ABSTRACT
Elevated level of glucolipotoxicity induces the loss of pancreatic β-cells functions and plays an important role in the development of type 2 diabetes (T2DM). Previous studies have indicated the importance of developing therapies against T2DM, while circular RNA (circRNA) has gained attraction as a modulator of pancreatic β-cell function. In the present study role of circPIP5K1A in dysfunctional β cells and mouse pancreas was comprehensively analyzed. INS-1E, as it has close similarity with naïve pancreatic β-cells, and clinical samples of T2DM patients were used to investigate the effect of circPIP5K1A, miR-552-3p, and Janus kinase 1 (JAK1). While, INS-1E cells were exposed to PAHG conditions (0.5 mM palmitic acid and 28 mM glucose) as studies have suggested that increased level of fatty acid and glucose resulted in autophagy activation of pancreatic β-cells that leads to T2DM. Key player of JAK1-STAT3 pathway and the level of Reactive Oxygen Species, inflammatory factors, and insulin secretion was detected to analyze the of the active association of circPIP5K1A, miR-552-3p with JAK1pathway. Our study has revealed the elevated level ofcircPIP5K1A and JAK1, but reduced level of miR-552-3pin the serum of T2DM patients. Furthermore, we also found that reduced expression ofcircPIP5K1A leads to decreased rate of inflammation, oxidative damage and apoptosisinINS-1E cells induced by glucolipotoxicity. CircPIP5K1A was available to competitively combine with miR-552-3p, while whose direct target was JAK1. In conclusion, our study suggested a novel involvement of circPIP5K1A in a cross talk between miR5523p/JAK1/STAT3 pathways in β-cells as a new therapeutic target for T2DM.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a long-term illness featured via insulin resistance and pancreatic β-cell dysfunction [Citation1,Citation2]. Glucose and fatty acid provide energy for proper functioning of the cells but their elevated level resulted in β-cell dysfunction which may leads to increase rate of β-cell apoptosis, inhibition of glucose-stimulated insulin secretion, which ultimately reduced the β-cell mass and induce T2DM [Citation2,Citation3]. Additionally, the fusion of glucolipotoxicity enhanced the damaging effects of chronic elevations of glucose and fatty acids on pancreatic β cells [Citation4,Citation5]. Therefore, it is essential to investigate the molecular mechanisms of β-cell adaptation for designing the new therapies for T2DM.
Circular RNAs (circRNAs) are newly discovered endogenous non-coding RNA [Citation6]. Meanwhile, circRNA forms a covalently closed loop with no free ends 3ʹ and 5ʹ [Citation7,Citation8]. Until now, circRNAs are famous for being extensively manifested in many tissues and eukaryotes and function in various biological and pathological processes [Citation9]. For instance, circRNAs are supposed to be involved in β-cell dysfunction mediated by pro-inflammatory cytokines, thereby advancing type 1 diabetes Mellitus (T1DM) [Citation10]. Circ-Tulp4 accelerates β-cell orientation to glucolipotoxicity via modulating soat1 [Citation2]. CircPIP5K1A is a newly discovered circRNAs linked with cancer [Citation8]. Li [Citation11]et al. reported that circPIP5K1A is upregulated in T1DM patients. Nevertheless, the latent regulatory mechanism of circPIP5K1A in β cells of T2DM requires further elucidation.
MicroRNA (miRNA) is a subgroup of non-coding RNA (ncRNA). MiRNA particularly combines with the 3ʹ-untranslated region (UTR) of messenger RNA (mRNA), control gene at the post-transcriptional level, and takes part in development of all kinds of diseases [Citation11–13]. Cell cycle is an important event in the process of cell proliferation while any abnormality in this process such as uncontrollable division leads to serious health conditions for instance in cancer [Citation11]. Till the date number of miRNAs have been claimed to be involved in the development and progression of pancreatic cancer [Citation14]. Furthermore, some studies have suggested that increased expression of miR-552-3p results in development and reoccurrence of ovarian cancer [Citation15].
Other than cancer metabolism related diseases, such as glycolipid metabolism is one of the main concern among health-related problems around the world as lipid and glucose metabolism irregularities resulted in glycolipid metabolic disorders such as diabetes. Fan et al. [Citation16] have suggested that miR-552-3p is the novel modulator of ameliorate hepatic glycolipid metabolic diseases by regulating LXRα- and FXR-related transcriptional activities in the nucleus. Ma et al. [Citation17] also have suggested that miR-552-3p is a novel inhibitor with a dual repressive mechanism, which makes miRNA-552-3P a potential therapy against hyperlipidemia. Lei et al. [Citation18] reported that miR-552-3p controls FXR’s and LXR’s transcriptional activity to mitigate liver glucose and lipid metabolism disorders. Janus kinase 1 (JAK1)-STAT3 signal pathway is a cytokine-induced signal transduction pathway found recently. JAK/STAT3 pathway consisted of tyrosine kinase-linked receptors, Janus kinases, and signal transducers and transcriptional activators [Citation19]. This pathway participates in numerous momentous biological processes like immune modulation, differentiation, and apoptosis [Citation20]. The studies of Francesca M Couto et al. [Citation16] emphasized that Exenatide (Ex-4) protects β cells from cytokine-induced cell death via repressing JAK1-STAT1. Arianna Mazzoliet al. [Citation21] announced that JNK1 ablation perfects the glucose tolerance of db/db mice via declining the loss of functional β cells in diabetes. However, whether miR-552-3p controls the β cells of T2DM through the JAK1-STAT3 pathway is still uncertain. These findings suggested that miR-552-3p may function as a possible target for metabolism-related diseases.
