Abstract
A halotolerant thermostable lipase was purified and characterized from the marine bacterium Oceanobacillus sp. PUMB02. This lipase displayed a high degree of stability over a wide range of conditions including pH, salinity, and temperature. It was optimally active at 30 °C and pH 8.0 respectively and was stable at higher temperatures (50–70 °C) and alkaline pH. The molecular mass of the lipase was approximately 31 kDa based on SDS-PAGE and MALDI-ToF fingerprint analysis. Conditions for enhanced production of lipase by Oceanobacillus sp. PUMB02 were attained in response surface method-guided optimization with factors such as olive oil, sucrose, potassium chromate, and NaCl being evaluated, resulting in levels of 58.84 U/ml being achieved. The biofilm disruption potential of the PUMB02 lipase was evaluated and compared with a marine sponge metagenome derived halotolerant lipase Lpc53E1. Good biofilm disruption activity was observed with both lipases against potential food pathogens such as Bacillus cereus MTCC1272, Listeria sp. MTCC1143, Serratia sp. MTCC4822, Escherichia coli MTCC443, Pseudomonas fluorescens MTCC1748, and Vibrio parahemolyticus MTCC459. Phase contrast microscopy, scanning electron microscopy, and confocal laser scanning microscopy showed very effective disruption of pathogenic biofilms. This study reveals that marine derived hydrolytic enzymes such as lipases may have potential utility in inhibiting biofilm formation in a food processing environment and is the first report of the potential application of lipases from the genus Oceanobacillus in biofilm disruption strategies.
Introduction
Marine ecosystems represent a large and as yet largely under explored reservoir of biodiversity with respect to industrially useful biocatalysts. These ecosystems can range from coastal environments to deep-sea hydrothermal vents with high hydrostatic pressure and temperatures as high as approximately 400 °C. Marine ecosystems are also subject to low temperatures with the average temperature of the deep oceans being around 3 °C.Citation1 The survival of microorganisms under these conditions is likely to have resulted in the development of quite novel microbial cellular biochemistry and metabolism, thereby ensuring that these bacteria are likely to possess unique enzyme systems, such as increased salt tolerance and cold adaptivity, which match many industrial requirements.Citation2
The biological relevance and large variability of lipids has led to the evolution of a wide variety of lipid-degrading enzymes throughout all the kingdoms of life. Among these lipases and/or esterases, which belong to the group of enzymes that catalyzes the cleavage of chemical linkages by the addition of a water molecule, are considered to be the most important biocatalysts due to their widespread biological functions and biotechnological potential.Citation3,Citation4 There has been great recent interest in lipase producing microorganisms primarily due to the utility of lipases in various industrial applications, where their stability, high activity levels in organic solvents, and enantio- and/or stereoselectivity are particularly attractive. These properties coupled with their broad substrate range have resulted in their use in waste treatment, textile processing, as additives in detergents, and in various biofuel production applications.Citation5 Thus, given the potential utility of lipases in various industrial applications, there is currently much interest in isolating novel enzymes particularly halophilic lipases for use in catalytic applications where high salt stability is desirable such as in the food and nutraceuticals industry, or in microalgae-based biofuel systems.Citation6
Biofilms are formed by the aggregation of bacterial cells on biotic and abiotic surfaces. Naturally existing biofilms are a major threat to human health, with up to 80% of clinical infections involving biofilm formation, while biofilms that form on indwelling medical devices are responsible for a large proportion of hospital-acquired infections.Citation7 Biofilm formation is also a major problem in membrane-based water treatment systems causing a decline in water fluxes and necessitating an increase in cleaning frequency.Citation8 Biofilm formation continues to be a problem in marine environments, leading to corrosion of metals and the undesirable colonization of surfaces by fouling microorganisms causing not only extensive material damage but also significant economic costs.Citation9 In addition, biofilm formation by food pathogens is common in the food industry, where attachment to food products or to surfaces that are in contact with products leads not only to economic losses but also to an increased risk of occurrence of bacterial food borne diseases within the consumer food chain.Citation10 Most of the current procedures for the removal of biofilm rely on stringent chemical cleaning regimes of the colonized surfaces, with a wide range of biocides, sanitizers, and disinfectants being used in controlling biofilms. However these chemical agents have little or no effect on established biofilms.Citation11 In addition, the use of toxic biocides such as triorganotins has been banned, thereby increasing research interest in non-toxic environmentally friendly alternatives, such as the use of enzymes (such as halotolerant antifouling enzymes that form the focus of this study), which represent a potentially attractive strategy for the control and removal of biofilms.
Enzymes are known to remove biofilms by destroying the physical integrity of the biofilm matrix of so called Extracellular Polymeric Substances (EPS).Citation12,Citation13 Commercially available proteases such as trypsin, proteinase K, and subtilisin have successfully been used to remove biofilms,Citation14-Citation16 while biofilms can also be removed using polysaccharide-degrading enzymes such as cellulases. For example cellulases from the fungus Penicillium faniculusum have been reported as one of the most effective enzymes in degrading mature biofilms of Pseudomonas aeruginosa.Citation17 Cellulases have also been shown to be effective in degrading the exopolysaccharide of Pseudomonas fluorescens.Citation17,Citation18 Lipases isolated from the wax moth (Galleria mellonella) have been shown to have bactericidal action against Mycobacterium tuberculosis (MBT) H37Rv and a lipase from bovine pancreas has been shown to degrade EPS and prevent biofilm formation in marine environments.Citation13 Lysobacter gummosus has also recently been reported to produce peptidases, which degrade biofilms formed by Staphylococcus epidermidis.Citation19
In this study, we examined the use of marine derived lipases as potential novel sources of antibiofilm agents and report on the characterization of a new halotolerant lipase isolated from Oceanobacillus sp. PUMB02. This lipase displayed a high degree of stability over a wide range of conditions including pH, salinity, and temperature and was optimally active at 30 °C and pH 8.0 respectively. Characterization of the lipase by the MALDI-TOF MS/MS finger-printing technique resolved the molecular mass as approximately 31 kDa. The biofilm disruption potential of the lipase was also evaluated and compared with a previously characterized marine sponge derived metagenomic lipase Lpc53E1Citation20; with both marine derived lipases displaying promising anti-biofilm activities against common food pathogens such as Bacillus cereus MTCC1272, Listeria sp. MTCC1143, Serratia sp. MTCC4822, Escherichia coli MTCC443, Pseudomonas fluorescens MTCC1748, and Vibrio parahemolyticus MTCC459.
Results
Screening and characterization of lipases
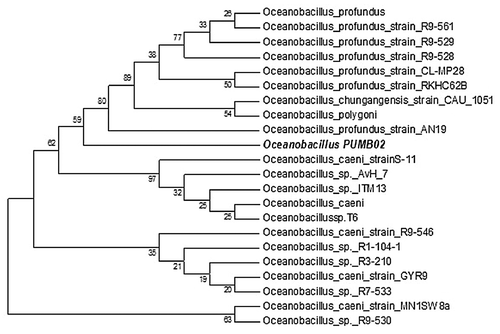
Forty four bacteria were isolated from the seawater samples and screened on both tributyrin and olive oil plates. Of these, 6 isolates were positive for lipase activity on tributyrin plates while one isolate PUMB02 was positive on both tributyrin and olive oil plates. The strain PUMB02 displayed a clear halo zone of 4 ± 1 mm (mean + S.D., n = 3) around the colony on tributyrin as well as on olive oil agar plates (). The PUMB02 strain was characterized as being Gram negative and was negative for catalase, oxidase, indole, methyl red and citrate production. It was positive in the Voges-Proskauer test and grew over a temperature range of 25–40 °C with optimal growth at 30 °C. On nutrient agar (supplemented with 2% NaCl) it produced slightly brown colored shiny surface umbellate transparent colonies (). BLASTn analysis of the 16S rRNA gene sequence of PUMB02 showed a sequence similarity of 98% with strains of Oceanobacillus sp. Based on the biochemical characteristics and phylogenetic analysis, the isolate PUMB02 was classified as Oceanobacillus sp. (). The seqmatch program of Ribosomal Database Project II (RDP II) showed that the sequence of PUMB02 was from the genus Oceanobacillus. The RDPII analysis indicated that the isolate had the closet match with Oceanobacillus caeni (S-11; AB275883) and Oceanobacillus sp. (ITM13; FR667183). In the maximum parsimony phylogenetic tree, the isolate PUMB02 located to a unique branch within the Oceanobacillus group (). All the closest branches of the phylogenetic tree were Oceanobacillus sp., Oceanobacillus profundus strains, and Oceanobacillus caeni strains. Based on the morphological, biochemical characteristics, and phylogenetic analysis, the isolate PUMB02 was classified as an Oceanobacillus sp.
