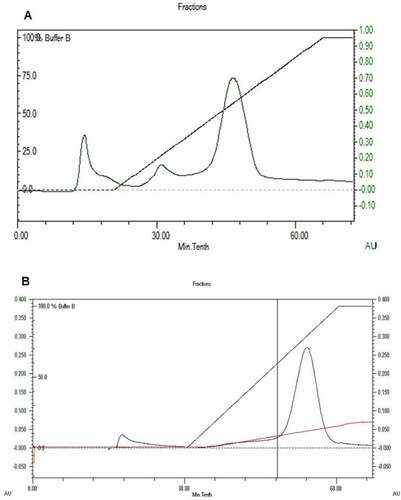
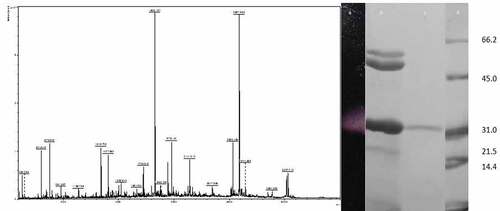
Figure 5. MALDI-TOF analysis of the tryptic digest fingerprint of PUMB02 lipase predicting the peptide mass to be 31kDa. Lane a: zymogram of PUMB02 lipase; Lane b: crude protein; Lane c: purified protein after size-exclusion chromatography; Lane d: molecular weight markers