Objective of this study was to investigate the role and molecular mechanism of circPIP5K1A in β cells of T2DM and to investigate the link among circPIP5K1A, miR-552-3p with JAK1-STAT3 pathway. We investigated the correlation between circPIP5K1A and miR-552-3p, and circPIP5K1A and JAK1. Meanwhile, we also investigated the binding ability of circPIP5K1A and miR-552-3p, and miR-552-3p and JAK1 3ʹUTR. Finally, we illustrated the role of circPIP5K1A in regulating miR-552-3p and indirectly affecting the JAK1 expression.
2 Materials and methods
2.1 Clinical sample
Human blood serum samples of healthy and T2DM patients were collected from Harvest of First Affiliated Hospital of Zhengzhou University. According to the diagnostic criteria of WHO (1999), oral glucose tolerance test (OGTT) has been conducted, with fasting blood glucose (FPG) ≥7.0 mmol/L, and postprandial 2 h blood glucose (2 h PG) ≥11.1 mmol/L was conducted. Exclusion standards were patients bearing hypertension, malignant tumors, polycystic ovary syndrome, lung diseases, and autoimmune diseases; patients carrying other diseases impacting glucose metabolism were excluded, like hyperthyroidism, acute metabolic syndrome, acromegaly, and Cushing’s syndrome or other stress conditions and smokers. Inclusion of subjects with FPG < 6.1 mmol/L and 2 h PG < 7.8 mmol/L was considered as healthy controls. Quick freezing of the sample was done in liquid nitrogen and then the sample was stored at −80°C for later use. The project was approved via the Ethics Committee of First Affiliated Hospital of Zhengzhou University. Signed consent from all participants has been obtained before sampling.
2.2 Cell culture
The rat β cell line INS-1E was purchased from the National Cell Resource Center (Beijing, China). INS-1E cells was cultured and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium consisting of glucose (11.1 mM), supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin, 100 µg/mL streptomycin, 1 mM sodium pyruvate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 50 μM 2-mercaptoethanol (all from Life Technologies, CA, USA). To induce glucolipotoxicity, INS-1E cells were exposed to palmitic acid (PA) and high glucose (PAHG) conditions for 24 h, which consisted of 0.5 mM PA and 28 mM glucose (both from Sigma-Aldrich, MO, USA) [Citation22].
2.3 Cell transfection
CircPIP5K1A (si-circPIP5K1A#1, si-circPIP5K1A#2, and si-circPIP5K1A#3), miR-552-3p mimic/inhibitor, JAK1 elevation plasmid, and the corresponding negative control (NC) were obtained from RiboBio (Guangzhou, China). According to the manufacturer’s protocol, INS-1E cells have been transfected with these oligonucleotides for 48 hours by using Lipofectamine 2000 (Invitrogen, CA, USA) [Citation14].
2.4 Cell counting kit (CCK-8) assay
Glucolipotoxicity of PAHG on INS-1E cells has been determined by CCK-8 (Beyotime Biotechnology, Shanghai, China) by following the manufacturer’s protocol. INS-1E cell has been cultured in 96-well plate with the cell density of 4000 cells/well and allowed to grow for next 4 days. Absorbance was read at 450 nm wavelength in a plate reader (DU650, Beckman Coulter, CA, USA). However, the cell viability graph has been plotted by using mean values [Citation23].
2.5 Flow cytometry
Apoptosis rate of INS-1E cells was examined by flow cytometry by using annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) according to the (BD Biosciences, CA, USA) protocol. In short, INS-1E cells were cultured in 6-well plate for 4 days. Then the cells were harvested, rinsed off, and resuspended in PBS (phosphate buffer saline). Rate of apoptotic cell death was measured by usingAnnexin V-FITC and PI double staining. Reactive oxygen species (ROS) production was also examined via fluorescence-activated cell sorting (FACS) analysis. For the detection of ROS, the cells were harvested, resuspended in PBS and incubated with 5 μM DCFDA, and analyzed immediately by flow cytometry, as described previously [Citation3].
2.6 Enzyme-linked immunosorbent assay (ELISA)
Total inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6 production in the serum was detected by using ELISA kit according to the manufacturer’s protocol (Sigma-Aldrich) [Citation24].