Figure 1. The growth of Oceanobacillus sp. PUMB02 on nutrient agar media supplemented with 2% NaCl (A). The hydrolysis zone on tributyrin plate (B) and olive oil plate (C).
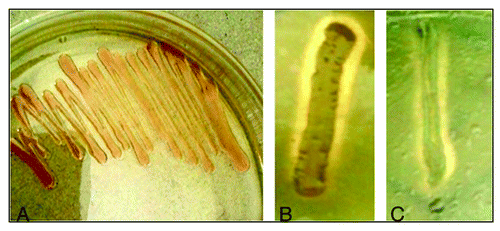
Figure 2. Maximum Parsimony bootstrapping phylogenetic tree of Oceanobacillus sp. PUMB02 and closest NCBI (Mega BLAST) relatives based on the 16S rRNA gene sequences. Phylogeny reconstruction was performed using Maximum Parsimony. Bootstrap values calculated from 1,000 re-samplings are shown at the respective nodes when the calculated values were 50% or greater.
Statistical optimization of lipase production by Oceanobacillus sp. PUMB02 by response surface method
Conventional optimization using one-factor-at-a-time experiments precludes the possible interactive effect of variables. To overcome this problem, optimization was performed by employing response surface methodology (RSM). RSM is a regression-based mathematical modeling tool, which is typically used to explore interactive responses of multiple factors influencing lipase production. Here 11 factors affecting lipase production were optimized through one factor at a time experiments. Based on this data, the critical control factors such as olive oil, sucrose, potassium chromate, and NaCl were selected for statistical optimization to determine the interactive effect of these factors on lipase production.
Eleven variables namely A:Coconut oil (ml/L), B:Sucrose(g/L), C:Olive oil (ml/L), D:Ferrous sulfate (mg/L), E:Yeast extract (g/L), F:Tween 20 (ml/L), G:Potassium chromate (mg/L), H:NaCl (% (V/V), J:Incubation time (h), K:pH and L:Temperature (°C), were selected for optimization by Placket Burman design. Experiments were conducted and the response value obtained and analyzed by ANOVA. The ANOVA results indicated the six factors which most affected lipase production at a significance level of P > 0.05 (Table S3). Four factors, namely olive oil, sucrose, potassium chromate, and NaCl, were selected for further optimization and interaction studies. The two factors of pH and temperature was set at a constant level of 8 and 30 °C respectively. The Central Composite Design (CCD) was performed with the aforementioned four factors and the responses were analyzed by the Design Expert Software. The results were analyzed by Design Expert Software and following quadratic regression equation analysis were obtained in terms of four chosen variables designated as A, Olive oil; B, Sucrose; C, Potassium chromate; and D, NaCl on lipase production.
Lipase activity (U/ml) = +58.84+3.35* A-0.068* B+0.74 * C-0.61*D+1.10*A* B+8.750E-003 * A * C+1.71 * A * D-1.64* B * C-1.17* B * D-1.16* C * D-5.41 *A2–0.87 * B2–1.15 * C2–1.29* D2
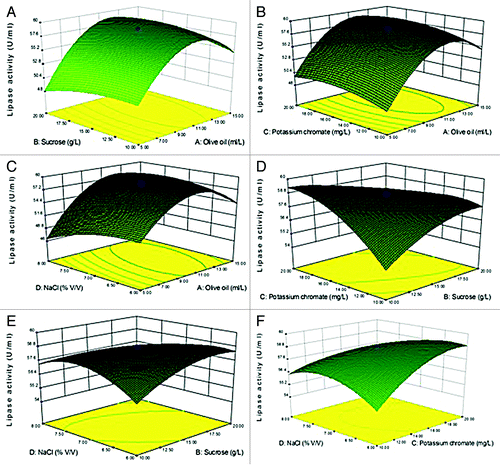
Three-D surface graphs were developed which predicts the response over a range in the design surface and it accounts for two factors at a time while keeping the other two at a fixed level (). The CCD ANOVA suggests which of the selected factors are significant with respect to maximum lipase production (Table S4). The maximum lipase production obtained in the RSM-guided experiments was 58.84 U/ml with factors such as olive oil, sucrose, potassium chromate and NaCl at concentrations of 10 ml/L, 15 g/L, 15 mg/L, and 7% (v/v) respectively. The coefficient of determination (R2) was found to be 0.9875, confirming the adequacy of the model. The observed close fit of the model to the experimental data provides good evidence that this model can be used to optimize lipase production.
Figure 3. 3-D contour plots of relative enzyme activity with four variables (A: Olive oil, B: Sucrose, C: Potassium chromate, and D: NaCl). (A) Sucrose (g/L) and Olive oil (ml/L), (B) Olive oil (ml/L) and Potassium chromate (mg/L), (C) Olive oil ml/L and NaCl (% v/v), (D) Sucrose (g/L) and Potassium chromate (mg/L), (E) Sucrose (g/L) and NaCl (% v/v), (F) Potassium chromate (mg/L) and NaCl (% v/v).
Purification of lipase PUMB02
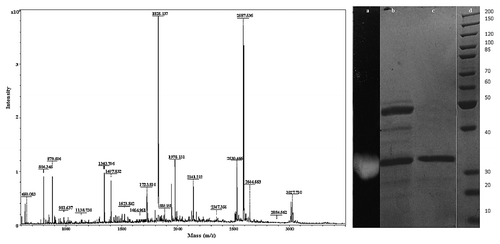
To purify the lipase from PUMB02, the cell free supernatant (CFS) was first subjected to ammonium sulfate precipitation, followed by dialysis, butyl-sepharose fast flow Pharmacia column separation, anion-exchange chromatography on DEAE cellulose, and gel filtration chromatography on Sephadex G-75 column. The highest lipase activity was detected in the 80–100% fraction of the ammonium sulfate precipitate. The recovered active fraction from the 80–100% ammonium sulfate precipitation of culture broth was then subjected to an anion exchange column eluted with a NaCl gradient which showed the protein had absorbed to the resin matrix (). The partially purified preparation was concentrated and applied to a gel filtration column. The protein was further purified using Sephadex G-75 and tested for lipase activity (). The enzyme was purified 34.56-fold after Sephadex G-75 chromatography with the active column fraction showing a specific activity of 5.53 U/mg protein (). The molecular mass of purified protein was approximately 31 kDa based on the results of SDS-PAGE and MALDI-TOF (). The MALDI-TOF analysis of the tryptic digest fingerprint matched with a lipase from Ornithinibacillus scapharcae in the NCBI database (accession number gi|497784402). PHYRE 2 was used to predict the 3D structure of the lipase (Table S1). The genera Oceanobacillus and Ornithinibacillus are related, with both being within the family Bacillaceae.
