ABSTRACT
Oral squamous cell carcinoma (OSCC) is a frequent threatening head and neck malignancy. Serine hydroxymethyltransferase 2 (SHMT2) was identified to be upregulated in OSCC and its high expression was associated with poor patient prognosis. This paper set out to assess the influence of SHMT2 on OSCC progression and the potential mechanisms related to interleukin enhancer-binding factor 2 (ILF2). First of all, reverse transcription-quantitative PCR (RT-qPCR) and western blot examined the expression of SHMT2 and ILF2 in OSCC cells. Cell Counting Kit-8 (CCK-8) and colony formation assays appraised cell proliferation. Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL) staining was to estimate the apoptotic rate of cells. Further, wound healing and transwell assays verified the migration and invasion of cells. Western blot was adopted to detect the expression of factors related to apoptosis, migration, and epithelial–mesenchymal transition (EMT). The possible interaction of SHMT2 and ILF2 was predicted by a Molecular INTeraction (MINT) and BioGRID databases and determined using co-immunoprecipitation (IP) assay. Subsequently, ILF2 was overexpressed to investigate whether SHMT2 regulated OSCC progression by binding to ILF2. Results implied that SHMT2 possessed increased expression in OSCC cells, and OSCC cell viability, migration, invasion, EMT were inhibited and apoptosis was potentiated after its silencing. ILF2 bound to SHMT2 and ILF2 expression was downregulated after SHMT2 silencing in OSCC cells. Importantly, ILF2 overexpression abolished the suppressive role of SHMT2 interference in the progression of OSCC. Collectively, SHMT2 could promote the progression of OSCC by binding to ILF2.
Graphical abstract

Introduction
It is generally accepted that oral squamous cell carcinoma (OSCC) is one of the most aggressive and dangerous head and neck cancer [Citation1]. As a frequent oral cancer, OSCC is believed to be the main cause of mastication and swallowing dysfunction in patients [Citation2]. The prognosis has not improved significantly due to its high recurrence rate and risk of lymph node metastasis over the past decade regardless of great advances in OSCC diagnosis and therapy [Citation3]. It has been reported that recurrence of OSCC is closely related to local expansion and nodal metastasis of tumor cells [Citation4]. Metastasis of OSCC occurs mainly through disruption of the basement membrane, invasion of the extracellular matrix and metastasis to the lymph or blood vessels [Citation5]. Therefore, it is crucial to investigate the process of cancer cell transformation so as to understand the pathogenesis of OSCC.
Serine and glycine anabolism is deemed essential in cancer cells [Citation6]. As a key metabolic enzyme, serine hydroxymethyltransferase 2 (SHMT2) is capable of converting the serine to glycine, which affects cancer cell regulation and metabolism [Citation7]. Extensive literature has demonstrated the important catalytic function of SHMT2 in various human cancers and its association with tumor growth [Citation8–11]. Moreover, it has been mentioned that up-regulation of SHMT2 predicted a poor prognosis of OSCC patients [Citation12]. However, the function of SHMT2 in OSCC is uncertain. Interleukin enhancer-binding factors 2 (ILF2), which also known as nuclear factor 45 (NF-45), a subunit of NF-AT (nuclear factor of activated T cells), has been documented to modulate mRNA stability to mediate cell growth [Citation13,Citation14]. The current investigation identified that ILF2 displays enhanced expression in a variety of cancers and that elevated expression of ILF2 is implicated in poor clinical outcomes [Citation15–18]. Nevertheless, what is not yet uncertain is the function of ILF2 in OSCC. By using TNMplot database, ILF2 was uncovered to possess increased expression in oral tumor tissues and was closely related to tumor metastasis. Analysis of MINT and BioGRID database revealed a possible interaction between ILF2 and SHMT2. Importantly, high expression of both ILF2 and SHMT2 was discovered to be closely associated with cancer metastasis [Citation19,Citation20]. Therefore, we make the speculation that SHMT2 may bind to ILF2 to function as a core participator in the process of OSCC.
In the present study, we aimed to investigate the function of SHMT2 on the malignant biological properties of OSCC cells. Subsequently, an in-depth study on the mechanism of SHMT2 related to ILF2 was conducted, so as to provide a new approach and theoretical basis for the treatment of OSCC.