2.7 Glucose-stimulated insulin secretion determination
To determine the glucose-stimulated insulin secretion, INS-1E cells were cultured in RPMI-1640 (containing 0.5 mM PA) supplemented with KRB buffer and 28 mM glucose for 4 d. The insulin secretion was determined by strictly following the instructions of ELISA kit (Sigma, MO, USA) [Citation24].
2.8 Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the INS-1E cells by using TRIzol reagent (Invitrogen)by using manufacturer’s protocol and quantified by spectrophotometry followed by reverse transcription of RNA into cDNA. RT-qPCR was performed via miScript SYBR-Green PCR Kit (Qiagen, Dusseldorf, Germany). The primer sequences of the target genes are listed in . U6 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. Determination of the result was via the 2−ΔΔCT method [Citation14].
Table 1. Primer sequence
2.9 Western blot
To elucidate the effect of PAHG on the INS-1E cells, the Western blot analysis has been performed. The INS-1E cells has been cultured and maintained in PRMI-1640 and exposed to PAHG conditions for the determined time period. After that, the cells were washed with PBS and trypsinized and incubated with protein lysis buffer for 30 minutes on ice. The samples were then centrifuged at 4°C for 10 min at 12000 rpm. Supernatant has been transferred to the new Eppendorf, and protein concentration was measured. Protein lysates were separated by using SDS-PAGE and PVDF technique of electrophoresis. PVDF membrane was blocked in 5% of BSA followed by incubation with relevant antibodies including primary anti-JAK1 (1: 1000), anti-p-STAT3 (1: 1000), anti-STAT3 (1: 1000), anti-GAPDH (1: 1000)), and then treatment with horseradish peroxidase-conjugated secondary antibody was visualization. However, all antibodies were obtained from Abcam (MA, USA) [Citation11].
2.10 Subcellular separation
Subcellular separation has been performed by using PARIS kit (Life Technologies). In line with the manufacturer’s protocol, the cells were assigned to nuclear and cytoplasmic parts prior to proceeding with the RNA isolation procedure. In the meantime, GAPDH or U6 was considered cytoplasmic and nuclear controls [Citation14].
2.11 The luciferase activity assay
To investigate the effect of PHAG condition on circPIP5K1A, miR-552-3p, and JAK1in INS-1E cells, luciferase assay has been performed. This pathway participates in numerous momentous biological processes like immune modulation, differentiation, and apoptosis. Prediction of the latent binding link among circPIP5K1A, miR-552-3p, and JAK1 was through the website of starbase (http://starbase.sysu.edu.cn). The 3ʹUTR sequence of circPIP5K1A or JAK1 consisting of miR-552-3p wild-type (WT) or mutant (MUT) binding sites was integrated into the pmirGLO vector (Promega, WI, USA) to form WT-circPIP5K1A or MUT-circPIP5K1A and WT-JAK1 or MUT-JAK1, separately. Briefly the transfection of miR-552-3p or mimic-NC has been done by using Lipofectamine 2000 (Invitrogen) by following the manufacturer’s protocol. After 48 h of transfection, luciferase activity was measured via dual-luciferase reporting kit (Promega) [Citation19].
2.12 RNA-pull down assay
RNA-pull down assay has been executed by using pierce magnetic RNA-protein pull down kit (Sigma Aldrich). Briefly, biotin-labeled miR-552-3p WT/MUT plasmids (50 nM each) were transfected into INS-1E cells for 24 h. Afterword, the cells were harvested rinsed off and incubated with M-280 streptavidin magnetic beads (Sigma). Then, biotin-bound proteins were pull down, extracted, and enrichment of circPIP5K1A was detected by RT-qPCR analysis while an antagonistic miR-552-3p probe was set up as an NC [Citation14].
2.13 RNA binding protein immunoprecipitation (RIP) assay
RNA binding protein immunoprecipitation (RIP) assay was executed by using Magna RNA RIP Kit (Merck KGaA, Darmstadt, Germany) by following the manufacturer’s protocol. INS-1E cells were lysed by using RIP lysis buffer (Beyotime) and incubated with magnetic beads conjugated with Argonaute-2 (Ago2, Millipore, MA, USA) or control immunoglobulin G (IgG, Millipore) at 4ʹC overnight. Detachment of the complex was with proteinase K (Sigma-Aldrich), and the bound RNA was identified by RT-qPCR [Citation14].
2.14 Statistical analysis
The data were statistically analyzed via SPSS 22.0 and presented as the mean ± standard deviation (SD) of three independent experiments. One-way analysis of variance (ANOVA) and Student’s t-test were applied to compare the differences the among groups. Pearson’s correlation was conducted to analyze the link among circPIP5K1A, miR-552-3p, and JAK1, and the value P < 0.05 was considered as statistically significant.