Figure 4. Purification of PUMB02 lipase. The dialyzed solution was purified with a fast-performance liquid chromatography (FPLC) system (BioRad) equipped with an anionic exchange (DEAE Sepharose) which was previously equilibrated with 50mM tris-HCl buffer (pH 7.4). The column was washed with 50 ml of the buffer and then eluted with a linear gradient of 0 to 1.0 M sodium chloride (NaCl) in the same buffer. Linear flow rate was 0.5 ml/min and fractions were collected every 2.0 min. The protease activity and protein concentration (A280) in each fraction were measured. (A) Butyl-sepharose anion exchange chromatography. (B) DEAE sephadex gel.
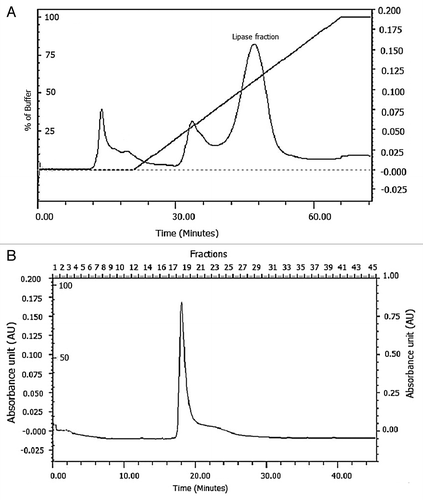
Table 1. Purification summary of lipase from Oceanobacillus PUMB02
FT-IR analysis
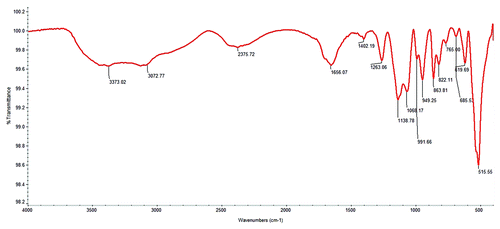
FT-IR analysis of the PUMB02 lipase showed a Broad Peak around 3600–3000 cm−1 corresponding to the hydrogen bonded stretching vibration of -N-H or O-H. The amide I band (1700–1600 cm−1) typically provides both qualitative and quantitative data on the composition of the secondary-structure of proteins, thus the peak observed here around 1700–1600 cm−1 is likely due to the overlapping of -C = O stretching and amide -N-H bending frequency and the other peak at 1507 cm−1 may be due to the N-H symmetric bending vibration. The peaks at 1404 cm−1and 1455 cm−1 are likely to correspond to the amide -C-N stretching band and the CH2- bending frequency respectively, while the strong broad peak around 1100–900 cm−1 is likely to correspond to the -C-O stretching vibration ().
Figure 6. FT-IR analysis showing Broad Peak around 3600–3000 cm−1corresponds to the hydrogen bonded stretching vibration of - -N-H or O-H. The peak around 1700–1600 cm−1 due the overlapping of -C = O stretching and amide -N-H bending frequency and another peak at 1507 cm−1 N-H symmetric bending vibration.CH2- bending frequency observed at 1455 cm−1.The peak at 1404 cm−1corresponds to the amide -C-N stretching band. The strong and broad peak around 1100–900 cm−1 corresponds to the -C-O stretching vibration.
Stability of lipase PUMB02
The enzyme was stable at higher temperatures (50–70 °C) retaining approximately 82% of its original activity at 70 °C for 3 h and almost 48% activity at 90 °C. Thus, the lipase shows stability at temperatures from 4 °C to 70 °C. The pH stability of the enzyme was assessed following incubation for 24 h at pH ranging from 3–12 in different buffer systems. The lipase was stable over a range of pH values retaining 70% activity at the lowest pH 3, while retaining around 60% residual activity at pH 12 (). Metal ions such as Ca2+, Fe3+and Mg2+ were found to enhance the enzyme activity while Al3+, Co2+, Mn2+, and Zn2+ ions inhibited the enzyme activity. Among the esters used, variable hydrolytic activity was observed toward p-nitrophenyl esters, with maximum hydrolytic activity being observed for p-nitrophenyl palmitate compared with other esters (Table S2).
Table 2. Stability characterization of lipase from Oceanobacillus
Evaluation of lipase for biofilm disruption and biofilm inhibitory concentration determination
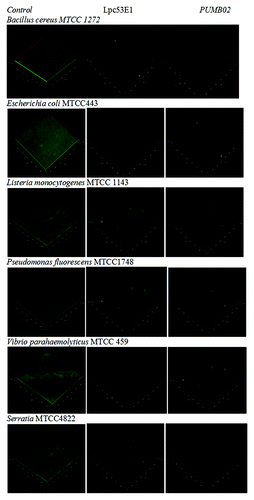
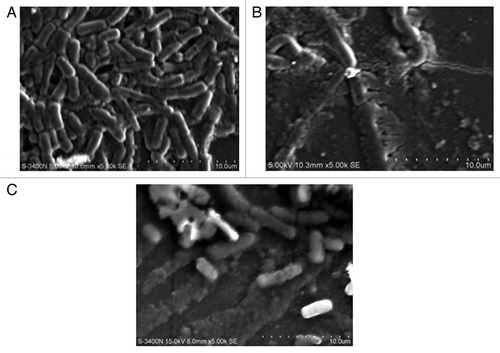
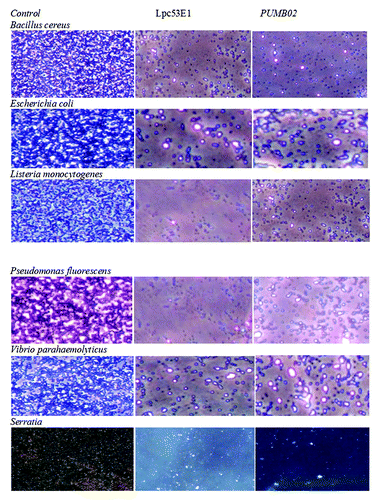
A range of biofilm inhibitory concentration (BIC) concentrations ranging from 50, 100, and 150 µl of lipases were tested for their biofilm disruption capabilities. Preformed biofilms of E. coli MTCC443, B. cereus MTCC1272, Pseudomonas fluorescens MTCC1748, Vibrio parahemolyticus MTCC459, Serratia sp MTCC4822, and Listeria sp. MTCC1143 were found to be effectively disrupted by the Lpc53E1 lipase and Oceanobacillus lipase respectively (). The lowest BIC of Lpc53E1 lipase was recorded against E. coli at 50 µl () whereas the BIC of the Oceanobacillus lipase was 150 µl against all tested pathogens (). The biofilm disrupting ability of the lipases determined from the microplate assay was subsequently confirmed using phase contrast microscopy where disruption of E. coli, B. cereus, Pseudomonas sp., Vibrio sp., S. typhimurium, and Listeria sp. was observed at a concentration of 150 µl for the PUMB02 lipase; while for the Lpc53E1 lipase concentrations of (50 µl) E. coli, (100µl) B. cereus, S. typhimurium, and (150µl) Pseudomonas sp., Vibrio sp., Listeria sp. were required for effective biofilm disruption respectively (). Confocal laser scanning microscopy (CLSM) was subsequently employed to monitor preformed biofilms of Bacillus, E. coli, Listeria, Pseudomonas, Vibrio, and Serratia on glass slides following 1 h treatment with PUMB02 and Lpc53E1 lipases. Image analysis indicates that both lipases resulted in an effective 90–95% disruption in biofilms in all the pathogens tested (). Scanning electron microscopy (SEM) was subsequently employed to further visualize the biofilm disruption effect of both lipase preparations. Clear inhibition of biofilm formation in V. parahemolyticus treated with the lipases is evident from the SEM image (), with the Lpc53E1 lipase being more effective in inhibiting biofilm formation () when compared with the Oceanobacillus lipase ().
Figure 7. Inhibition of pre-formed biofilms of E. coli, B. cereus, Pseudomonas sp., Vibrio sp., Serratia, and Listeria sp by lipases. (A) The BIC of Oceanobacillus PUMB02 lipase was found at the concentration 150µl for E. coli, B. cereus, Pseudomonas sp., Vibrio sp., S. typhi, and Listeria sp. (B) The BIC of Lpc53E1 lipase was 50 µl for E. coli followed by 100 µl B. cereus and S. typhi and 150 µl for Pseudomonas sp., Vibrio sp., and Listeria sp.
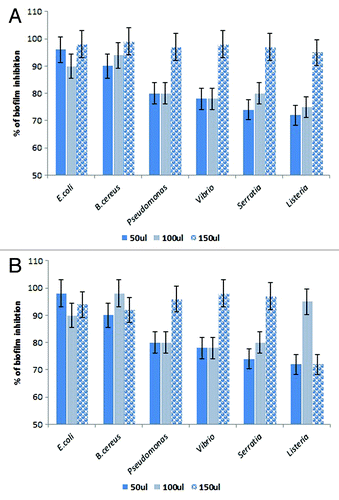
Figure 8. Phase contrast microscopic images showing antibiofilm effect of lipases against biofilm forming pathogens at their BIC.
Discussion
In this paper we report on the isolation, purification and biochemical characterization of a halotolerant thermostable lipase from the marine bacterium Oceanobacillus sp. PUMB02, which was isolated from seawater samples collected from Pondicherry on the east coast of India. We chose this marine environment as it has long been recognized as an untapped source of novel biocatalysts.Citation2 In addition a number of lipases have to date been isolated from both marine bacteria and marine metagenomic libraries which have novel biochemical characteristics.Citation20-Citation22 Lipases from microorganisms are currently the focus of much interest given their large potential in a wide variety of industrial applications in the food, pharmaceutical, cosmetics, fine chemical and dairy industries amongst others coupled with their potential use in anti biofoulingCitation8,Citation23 and anti-biofilm strategies.Citation24
Following screens involving both tributyrin and olive oil plates a bacterial strain (PUMB02) with good activity was isolated, which, following phylogenetic analysis, was classified as an Oceanobacillus species. Oceanobacillus is a genus in the family Bacillaceae which include the genus Ornithinibacillus which can be differentiated based on the unique feature of the presence of l-ornithine in the peptidoglycan.Citation25 The full taxonomic description of PUMB02 is beyond the scope of this report and will be considered in more detail in further research. Oceanobacillus iheyensis is the type species of the Oceanobacillus genus and is an extremely halotolerant and alkaliphilic deep sea species.Citation26
It is not surprising then perhaps that maximum lipase production in Oceanobacillus PUMB02 was observed up to 7% NaCl and that upon purification and subsequent biochemical characterization that the lipase displayed a high level of stability over a wide range of both pH (3–11) and temperature (10–70 °C). This phenomenon of marine derived halotolerant lipases having a high level of stability over a range of pH and temperatures is common; with the Lpc53E1 lipase which was isolated from the metagenome of the marine sponge Haliclona simulans similarly displaying good activity across broad pH (3–12) and temperature (4–60 °C) ranges.Citation16 While another metagenome derived lipase LipC12, isolated from a metagenomic library constructed from fat-contaminated soil collected from a wastewater treatment plant has also been reported to be stable up to 3.7 M NaCl and from pH 6 to 11 and to be active within the pH 4.5 to 10 range.Citation27
Characterization of the PUMB02 lipase by MALDI-TOF MS/MS fingerprint analysis showed partial similarity with a hypothetical protein (with an accession number gi498337881) from Oceanobacillus and a lipase from Ornithinibacillus scapharcae in the NCBI database (with an accession number gi497784402). The molecular mass of the purified PUMB02 lipase was approximately 31 kDa based on MALDI-TOF fingerprint and SDS-PAGE analysis. The molecular mass of lipases varies between strains and typical ranges which have been reported include B. thermocatenulatus (16 kDa),Citation28 Bacillus sp. H-257 (24 kDa),Citation29 Penicillium expansum (25 kDa),Citation30 Pseudomonas pseudoalcaligenes F-111 (32 kDa),Citation31 to Geotrichum candidum (56 kDa).Citation32
Given that marine bacteria are known to possess anti-biofilm activities, with Pseudomonas haloplanktis TAC125 recently being reported to inhibit biofilm formation in Staphylococcus epidermidisCitation33 and another Oceanobacillus strain, Oceanobacillus iheyensis BK6 being reported to produce extracellular polymeric substances (EPS) with anti-biofilm activity against a pathogenic strain of Staphylococcus aureusCitation34; we decided to examine the biofilm disruption potential of the Oceanobacillus PUMB02 lipase together with the aforementioned halotolerant recombinant Lpc53E1 lipase and to specifically target their activity against food pathogens.
Many common food pathogens such as Listeria, Pseudomonas, Bacillus, and Salmonella are potential biofilm forming microbes within the food processing sector, which tends to be a “reservoir” for these pathogens by providing an environment which is conducive to biofilm formation.Citation35,Citation36 Cross-contamination of foods from these persistent pathogen “reservoirs” is a major risk factor in the food processing environment with respect to the spread of food borne diseases within the consumer food chain. For example, contamination of food processing equipment with biofilms has been reported to be a contributing factor in 59% of food-borne disease outbreaks investigated in France.Citation37 The biofilm phenotype typically confers an increased resistance to not only environmental stresses such as UV light but also resistance to various chemical cleaning agents including disinfectants, which are typically used in the food processing area, which makes elimination of these biofilms extremely challenging.Citation10 Enzymes have the potential to be employed in the removal of biofilms formed by food related microorganisms, with proteinase containing disinfectants being reported to be effective against biofilms of Lactobacillus bulgaricus, Lactobacillus lactis, and Streptococcus thermophilus.Citation38
Both the Oceanobacillus PUMB02 lipase and the recombinant Lpc53E1 lipase were effective in disrupting preformed biofilms of E. coli, B. cereus, Pseudomonas sp., Vibrio sp., Serratia sp., and Listeria sp; with the PUMB02 lipase being active against all biofilms tested, while the Lpc53E1 lipase was particularly active against E. coli biofilms (). Various microscopic based approaches including phase contrast microscopy, confocal laser scanning microscopy and scanning electron microscopy was subsequently employed to assess and confirm the effective nature of the biofilm disruption and further visualize the disruptive effect of both the lipase preparations.
As previously mentioned enzymes are known to disrupt biofilms by destroying the physical integrity of the biofilm matrix or so called Extracellular Polymeric Substances (EPS).Citation13,Citation39,Citation40 They achieve this by weakening the protein, carbohydrate, and lipid moieties within the overall EPS structure. Lipids are typically located in the matrix of the biofilm and are involved in surface activity and bacterial attachment, facilitating biofilm development as well as in structural maintenance.Citation41 Thus the disruption of the biofilms observed here by both lipase preparations may have resulted from a loss in the overall integrity of the lipid component of the biofilms resulting in concomitant effects on subsequent biofilm development and maintenance.
The anti-biofilm activity of the marine metagenomic derived Lpc53E1 lipase is particularly interesting. This lipase was isolated from a marine sponge metagenomic library.Citation20 Marine sponges are known to play host to a wide variety of different microbial phyla where they play significant roles in the host’s physiology.Citation42,Citation43 Sponges like other sessile marine organisms are exposed to the threat of microfouling and surface colonization, such as biofilm formation.Citation44 Therefore it is tempting to speculate that sponges may protect their surfaces through the coordinated (sponge-microbial) synthesis of antibiofilm compounds such as for example lipases.
In conclusion this is the first report to our knowledge of the potential application of lipases from the genus Oceanobacillus and of a recombinant metagenome derived lipase in the disruption of biofilms derived from potential food pathogens. Thus it is clear that marine lipases may have potential utility in inhibiting biofilm formation in the food processing area.