3 Results
Studies have suggested that miR-552-3p is a novel inhibitor [Citation18] and is involved in numerous momentous biological processes such as immune modulation, differentiation, and apoptosis [Citation20]. However, Arianna Mazzoli et al. [Citation21] found that decreased expression of JNK1 resulted in improved glucose tolerance in db/db mice by downregulating the dysfunctional β cells in diabetes. However, whether miR-552-3p controls the β cells of T2DM through the JAK1-STAT3 pathway still needs to be addressed. The aim to conduct this study was to evaluate the molecular mechanism of circPIP5K1A in β cells of T2DM, and how it regulates the miR-552-3p with JAK1-STAT3 pathway.
3.1 Elevated circPIP5K1A and JAK1, but reduced expression of miR-552- in the serum sample of T2DM patients
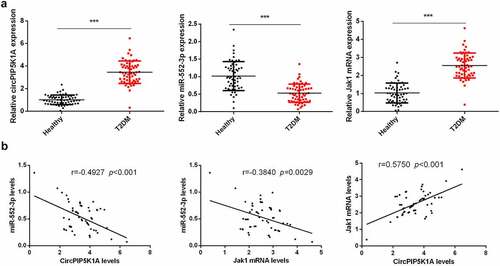
Relative expression of circPIP5K1A, JAK1, and miR-552-3p in blood samples of T2DM patients was measured, while healthy individual’s blood samples were used as control. Our findings manifested that level of circPIP5K1A and JAK1 in the serum of T2DM patients was significantly upregulated and miR-552-3p level was apparently reduced as compared to the control (). We further suggested that serum circPIP5K1A with miR-552-3p and miR-552-3p with JAK1 were negatively linked, while circPIP5K1A was positively correlated with JAK1 (). These results provided the first evidence that elevated circPIP5K1A was vital in the development of T2DM.
Figure 1. Elevated circPIP5K1A and JAK1 but reduced miR-552-3p are presented in the serum sample of T2DM patients.
3.2 Downregulated circPIP5K1A represses the inflammation, oxidative damage, and apoptosis of INS-1 β cell induced via glucolipotoxicity
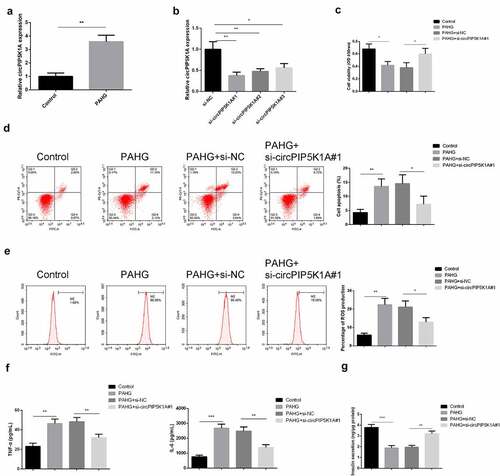
Glucolipotoxicity injury of pancreatic β cells is a key pathological feature of T2DM. In order to investigate the glucolipotoxicity-induced circPIP5K1A expression in INS-E1 β cell, INS-1E cells were exposed to PAHG conditions, and detection of circPIP5K1A was conducted. The results manifested that circPIP5K1A was enhancive under PAHG conditions (). To confirm our findings that PAHG was directly associated with increased expression of circPIP5K1A, transfection of si-circPIP5K1A inINS-1E cells has been performed. Outcomes suggested that si-circPIP5K1A#1 manifested higher silencing efficiency (), so si-circPIP5K1A#1 was selected for subsequent experiments. Our findings suggested that exposure of INS-1E cells to PAHG leads to increased rate of the cell proliferation, and silencing of circPIP5K1A can reverse the damaging effects of PAHG in INS-1E cells (). The results of FACS analysis of ROS identified that silencing of circPIP5K1A have the potential to reverse the effects of PAHG-induced oxidative damage in INS-1E cells (). Detection of the inflammatory factors clarified that after INS-1E cells’ exposure to PAHG, TNF-α and IL-6 was elevated, while silencing circPIP5K1A could reduce inflammation reaction (). After measuring insulin secretion, it came out that PAHG introduction lessened the insulin secretion response of INS-1E cells to 16.7 mM glucose by about 50%, while depressive circPIP5K1A could partially turn around this response (). The results clarified that silencing of circPIP5K1A could reduce the toxic effect of glycolipid toxicity on INS-1 β cell.
Figure 2. Downregulated circPIP5K1A restrains the inflammation, oxidative damage, and apoptosis of INS-1 β cell induced via glycolipid toxicity.
3.3 CircPIP5K1A adsorbs miR-552-3p in INS-1E cells
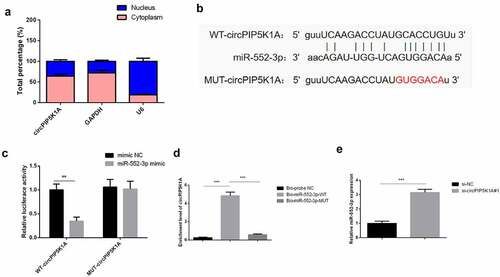
In order to further understand how circPIP5K1A plays a key role in INS-1E cells, a sub-cellular hierarchical analysis was first performed to detect its cellular location in INS-1E cells. In line with the results, circPIP5K1A seemed to be majorly situated in INS-1E cells’ cytoplasm (). Therefore, it was speculated that circPIP5K1A might post-transcriptionally control the gene via acting as a competitive endogenous RNA (ceRNA) in INS-1E. Through starbase (http://starbase.sysu.edu.cn/) was found a binding site of circPIP5K1A with miR-552-3p (). Moreover, upregulated miR-552-3p apparently reduced the luciferase activity of pmirGLO-circPIP5K1A-WT, while no apparent transformations in the luciferase activity of MUT existed in disparate groups (). An experiment was conducted to verify that circPIP5K1A could be applied as a ceRNA to adsorb miR-552-3p. It was emphasized that the enrichment of circPIP5K1A in the Bio-miR-552-3p-WT was clearly elevated vs. that of Bio-miR-552-3p-MUT and Bio-probe NC groups (). And depression of circPIP5K1A was available to elevate miR-552-3p (). The above results emphasized that circPIP5K1A modulated miR-552-3p via competitively combining with miR-552-3p.
Figure 3. CircPIP5K1A adsorbs miR-552-3p in INS-1E cells.
3.4 Silencing of miR-552-3p reverse the depletive effects of circPIP5K1A by reducing glucolipotoxicity on INS-1 β cell
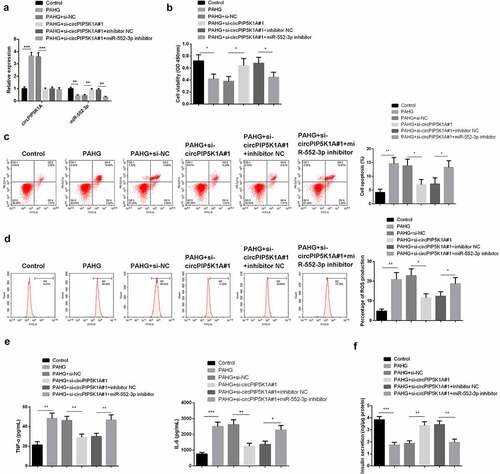
For clarifying whether circPIP5K1A’s reduction of glucolipotoxicity in INS-1E cell is depended on the modulation of miR-552-3p, application of si-circPIP5K1A#1 + miR-552-3p inhibitor was to transfect INS-1E cells. The analysis manifested that miR-552-3p expression was reduced under PAHG conditions, while silencing of circPIP5K1A restored miR-552-3p (). Additionally, in PAHG-induced INS-1E cells, restrained miR-552-3p could reverse the motivation in cell proliferation via repressive circPIP5K1A (). Detecting cellular inflammatory factors manifested in PAHG-induced INS-1E cells, downregulation of circPIP5K1A could restrain ROS, TNF-α, and IL-6, while simultaneously reducing circPIP5K1A and miR-552-3p could reverse this effect (). Insulin secretion measurement revealed that depressive circPIP5K1A and miR-552-3p clearly restored the insulin secretion affected via si-circPIP5K1A#1 transfection (). The above results clarified in PAHG-induced INS-1E cells, restrained miR-552-3p could reverse the mitigation of declined circPIP5K1A on cell inflammation, oxidative damage, and apoptosis induced by glucolipotoxicity.
Figure 4. Downregulating miR-552-3p turns around that dissipative circPIP5K1A reduces the toxic effect of glycolipid toxicity on INS-1 β cell.
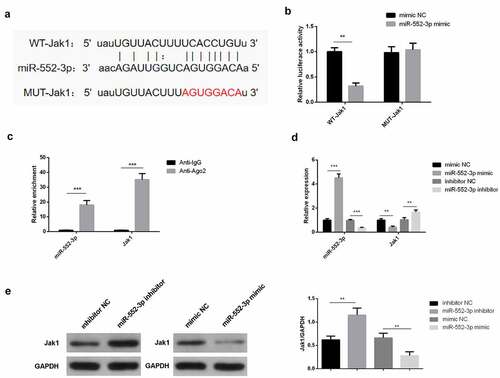
3.5 JAK1 performs as a targeted mRNA downstream of miR-552-3p
Through the starbase website was discovered a binding site of miR-552-3p with JAK1 (). It has been found that the combination of JAK1 with miR-552-3p did exist (), which was consistent with the results of the RIP assay (). Meanwhile, miR-552-3p promotes the expression of JAK1 while miR-552-3P inhibitor downregulated the JAK1 (). In short, miR-552-3p directly targeted JAK1 and negatively modulated its expression.
Figure 5. JAK1 acts as a targeted mRNA of miR-552-3p.