Materials and Methods
Isolation and identification of lipase producing microorganism
Seawater samples were collected in 1000 ml sterile sample bottles at 10 min depth from Pondicherry coast at 11° 55′ 51.47″ N, 79° 47′ 6.65″ E located on the east coast of India. The samples were transported to the laboratory under ice within 30 min and stored at 4 °C. Samples were serially diluted in sterile aged seawater and plated on Zobell marine agar, and nutrient agar supplemented with 2% NaCl. The colonies were sub-cultured and single colonies were stored as glycerol stock. The isolates were screened on LB agar supplemented with 1% tributyrin and olive oil respectively. Briefly, 1% of tributyrin and olive oil were sonicated in 50 ml of distilled water for 15 min at the interval of 3 min mixed with 50 ml LB agar supplemented with 2% NaCl to obtain a final volume of 100 ml LB agar. Isolated colonies were streaked on tributyrin and olive oil plates and incubated at 30 °C for 48 h. The colonies showing a clear halo formed by the hydrolysis of tributyrin and olive oil were selected for further characterization. Biochemical, morphological and physiological characteristics of the potential lipase producer PUMB02 were determined by adopting standard methods,Citation45,Citation46 with identification being confirmed following phylogenetic analysis. Briefly, genomic DNA from strain PUMB02 was extracted with a modification of the previously described method of Enticknap et al.Citation47 Universal primers 8F (5′- AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′- GGT TAC CTT GTT ACG ACT T-3′) were used to target the 16S rRNA gene from the isolate with the 16S rRNA gene product being subsequently cloned using the TOPO TA cloning kit according to the manufacturer’s instructions. The 16S rRNA sequence of the isolate was then analyzed using the megablast tool of GenBank (http://www.ncbi.nlm.nih.gov/).Citation48,Citation49 Taxonomic affiliation of the 16S rRNA sequences of the isolate PUMB02 was confirmed with classifier program of RDPII. Multiple alignments of these sequences were performed using Clustal W 1.83 version of EBI (http://www.ebi.ac.uk/cgi-bin/clustalw/) with 0.5 transition weight. Phylogenetic trees were constructed in MEGA 6.0 version (http://www.megasoftware.net) using the maximum parsimony algorithm. The maximum parsimony bootstrapping was performed with 1,000 replicates with pseudorandom number generators.
Metagenomic lipase Lpc53E1
Metagenomic library construction and screening for the isolation of lipase Lpc53E1 has previously been reported in Selvin et al.Citation20 Briefly, a fosmid metagenomic library was constructed from the marine sponge Haliclona simulans and a positive clone pCC1FOS53E1 was obtained by screening on LB agar containing 1% tributyrin. The putative lipase gene Lpc53E1, which encodes a 387 amino acid protein that heterologously expressed in E. coli DH5α cells, was confirmed to possess lipolytic activity.
Production medium
The Oceanobacillus sp. PUMB02 inoculum was prepared by the addition of sterile distilled water to the freshly grown LB slants, and 5 ml of cell suspension was inoculated onto 2 l of sterilized fermentation medium and incubated at 30 °C for 48 at 200 rpm in a shaking incubator. The composition of the fermentation medium was [g/l] 0.1g KH2PO4, 0.02 g NaCl, 0.02 MgSO4, 0.3g yeast extract, 5ml tributyrin, pH 7.2. The production medium obtained after incubation was harvested by centrifugation at 10 621 xg for 30 min at 4 °C. The cell free supernatant (CFS) was then tested for lipase activity and the protein content of the CFS was estimated by the Biuret method.Citation50
Lipase assay
Titrimetric method
Titration of lipase was performed according to Freire et al.Citation51 Briefly one ml of enzyme was added to 19 ml of 5% (w/v) olive oil and 5% (w/v) arabic gum in phosphate buffer in 100 mM Phosphate buffer, pH 7.0, and incubated at 35 °C and 200 rpm for 15 min. An acetone-ethanol mixture (1:1 v/v) was added, and titrated against sodium hydroxide up to a final pH of 10. One unit of lipase was defined as the amount of enzyme that causes the release of 1 μmol of fatty acid per min under the standard assay conditions.
Spectrophotometric assay with p-nitrophenyl palmitate as substrate
Lipase hydrolytic activity of the crude extract was determined in triplicate by hydrolysis of p-nitrophenyl palmitate (p-NPP) at a wavelength of 410 nm according to Winkler and Stuckmann.Citation52 The samples were centrifuged for 10 min at 10 621 xg before reading the absorbance at 410 nm (PG Instruments, UK). Lipase activity unit (IU) was defined as micromoles of p-NPP released min−1 by mg−1 of protein under given conditions.
Statistical optimization by response surface method
A number of individual factors were used for the initial screening by the Plackett-Burman design. In this, a set of 12 experiments was constructed using the Design expert (version 8.07.1) software (Stat-Ease Corporation) for 11 factors (Table S1). The selected 11 factors are A, Coconut oil (ml/L); B, Sucrose (g/L); C, Olive oil (ml/L); D, Ferrous sulfate (mg/L); E, Yeast extract (g/L); F, Tween 20 (ml/L); G, Potassium chromate (mg/L); H, NaCl (% [v/v]); J, Incubation time (h); K, pH; and L, Temperature (°C), which are all considered to be important factors for lipase production. The Plackett-Burman design was used to test each component at two concentration levels, low (-) and high (+). All these experiments were performed in 200 ml Erlenmeyer flasks containing 100 ml of production medium and the response on lipase activity was measured in each experimental conditions. Followed by Plackett-Burman design, a 2n factorial Central Composite Design (CCD) was developed to optimize the concentration of the most significant factors to improve the lipase yield. The most significant factors such as olive oil, sucrose, potassium chromate, and NaCl were selected in the three level concentrations with a set of 30 experiments (Table S2). The other significant factors including pH and temperature were set at a constant level. Response data were analyzed and 3D contour plots generated, indicating the optimum concentrations and interaction among these factors for lipase production.
Purification of lipase PUMB02
Lipase was produced under the optimized conditions and the CFS was collected (crude lipase) by centrifugation at 10 621 x g for 20 min at 4 °C and assayed for lipase and protein content. All purification steps were performed at 4 °C. The crude enzyme was precipitated by adding ammonium sulfate crystals up to 80% saturation; the precipitate was collected after centrifugation at 15 294 xg for 15 min and dissolved in 50 mM TRIS-HCl buffer at (pH 7.5) to achieve a 1/4th of the original volume. The re-dissolved solution was dialyzed against 50 mM TRIS-HCl buffer at (pH 7.5) overnight and applied to a fast Flow (Pharmacia) column (1.6 × 20 cm) pre-equilibrated with the same buffer. Bound proteins were eluted by decreasing the concentration of (NH4)2SO4 from 0.5 to 0 M at a flow rate of 1 ml/min. The active fractions were then pooled, concentrated by using a filter membrane (Millipore) and kept in the refrigerator at 4 °C for further purification. The partially purified lipase from the ammonium sulfate fraction was loaded onto a DEAE-cellulose column previously equilibrated with 50 mM TRIS-HCl buffer (pH 7.5). After washing in two bed volumes of initial buffer, the elution was performed with the negative linear gradient of 0.02, 0.04, 0.06, 0.08 and 0.1N NaCl at a flow rate of 0.5 ml/min. Fractions showing lipase activity were pooled and subjected to Sephadex G-75 column chromatography for further purification.