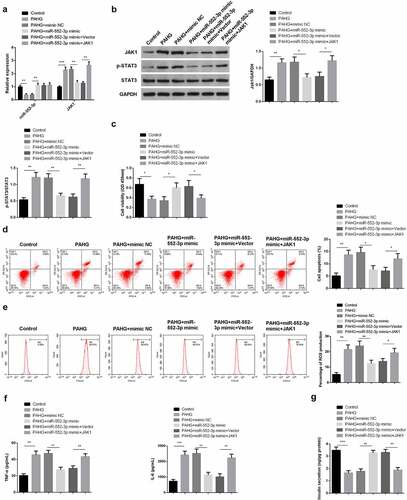
3.6 MiR-552-3p restrains the inflammation, oxidative damage, and apoptosis of INS-1 β cell induced via glucolipotoxicity by targeting the JAK1-STAT3 pathway
For further clarifying the function and molecular mechanism of miR-552-3p in glucolipotoxicity-induced INS-1 β cell, transfection of miR-552-3p mimic with JAK1 elevation vectors was into PAHG-treated INS-1E cells. Meanwhile, detecting miR-552-3p and JAK1-STAT3 pathway was conducted. The results affirmed that co-transfection of miR-552-3p mimics and JAK1 overexpression plasmid significantly reverse the effects of miR-552-3p mimics by upregulating the expression of JAK1 and leads to the phosphorylation of STAT3 (). Additionally, in PAHG-induced INS-1E cells, miR-552-3p could induce the motivation in cell advancement, which was turned around via JAK1 (). Detecting cellular inflammatory factors manifested in PAHG-induced INS-1E cells, miR-552-3p repressed ROS, TNF-α, and IL-6, while simultaneous upregulation of miR-552-3p and JAK1 reversed this effect (). Measuring insulin secretion revealed that miR-552-3p facilitated insulin secretion, while JAK1 was opposite (). The above results clarified that in PAHG-induced INS-1E cells, miR-552-3p reduced the toxic effect of glucolipotoxicity on INS-1E cells via targeting the JAK1-STAT3 pathway.
Figure 6. MiR-552-3p refrains the inflammation, oxidative damage, and apoptosis of glycolipid toxicity-stimulated INS-1 β cells via targeting the JAK1-STAT3 pathway.
4 Discussion
Progressive decrease in pancreatic β cells function may lead toward chronic diseases such as T2DM [Citation5]. A study has also manifested that elevated level of glucose and fatty acid are the point of concern for increasing ratio of T2DM-related deaths [Citation25]. Owing to numerous inconsistent reports the exact mechanism of glucolipotoxicity in pancreatic β cells is still remaining unclear. Hence, the aim of this study was to clarify the mechanism in which circPIP5K1A controlled glucolipotoxicity in INS-1E cells through the miR-552-3p/JAK1-STAT3 axis.
Although its reported that circRNAs has played a significant role in the development of certain life-threatening diseases such as cancer and diabetes [Citation26,Citation27]. Recently, Wang et al. reported that 1,020 elevated and 902 reduced circRNAs are identified in β cells introduced with cytokines, and repressive circRNA 000286 and 01727 in β cells can stimulate apoptosis by affecting insulin biosynthesis and secretion [Citation28]. Refrained hsa_circ_0054633 prevents HG-induced human pancreatic β-cell apoptosis and impaired insulin secretion via controlling the miR-409-3p/caspase-8 axis [Citation29]. The circLRP6/miR-9-5p/PRMT axis impacts the cell proliferation, insulin stimulation, and oxidative stress of INS-1E cells attacked by streptozotocin (STZ) [Citation30].
Till date, CDR1, cerebellum degeneration-related antigen 1, also termed as ciRS-7, is most known diabetes-related circRNA, and it is produced from a natural antisense strand of CDR1. Studies have shown that increased expression of CDRI may lead to the elevated secretion level of insulin, and this increased insulin secretion subsequently repaired the dysfunctional β cells [Citation27]. miR-7 is a powerful inhibitor [Citation27] of CDR1and stimulate the proliferation of β cells and increased the secretion of insulin via encouraging the expression of Pax6 and Myrip genes [Citation27]. On the other hand, in vivo studies in transgenic mice have shown that increased expression of miR-7a resulted in decreased level of insulin secretion in β cell followed by dedifferentiation of β cells [Citation31]. Stoll et al. investigated the expression of circHIPK3 and ciRS-7/CDR1 and found that decreased expression of circHIPK3 and ciRS-7/CDR1 was reduced in the islets of diabetic transgenic mice [Citation32]. While, silencing of circHIPK3 and ciRS-7/CDR1 in the small patches of normal mice caused impaired insulin secretion, poor rate of β-cell proliferation, and lower survival rate, indicating a possible role of transformed expression of circHIPK3 and ciRS-7 in the development of diabetes mellitus [Citation32]. These findings goes parallel with those of Cao et al., and it stated that the decreased expression of circHIPK3 in diabetic patients was significantly associated with high glucose-treated HUVECs and primary aortic endothelial cells (HAECs) [Citation33]. These findings further suggested that circular RNAs might be possible remedial targets in diabetes.