SDS-PAGE and in-gel-digestion with trypsin
The protein purity and molecular mass was determined using SDS-PAGE.Citation53 Electrophoresis was performed in 15% polyacrylamide gels at a constant voltage (60 V) and the proteins were detected by silver stainingCitation54. “In-gel-digestion” with trypsin was performed in SDS-PAGE gels that were stained with Coomassie brilliant blue G-250. Protein bands were excised from the gel and rinsed three times for 10 min with water (HPLC grade, Merck). Reduction was performed with 0.1 M Tris (pH 8.5) containing 0.01 M ethylenediaminetetra acetic acid, 6 M guanidine HCl, and 25 mM dithiothreitol for 30 min at 37 °C. The proteins were subsequently alkylated with 125 mM iodoacetamide in the dark for an additional period of 1 h at 37 °C. Gel pieces were equilibrated twice with 100 ml of 50 mM ammonium bicarbonate (NH4HCO3, pH 7.8) for 10 min, shrunk with 100 ml of acetonitrile, rehydrated with 100 ml of 50 mM NH4HCO3 (pH 7.8), and finally shrunk with acetonitrile. After air-drying, gel pieces were re-swollen in a digestion buffer, containing 20 ml of 50 mM NH4HCO3, and 0.05 mg of trypsin (Sigma-Aldrich) at 37 °C for 16 h. Peptides were extracted by subsequent incubation with 50 mM NH4HCO3, 0.1% trifluoroacetic acid for 20 min at room temperature and finally with 0.1% TFA: acetonitrile (2:3, v/v). The pellet was dissolved in 10 ml of 0.1% TFA.
MALDI-TOF analysis
The digested protein was applied onto the target plates and subjected to MALDI-TOF analysis (Applied Biosystems). The matrix used was α-hydro-cyano-cinnammic acid (CHCA). The Peptide mass fingerprints obtained from the MALDI-TOF-MS was used to search the NCBI nr protein sequence database using Mascot software (Matrix Science, http://www.matrixscience.com). For each Mascot peptide fingerprint search, the target spectrum was queried against a second (decoy) database with characteristics similar to those of the first. Search parameters included a maximum allowed peptide mass error of 100 ppm with consideration of one missed cleavage per peptide. Accepted modifications included carbamido-methylation of cysteine residues (from iodoacetamide exposure) and carboxymethyl (C), a common modification that typically occurs during SDS-PAGE analysis. The criteria for positive identification of proteins were set with statistical significance (P< 0.05) of the match when tested by Mascot, and the matched peptides covered at least 12% of the whole protein sequence.
Characterization of the lipase
FT-IR analysis was performed to determine the conformation of the lipase on a Thermo Nicolet 6700 FT-IR Spectrophotometer using potassium bromide (KBr) thin films. Sample and KBr was mixed in a ratio of 1:100 and finely ground in a mortar and pestle. The ground mixture was dried and then applied between two disks to make a pellet. The pellet was subsequently analyzed and the data reported in frequency of absorption (cm−1).Citation55
Effect of pH, temperature, and metal ions on the purified enzyme
The temperature stability of the purified lipase obtained from Oceanobacillus sp. PUMB02 collected following column chromatography was assessed at a wide range of temperatures (10- 90 °C) at pH 7 for 3 h. The pH stability was determined by performing the assay at pH values ranging from 3 to 12 at 30 °C for 24 h. Different buffer system were used namely; sodium acetate buffer (pH 3–6), phosphate buffer (pH 7), and glycine-NaOH buffer (pH 8–12).Citation17 Residual activity was determined as previously described. To determine the effects of metal ions like Ca2+, Fe3+, Mg2+, Al3+, Co2+, Mn2+, and Zn2+ on enzyme activity, different concentrations of these metal ions at concentrations up to 10 mM, were added directly to the standard p-NPP assay mixture and the residual activity was calculated.
Substrate specificity of lipase
The substrate specificity of the enzyme was assessed by employing different substrates, namely: 4-nitrophenyl palmitate, 4-nitrophenyl laurate, 4-nitrophenyl caprylate, 4-nitrophenyl formate, and 4-nitrophenyl. The substrate concentration used was 20 mM and the enzyme activity was expressed as U/ml. One unit (U) of lipase activity was defined as µM of p-nitrophenol released by hydrolysis of p-NPP by one ml of enzyme at 30 °C under the assay conditions.
Determination of biofilm inhibitory concentration (BIC)
The BIC was determined as the lowest concentration which produces visible inhibition under phase-contrast microscopic observation and significant reduction (P ˂ 0.05) in OD value of microplate assay at 580 nm (Baldassarri et al. 2006). The BIC of the Oceanobacillus sp. PUMB02 and Lpc53E1 lipases against the test bacteria such as Vibrio parahemolyticus, Pseudomonas fluorescence, Bacillus cereus, Salmonella typhimurium, Escherichia coli, and Listeria monocytogenes was determined by the broth microdilution assay in sterile 96 well microtiter plates (Tarsons). Briefly, pre-grown (36 h) bacterial cell cultures were added to the microtiter plate wells containing nutrient broth to achieve final cell numbers of 1 × 107 cells/mL. The purified PUMB02 lipase contained 1.12 µg protein per µl whereas the dialysed active ammonium sulfate fraction of expressed Lpc53E1 lipase contained 1.6 µg protein per µl. Lipases were added to these wells at varying concentrations (50, 100, 150, 200, 250, and 300 µl in triplicate). The plates were incubated at 30 °C for 48 h and following the incubation period, growth in the presence of the lipase was estimated at OD600 using a microtiter plate reader (Multiskan, Thermo Lab systems). Wells without lipase and those lacking the cells were used as controls.
Evaluation of biofilm disruption potential by a microtiter plate assay
The biofilm test strains were cultured overnight in LB broth (Himedia) and their microtiter plate adherence was determined as per Stepanovic et al.Citation56,Citation57 with suitable modifications. Briefly, cultures were grown in LB broth for 18 h at 37 °C and incubated on a rotary shaker at 200 rpm. The cultures were then centrifuged at 10 621 xg for 5 min at 4 °C and rinsed in phosphate buffered saline (PBS, pH 7.2) three times, then resuspended in LB broth to a turbidity equivalent to 0.5 M McFarland standard. Wells of sterile 96-well U-bottomed microtiter plates were filled with 80 μL of LB broth, 10 μL of each cell suspension and 10 μL each of the test concentration/controls. An aliquot of 100 µl each of cells and lipase and/or control mix was maintained in triplicates. The control was set with a newly developed biofilm, negative control wells were filled with culture broth and 10 μg mL−1 CuSO4 was used as a positive control. All the triplicate wells were set with purified lipases PUMB02 and Lpc53E1. The control and treated wells were set in a Latin-square arrangement to eliminate any potential extraneous variability row-column effects in the microtiter plates, and experiments were repeated six times to statistically validate the results. Plates were placed on a platform shaker to obtain dynamic culture conditions. The initial OD was measured in a microplate plate reader (Labnics) at 595 nm immediately after inoculation and after 18 h of incubation at 37 °C. The plates were then prepared to quantitate the amount of preformed biofilm. The supernatant of the wells were gently removed and washed thrice with 100 µl of PBS using a multichannel pipette. The wells were air-dried and stained with 200 μL of a 0.4% crystal violet solution (w/v) for 10 min. The stain was then removed from the wells and the wells were rinsed twice with 200 μL of deionized water. The wells were allowed to air dry before solubilisation of the crystal violet with 200 μL of dimethyl sulfoxide. The OD was determined at 595 nm in a microplate reader (Labnics). One of the quadruplicate plate was stained with crystal violet and observed under a phase-contrast microscope with × 40 magnifications (Optica) to evaluate the disruption of biofilms.
Confocal laser scanning microscopy (CLSM)
To prepare culture for confocal imaging, the cells were grown in LB broth at 37 °C for 18 h. This fresh culture was used to form biofilm on glass slides and the pre-formed biofilm was treated for 1 h with lipases PUMB02 and Lpc53E1. Untreated biofilms were used as controls and the biofilm coverage thus formed on glass slides were stained with 0.1% acridine orange and subjected to visualization in a CLSM (LSM 710, Carl Zeiss). The 488-nm Ar laser and 500–640 nm band pass emission filter were used to excite and detect the stained cells. Multiple (20) images were scanned and processed using Zen 2009 image software.