In this study, increased expression of circPIP5K1A has been found in serum samples of T2DM patients as compared to controls. However, studies have shown that increased expression of circPIP5K1A is involved in upregulation of cell proliferation by modulating the cell cycle and apoptosis [Citation22]. On the one hand, in our study, we have found that glucolipotoxicity-induced downregulation of circPIP5K1A repressed the inflammation, oxidative damage, and apoptosis of INS-1E cells. These findings assured that circPIP5K1A might be crucial in the pathogenesis of T2DM.
MiRNAs always interact with circRNAs and play a bridge role in perfecting disease signaling pathways [Citation34]. Circ_001209 worsens diabetic retinal vascular dysfunction via mediating miR-15b-5p/COL12A1 [Citation35]. Silencing of circANKRD36 targets miR-145 via XBP1 to restrain STZ-caused insulin resistance and inflammation in diabetic rats [Citation23]. CircEIF4G2 aggravates the renal fibrosis of diabetic nephropathy through sponging miR-218 [Citation36]. Meanwhile, the study clarified that circPIP5K1A motivated the cell inflammation, oxidative damage, and apoptosis induced by glucolipotoxicity via absorbing miR-552-3p. Moreover, miR-552-3p targeted JAK1 3ʹ UTR and negatively controlled its expression. A former study emphasizes that JNK1 ablation mitigates glucose tolerance in db/db mice via lessening the loss of functional β cells in db/db obesity-driven diabetes [Citation37]. Additionally, T cell protein tyrosine phosphatase non-receptor type 2 can help decline periodontal inflammation in T2DM via refraining the dephospho-protein substrate in the JAK1/STAT3 pathway [Citation38]. Similarly, our results also demonstrated that miR-552-3p restrained the inflammation, oxidative damage, and apoptosis of glucolipotoxicity-stimulated INS-1 β cell via targeting the JAK1-STAT3 pathway. Hence, it can be concluded that depressive circPIP5K1A via miR-552-3p/JAK1-STAT3 axis reduces the mechanism of INS-1E cytotoxicity induced by glucolipotoxicity.
5 Conclusions
In general, in T2DM tissues and cells, circPIP5K1A and JAK1 were upregulated, but miR-552-3p was downregulated. Repressing circPIP5K1A via miR-552-3p/JAK1-STAT3 axis restrains the inflammation, oxidative damage, and apoptosis of glycolipid toxicity-stimulated INS-1E cells. CircPIP5K1A is expected to become a latent clinical biomarker of T2DM, offering new ideas for further understanding of the molecular pathology of T2DM. It is of great significance for formulating specific targeted therapy strategies for T2DM.
Highlights
1. Elevated circPIP5K1A and JAK1 but reduced miR-552-3p were presented in the serum of T2DM patients.
2. Downregulating circPIP5K1A reduced the toxic effect of glucolipotoxicity on INS-1 β cells.
3. CircPIP5K1A adsorbed miR-552-3p, which targeted JAK1.
4. CircPIP5K1A modulated INS-1E cell dysfunction induced via glucolipotoxicity through miR-552-3p/JAK1-STAT3 axis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Li R, Sun X, Li P, et al. GLP-1-induced AMPK activation inhibits PARP-1 and promotes LXR-mediated ABCA1 expression to protect pancreatic beta-cells against cholesterol-induced toxicity through cholesterol efflux. Front Cell Dev Biol. 2021;9:646113.
- Wu L, Xiong L, Li J, et al. Circ-Tulp4 promotes beta-cell adaptation to lipotoxicity by regulating soat1 expression. J Mol Endocrinol. 2020;65(4):149–161.
- Alnahdi A, John A, Raza H. N-acetyl cysteine attenuates oxidative stress and glutathione-dependent redox imbalance caused by high glucose/high palmitic acid treatment in pancreatic Rin-5F cells. PLoS One. 2019;14(12):e0226696.
- Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45(3):273–283.
- Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes–a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143(2):339–342.
- Meng X, Li X, Zhang P, et al. Circular RNA: an emerging key player in RNA world. Brief Bioinform. 2017;18(4):547–557.
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338.
- Zheng S, Zhang X, Odame E, et al. CircRNA-protein interactions in muscle development and diseases. Int J Mol Sci. 2021;22(6):3262.
- Guo JU, Agarwal V, Guo H, et al. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15(7):409.
- Wang F, Zhang M. Circ_001209 aggravates diabetic retinal vascular dysfunction through regulating miR-15b-5p/COL12A1. J Transl Med. 2021;19(1):294.
- Li Y, Zhou Y, Zhao M, et al. Differential profile of plasma circular RNAs in type 1 diabetes mellitus. Diabetes Metab J. 2020;44(6):854–865.
- Zheng K, Xie H, Wu W, et al. CircRNA PIP5K1A promotes the progression of glioma through upregulation of the TCF12/PI3K/AKT pathway by sponging miR-515-5p. Cancer Cell Int. 2021;21(1):27.
- Song H, Xu Y, Xu T, et al. CircPIP5K1A activates KRT80 and PI3K/AKT pathway to promote gastric cancer development through sponging miR-671-5p. Biomed Pharmacother. 2020;126:109941.
- Ma Y, Cong X, Zhang Y, et al. CircPIP5K1A facilitates gastric cancer progression via miR-376c-3p/ZNF146 axis. Cancer Cell Int. 2020;20(1):81.
- Ma N, Fan L, Dong Y, et al. New PCSK9 inhibitor miR-552-3p reduces LDL-C via enhancing LDLR in high fat diet-fed mice. Pharmacol Res. 2021;167:105562.
- Fan L, Lai R, Ma N, et al. miR-552-3p modulates transcriptional activities of FXR and LXR to ameliorate hepatic glycolipid metabolism disorder. J Hepatol. 2021;74(1):8–19.
- Zhou Y, He H, Xu H, et al. Association of oncogenic bacteria with colorectal cancer in South China. Oncotarget. 2016;7(49):80794–80802.
- Wang X, Dou N, Wang J, et al. FOXM1-induced miR-552 expression contributes to pancreatic cancer progression by targeting multiple tumor suppressor genes. Int J Biol Sci. 2021;17(4):915–925.
- Zhao W, Han T, Li B, et al. miR-552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12(1):121.
- Sun L, Wei Y, Wang J. Circular RNA PIP5K1A (circPIP5K1A) accelerates endometriosis progression by regulating the miR-153-3p/Thymosin Beta-4 X-Linked (TMSB4X) pathway. Bioengineered. 2021;12(1):7104–7118.
- Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009.
- El-Assaad W, Buteau J, Peyot M-L, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144(9):4154–4163.
- Ma J, Wu Y, He Y. Silencing circRNA LRP6 down-regulates PRMT1 to improve the streptozocin-induced pancreatic beta-cell injury and insulin secretion by sponging miR-9-5p. J Bioenerg Biomembr. 2021;53(3):333–342.
- Juan-Mateu J, Rech TH, Villate O, et al. Neuron-enriched RNA-binding proteins regulate pancreatic beta cell function and survival. J Biol Chem. 2017;292(8):3466–3480.
- Zhu XY, Guo Q, Zhu M, et al. HLX affects cell cycle and proliferation in AML cells via the JAK/STAT signaling pathway. Oncol Lett. 2020;20(2):1888–1896.
- Maiese K. Disease onset and aging in the world of circular RNAs. J Transl Sci. 2016;2(6):327–329.
- Xu H, Guo S, Li W, et al. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci Rep. 2015;5(1):12453.
- Couto FM, Minn AH, Pise-Masison CA, et al. Exenatide blocks JAK1-STAT1 in pancreatic beta cells. Metabolism. 2007;56(7):915–918.
- Mazzoli A, Sardi C, Breasson L, et al. JNK1 ablation improves pancreatic beta-cell mass and function in db/db diabetic mice without affecting insulin sensitivity and adipose tissue inflammation. FASEB Bioadv. 2021;3(2):94–107.
- Zhang D, Xie T, Leung PS. Irisin ameliorates glucolipotoxicity-associated beta-cell dysfunction and apoptosis via AMPK signaling and anti-inflammatory actions. Cell Physiol Biochem. 2018;51(2):924–937.
- Latreille M, Hausser J, Stützer I, et al. MicroRNA-7a regulates pancreatic beta cell function. J Clin Invest. 2014;124(6):2722–2735.
- Stoll L, Sobel J, Rodriguez-Trejo A, et al. Circular RNAs as novel regulators of beta-cell functions in normal and disease conditions. Mol Metab. 2018;9:69–83.
- Cao Y, Yuan G, Zhang Y, et al. High glucose-induced circHIPK3 downregulation mediates endothelial cell injury. Biochem Biophys Res Commun. 2018;507(1–4):362–368.
- Wang Z, Deng C, Zheng Y. Involvement of circRNAs in proinflammatory cytokines-mediated beta-cell dysfunction. Mediators Inflamm. 2021;2021:5566453.
- Sun R, Xue W, Zhao J. Hsa_circ_0054633 mediates apoptosis and insulin secretion in human pancreatic beta cells through miR-409-3p/caspase-8 axis. Diabetes Res Clin Pract. 2021;176:108837.
- Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51.
- Lu J, Pang L, Zhang B, et al. Silencing circANKRD36 inhibits streptozotocin-induced insulin resistance and inflammation in diabetic rats by targeting miR-145 via XBP1. Inflamm Res. 2021;70(6):695–704.
- Xu B, Wang Q, Li W, et al. Circular RNA circEIF4G2 aggravates renal fibrosis in diabetic nephropathy by sponging miR-218. J Cell Mol Med. 2020. DOI:10.1111/jcmm.16129.