Scanning electron microscope (SEM) analysis
A representative strain of Vibrio sp. was used for SEM analysis. An 18 h culture of Vibrio sp. was diluted to 0.1 OD600 and biofilm of Vibrio was allowed to form on pre-sterilized glass surfaces placed in different wells of a 24 well microtiter plate containing 3 ml LB broth. Biofilms which were not subjected to enzyme treatments were used as control. The preformed Vibrio biofilm was fixed for 4 h with 4% glutaraldehyde and washed three times with 0.1 M PBS in the intervals of 5 min, then fixed to the black transparent in 2% osmium tetroxide. Samples were dehydrated and examined using a scanning electron microscopy (Hitachi, Model: S-3400N).
Additional material
Download Zip (264.1 KB)Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
J.K. and A.D.W.D. wish to acknowledge supported by the PharmaSea project (www.pharma-sea.eu) funded by the EU Seventh Framework Programme (contract no. 312184) and by the Beaufort Marine Research Award, part of the Sea Change Strategy and the Strategy for Science Technology and Innovation (2006–2012), with the support of the Marine Institute under the Marine Research Sub-Program of the National Development Plan 2007–2013.
References
- Siddiqui KS, Poljak A, Guilhaus M, De Francisci D, Curmi PM, Feller G, D’Amico S, Gerday C, Uversky VN, Cavicchioli R. Role of lysine versus arginine in enzyme cold-adaptation: modifying lysine to homo-arginine stabilizes the cold-adapted alpha-amylase from Pseudoalteramonas haloplanktis.. Proteins 2006; 64:486 - 501; http://dx.doi.org/10.1002/prot.20989; PMID: 16705665
- Trincone A. Marine biocatalysts: enzymatic features and applications. Mar Drugs 2011; 9:478 - 99; http://dx.doi.org/10.3390/md9040478; PMID: 21731544
- Bornscheuer UT. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev 2002; 26:73 - 81; http://dx.doi.org/10.1111/j.1574-6976.2002.tb00599.x; PMID: 12007643
- López-López O, Cerdán ME, Gonzalez-Siso MI. New Extremophilic Lipases and Esterases from Metagenomics. Curr Protein Pept Sci 2014; http://dx.doi.org/10.2174/1389203715666140228153801; PMID: 24588890
- Gurung N, Ray S, Bose S, Rai V. A broader view: microbial enzymes and their relevance in industries, medicine and beyond. Biomed Res Int 2013; 329-121
- Schreck SD, Grunden AM. Biotechnological applications of halophilic lipases and thioesterases. Appl Microbiol Biotechnol 2014; 98:1011 - 21; http://dx.doi.org/10.1007/s00253-013-5417-5; PMID: 24318008
- Wang Y, Dai Y, Zhang Y, Hu Y, Yang B, Chen S. Effects of quorum sensing autoinducer degradation gene on virulence and biofilm formation of Pseudomonas aeruginosa.. Sci China C Life Sci 2007; 50:385 - 91; http://dx.doi.org/10.1007/s11427-007-0044-y; PMID: 17609896
- Khan MM, Stewart PS, Moll DJ, Mickols WE, Nelson SE, Camper AK. Characterization and effect of biofouling on polyamide reverse osmosis and nanofiltration membrane surfaces. Biofouling 2011; 27:173 - 83; http://dx.doi.org/10.1080/08927014.2010.551766; PMID: 21253926
- Dusane DH, Damare SR, Nancharaiah YV, Ramaiah N, Venugopalan VP, Kumar AR, Zinjarde SS. Disruption of microbial biofilms by an extracellular protein isolated from epibiotic tropical marine strain of Bacillus licheniformis.. PLoS One 2013; 8:e64501; http://dx.doi.org/10.1371/journal.pone.0064501; PMID: 23691235
- Winklestroter LK, dos Reis Teixeira FB, Silva EP, Alves VF, De Martinis EPC. Unravelling microbial biofilms of importance for food microbiology. Microb Ecol 2014; http://dx.doi.org/10.1007/s00248-013-0347-4
- Walker SL, Fourgialakis M, Cerezo B, Livens S. Removal of microbial biofilms from dispenses equipment: the effect of enzymatic pre-digestion and detergent treatment. J Inst Brew 2007; 113:61 - 6; http://dx.doi.org/10.1002/j.2050-0416.2007.tb00257.x
- Xavier JB, Picioreanu C, Rani SA, van Loosdrecht MCM, Stewart PS. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix--a modelling study. Microbiology 2005; 151:3817 - 32; http://dx.doi.org/10.1099/mic.0.28165-0; PMID: 16339929
- Zanaroli G, Negroni A, Calisti C, Ruzzi M, Fava F. Selection of commercial hydrolytic enzymes with potential antifouling activity in marine environments. Enzyme Microb Technol 2011; 49:574 - 9; http://dx.doi.org/10.1016/j.enzmictec.2011.05.008; PMID: 22142734
- Regina VR, Søhoel H, Lokanathan AR, Bischoff C, Kingshott P, Revsbech NP, Meyer RL. Entrapment of subtilisin in ceramic sol-gel coating for antifouling applications. ACS Appl Mater Interfaces 2012; 4:5915 - 21; http://dx.doi.org/10.1021/am301554m; PMID: 23020255
- Berg CH, Kalfas S, Malmsten M, Arnebrant T. Proteolytic degradation of oral biofilms in vitro and in vivo: potential of proteases originating from Euphausia superba for plaque control. Eur J Oral Sci 2001; 109:316 - 24; http://dx.doi.org/10.1034/j.1600-0722.2001.00099.x; PMID: 11695752
- Green JBD, Fulghum T, Nordhaus MA. Review of immobilized antimicrobial agents and methods for testing. Biointerphases 2011; 6:CL2 - 43; http://dx.doi.org/10.1116/1.3645195; PMID: 22239816
- Loiselle M, Anderson KW. The use of cellulase in inhibiting biofilm formation from organisms commonly found on medical implants. Biofouling 2003; 19:77 - 85; http://dx.doi.org/10.1080/0892701021000030142; PMID: 14618691
- Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am J Infect Control 2004; 32:170 - 6; http://dx.doi.org/10.1016/j.ajic.2003.10.009; PMID: 15153929
- Gökçen A, Vilcinskas A, Wiesner J. Biofilm-degrading enzymes from Lysobacter gummosus.. Virulence 2014; 5:378 - 87; http://dx.doi.org/10.4161/viru.27919; PMID: 24518560
- Selvin J, Kennedy J, Lejon DPH, Kiran GS, Dobson ADW. Isolation identification and biochemical characterization of a novel halo-tolerant lipase from the metagenome of the marine sponge Haliclona simulans.. Microb Cell Fact 2012; 11:72; http://dx.doi.org/10.1186/1475-2859-11-72; PMID: 22657530
- Kim JT, Kang SG, Woo JH, Lee JH, Jeong BC, Kim SJ. Screening and its potential application of lipolytic activity from a marine environment: characterization of a novel esterase from Yarrowia lipolytica CL180. Appl Microbiol Biotechnol 2007; 74:820 - 8; http://dx.doi.org/10.1007/s00253-006-0727-5; PMID: 17119955
- Dinica RM, Furdui B, Ghinea IO, Bahrim G, Bonte S, Demeunynck M. Novel one-pot green synthesis of indolizines biocatalysed by Candida antarctica Lipases. Mar Drugs 2013; 11:431 - 9; http://dx.doi.org/10.3390/md11020431; PMID: 23389089
- Khan M, Danielsen S, Johansen K, Lorenz L, Nelson S, Camper A. Enzymatic cleaning of biofouled thin-film composite reverse osmosis (RO) membrane operated in a biofilm membrane reactor. Biofouling 2014; 30:153 - 67; http://dx.doi.org/10.1080/08927014.2013.852540; PMID: 24329165
- Prabhawathi V, Boobalan T, Sivakumar PM, Doble M. Antibiofilm Properties of Interfacially Active Lipase Immobilized Porous Polycaprolactam Prepared by LB Technique. PLoS One 2014; 9:e96152; http://dx.doi.org/10.1371/journal.pone.0096152; PMID: 24798482
- Kämpfer P, Falsen E, Lodders N, Langer S, Busse HJ, Schumann P. Ornithinibacillus contaminans sp. nov., an endospore-forming species. Int J Syst Evol Microbiol 2010; 60:2930 - 4; http://dx.doi.org/10.1099/ijs.0.021337-0; PMID: 20118298
- Lu J, Nogi Y, Takami H. Oceanobacillus iheyensis gen. nov., sp. nov., a deep-sea extremely halotolerant and alkaliphilic species isolated from a depth of 1050 m on the Iheya Ridge. FEMS Microbiol Lett 2001; 205:291 - 7; http://dx.doi.org/10.1111/j.1574-6968.2001.tb10963.x; PMID: 11750818
- Glogauer A, Martini VP, Faoro H, Couto GH, Müller-Santos M, Monteiro RA, Mitchell DA, de Souza EM, Pedrosa FO, Krieger N. Identification and characterization of a new true lipase isolated through metagenomic approach. Microb Cell Fact 2011; 10:54; http://dx.doi.org/10.1186/1475-2859-10-54; PMID: 21762508
- Schmidt-Dannert C, Sztajer H, Stöcklein W, Menge U, Schmid RD. Screening, purification and properties of a thermophilic lipase from Bacillus thermocatenulatus.. Biochim Biophys Acta 1994; 1214:43 - 53; http://dx.doi.org/10.1016/0005-2760(94)90008-6; PMID: 8068728
- Imamura S, Kitaura S. Purification and characterization of a monoacylglycerol lipase from the moderately thermophilic Bacillus sp. H-257. J Biochem 2000; 127:419 - 25; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a022623; PMID: 10731713
- Stöcklein W, Sztajer H, Menge U, Schmid RD. Purification and properties of a lipase from Penicillium expansum.. Biochim Biophys Acta 1993; 1168:181 - 9; http://dx.doi.org/10.1016/0005-2760(93)90123-Q; PMID: 8504153
- Lin SF, Chiou CM, Yeh CM, Tsai YC. Purification and partial characterization of an alkaline lipase from Pseudomonas pseudoalcaligenes F-111. Appl Environ Microbiol 1996; 62:1093 - 5; PMID: 8975602
- Veeraragavan K, Colpitts T, Gibbs BF. Purification and characterization of two distinct lipases from Geotrichum candidum.. Biochim Biophys Acta 1990; 1044:26 - 33; http://dx.doi.org/10.1016/0005-2760(90)90214-I; PMID: 2340308
- Papa R, Parrilli E, Sannino F, Barbato G, Tutino ML, Artini M, Selan L. Anti-biofilm activity of the Antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res Microbiol 2013; 164:450 - 6; http://dx.doi.org/10.1016/j.resmic.2013.01.010; PMID: 23411371
- Kavita K, Singh VK, Mishra A, Jha B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis.. Carbohydr Polym 2014; 101:29 - 35; http://dx.doi.org/10.1016/j.carbpol.2013.08.099; PMID: 24299745
- Kregiel D. Advances in biofilm control for food and beverage industry using organo-silane technology: a review. Food Contr 2014; 40:32 - 40; http://dx.doi.org/10.1016/j.foodcont.2013.11.014
- Tan SYE, Chew SC, Tan SYY, Givskov M, Yang L. Emerging frontiers in detection and control of bacterial biofilms. Curr Opin Biotechnol 2014; 26:1 - 6; http://dx.doi.org/10.1016/j.copbio.2013.08.002; PMID: 24679251
- Midelet G, Carpentier B. Impact of cleaning and disinfection agents on biofilm structure and on microbial transfer to a solid model food. J Appl Microbiol 2004; 97:262 - 70; http://dx.doi.org/10.1111/j.1365-2672.2004.02296.x; PMID: 15239692
- Augustin M, Ali-Vehmas T, Atroshi F. Assessment of enzymatic cleaning agents and distinfectants against bacterial biofilms. J Pharm Sci 2004; 18:55 - 64
- Johansen C, Falholt P, Gram L. Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol 1997; 63:3724 - 8; PMID: 9293025
- Lequette Y, Boels G, Clarisse M, Faille C. Using enzymes to remove biofilms of bacterial isolates sampled in the food-industry. Biofouling 2010; 26:421 - 31; http://dx.doi.org/10.1080/08927011003699535; PMID: 20198521
- Bogino PC, Oliva MdeL, Sorroche FG, Giordano W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci 2013; 14:15838 - 59; http://dx.doi.org/10.3390/ijms140815838; PMID: 23903045
- Baker PW, Kennedy J, Dobson ADW, Marchesi JR. Phylogenetic diversity and antimicrobial activities of fungi associated with Haliclona simulans isolated from Irish coastal waters. Mar Biotechnol (NY) 2009; 11:540 - 7; http://dx.doi.org/10.1007/s10126-008-9169-7; PMID: 19083060
- Jackson SA, Kennedy J, Morrissey JP, O’Gara F, Dobson AD. Pyrosequencing reveals diverse and distinct sponge-specific microbial communities in sponges from a single geographical location in Irish waters. Microb Ecol 2012; 64:105 - 16; http://dx.doi.org/10.1007/s00248-011-0002-x; PMID: 22281804
- Hellio C, Tsoukatou M, Maréchal JP, Aldred N, Beaupoil C, Clare AS, Vagias C, Roussis V. Inhibitory effects of mediterranean sponge extracts and metabolites on larval settlement of the barnacle Balanus amphitrite. Mar Biotechnol (NY) 2005; 7:297 - 305; http://dx.doi.org/10.1007/s10126-004-3150-x; PMID: 15971089
- Collins CH, Lyne PM, Grange JM. Microbiological methods. Butterworths, London, 1989; 480.
- Cappucino JG, Sherman N. Microbiology: a laboratory manual. Pearson Education, Singapore 2004; 491.
- Enticknap JJ, Kelly M, Peraud O, Hill RT. Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl Environ Microbiol 2006; 72:3724 - 32; http://dx.doi.org/10.1128/AEM.72.5.3724-3732.2006; PMID: 16672523
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403 - 10; http://dx.doi.org/10.1016/S0022-2836(05)80360-2; PMID: 2231712
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25:3389 - 402; http://dx.doi.org/10.1093/nar/25.17.3389; PMID: 9254694
- Chykin S. Biochemistry laboratory techniques. John Wiley and Sons. NewYork, London, Sydney. 1966; 88-101.
- Freire DMG, Teles EMF, Bon EPS, Sant’ Anna GL Jr.. Lipase production by Penicillium restrictum in a bench-scale fermenter : effect of carbon and nitrogen nutrition, agitation, and aeration. Appl Biochem Biotechnol 1997; 63-65:409 - 21; http://dx.doi.org/10.1007/BF02920442; PMID: 18576099
- Winkler UK, Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens.. J Bacteriol 1979; 138:663 - 70; PMID: 222724
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680 - 5; http://dx.doi.org/10.1038/227680a0; PMID: 5432063
- Heukeshoven J, Dernick R. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis 1985; 6:103 - 12; http://dx.doi.org/10.1002/elps.1150060302
- Gumel AM, Annuar MSM, Heidelberg T. Enzymatic synthesis of 6-O-glucosyl-poly(3-hydroxyalkanoate) in organic solvents and their binary mixture. Int J Biol Macromol 2013; 55:127 - 36; http://dx.doi.org/10.1016/j.ijbiomac.2012.12.028; PMID: 23305702
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 2000; 40:175 - 9; http://dx.doi.org/10.1016/S0167-7012(00)00122-6; PMID: 10699673
- Kiran GS, Sabarathnam B, Selvin J. Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei.. FEMS Immunol Med Microbiol 2010; 59:432 - 8; PMID: 20528933