Materials and methods
Bioinformatics analysis
The relationship of SHMT2 and ILF2 was predicated by MINT (https://mint.bio.uniroma2.it/index.php/results-interactions/?id=SHMT2) and BioGRID (https://thebiogrid.org/108936/summary/homo-sapiens/) databases [Citation21,Citation22]. TNMplot database (https://www.tnmplot.com/) analyzed ILF2 expression in the oral tumor [Citation23].
Cell lines
Normal human oral epithelial cell line (HOK) and several human OSCC cell lines (HN4, SCC-9, and CAL-27) were obtained from BioVector NTCC Inc. HN6 cells were provided by National Institutes of Health of the United States of America. HOK cells were maintained in Oral Keratinocyte Medium (OKM, ScienCell). The culture medium for the other cells was Dulbecco’s modified Eagle medium (DMEM; Wako, Osaka, Japan). All mediums containing 10% fetal bovine serum (FBS) were used to culture cells. Cells were cultured in a humidified incubator with 5% CO2 at 37°C.
Plasmid transfection
Small interference RNA (siRNA) targeting SHMT2 (si-SHMT2-1/2), its negative control (si-NC), pcDNA3.1 plasmid carrying overexpression of ILF2 (Oe-ILF2) and the empty vector (Oe-NC) were all synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The above vectors underwent transfection treatment with the aid of Lipofectamine 3000 (Life Technologies). Reverse transcription-quantitative PCR (RT-qPCR) and western blot were to test SHMT2 and ILF2 expression after transfection.
Cell Counting Kit-8 (CCK-8) assay
Transfected CAL-27 cells were grown in 96-well plates at 37°C. After the supplementation of 10 µl of CCK-8 solution (Dojindo, Japan) at 24, 48 and 72 h of incubation, the cells were incubated for additional 4 h. The detection of optical density at 450 nm was undertaken utilizing a spectrophotometer (Bio-Tek, USA).
Colony formation assay
CAL-27 cells were cultured in 60-mm dishes at 37°C for 10 days. When colonies were seen, the culture medium was discarded. After washed in phosphate buffer saline (PBS) twice, cells were subjected to methanol fixation followed by 0.1% crystal violet staining. Finally, the number of colonies was counted under a light microscope (Olympus Corporation).
RT-qPCR
With the application of Total RNA Extraction Kit (R1200, Solarbio Life Science, Shanghai, China), total RNA was extracted from cells and subsequently subjected to reverse transcription into complementary DNA (cDNA) using VersoTM cDNA kit (AB-1453, ABgene). PCR reaction was conducted by means of SYBR qPCR Master Mix (Q511-02, Vazyme, Nanjing, China) on a GeneAmp 7500 system (Applied Biosystems). The primers for selected genes were: SHMT2, forward, 5’-TCAAGCGGATATCAGCCACG-3’, reverse, 5’-AGCAGTCAGTGCCAGGTTG-3’. ILF2, forward, 5’-TTTTAAGGCGCCATGAGGGG-3’, reverse, 5’-AGGCCATTTCACACTAAACCAG-3’. Glyceraldehyde-phosphate dehydrogenase GAPDH, forward, 5’-ACAACTTTGGTATCGTGGAAGG-3’, reverse, 5’- GCCATCACGCCACAGTTTC-3’. The following PCR reaction conditions were used: 95°C for 5 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min, extension at 72°C for 1 min. Relative mRNA levels were estimated by means of 2−ΔΔCt with GAPDH serving as an endogenous reference [Citation24].
Western blot
The proteins collected from the radioimmunoprecipitation (RIPA) lysis buffer (Millipore) were subjected to separation with the use of 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and moved to polyvinylidene fluoride (PVDF) membranes. The membranes were probed overnight with primary antibodies including SHMT2 (Abcam, ab155230, 1:5000), ILF2 (Abcam, ab154791, 1:1000), Bax (Abcam, ab32503, 1:1000), cleaved caspase-3 (Abcam, ab32042, 1:500), Bcl-2 (Abcam, ab32124, 1:1000), matrix metallopeptidase (MMP)2 (Abcam, ab92536, 1:1000), MMP9 (Abcam, ab76003, 1:1000), Vimentin (Abcam, ab92547, 1:1000), E-cadherin (Abcam, ab40772, 1:10,000), GAPDH (Abcam, ab9485, 1:2500) at 4°C following impeded with 5% nonfat milk for 1.5 h. Then, secondary antibody (Abcam, ab6721, 1:2000) was employed to incubate the membrane for 1 h at room temperature. The visualization of protein blots was presented with the employment of electrochemiluminescence (ECL) reagents (W1A003a, Wanleibio, Shenyang, China). Using Image J software (National Institutes of Health), protein levels were quantified.
Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL) assay
TUNEL was carried out for the detection of apoptosis in CAL-27 cells in line with the directions of the supplier. Briefly, CAL-27 cells underwent 30 min fixation (4% paraformaldehyde) and 5 min permeabilization in a solution supplemented with 0.1% Triton X-100. After two washes with PBS, the cells were added with TUNEL Apoptosis Assay Kit (C1088, Beyotime) for 60 min incubation in the dark at 37°C, followed by being mounted with mounting medium (Vector Laboratories, Inc.) containing 4’,6-diamidino-2-phenylindole (DAPI). Cells showing green fluoresce were imaged by a fluorescence microscope (Olympus Corporation).
Wound healing assay
A 200 µl pipette was used to create a scratch on the fused monolayer of cells when CAL-27 cells inoculated in 6-well plates were cultured to 80% confluence. After washing with PBS, cells were cultivated in serum-starved medium under the normal condition. The width of the scratch at 0 and 24 h was recorded through a light microscope (Olympus Corporation).
Transwell assay
CAL-27 cells were cultured at a density of 1 × 105 in 24-well plates and subsequently inoculated into the upper chambers that had been pre-coated with 50 mg/L Matrigel (Corning), with a serum-free medium. The medium consisting of 10% FBS was loaded to the lower chamber. The cells crossing the membrane were dyed with crystal violet. Afterward, CAL-27 cells in the five fields selected randomly were photographed and counted with the employment of a light microscope (Olympus Corporation).
Co-immunoprecipitation (IP)
The transfected cells were lysed on ice for 30 min in RIPA lysis buffer containing protease inhibitors. The lysed protein samples were added to 10 µl of pre-treated protein A agarose-coupled antibodies, including anti-IgG, anti-SHMT2 (Abcam, ab155230, 1:5000), or anti-ILF2 (Abcam, ab154791, 1:1000).
Statistical analysis
Experiment data analysis was done with the adoption of GraphPad Prism software (version no: 8.0; La Jolla, CA, USA). The comparisons among groups were done with the application of Student’s t-test and one-way analysis of variance (ANOVA). All data were presented as the mean ± standard deviation (SD) and the data showed be statistically significant when p < 0.05.
Results
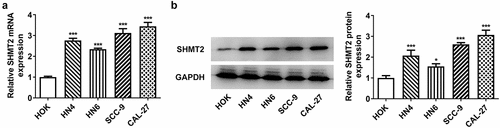
SHMT2 is highly expressed in OSCC cells
It has been reported that SHMT2, a key metabolic enzyme, can regulate cancer cell regulation and metabolism [Citation7]. It is noteworthy that up-regulation of SHMT2 predicts a poor prognosis of OSCC patients [Citation12]. This study was the first to explore the function of SHMT2 in OSCC. First of all, it can be seen in that SHMT2 expression was highly expressed in the OSCC cells including HN4, HN6, SCC-9, and CAL-27 compared with normal oral keratinocyte HOK. CAL-27 cell line was selected in the following experiments as it displayed the highest expression of SHMT2.
SHMT2 knockdown suppresses proliferation and promotes apoptosis of OSCC cells
For the sake of figuring out whether SHMT2 has effects on the proliferation and apoptosis of OSCC cells, we firstly knocked down SHMT2 expression in CAL-27 cells. As indicated in , SHMT2 expression was successfully declined in CAL-27 cells after its knockdown, and si-SHMT2-2-transfected cells were chosen in the follow-up experiments for the reason that cells transfected with si-SHMT2-2 expressed lower level of SHMT2 than those transfected with si-SHMT2-1. Besides, the SHMT2-silenced cells exhibited reduced cell viability and colony formation ability (). What’s more, si-SHMT2 induced an elevated apoptosis level in CAL-27 cells (). Accordingly, the up-regulated protein levels of pro-apoptotic Bax and cleaved caspase-3 as well as the down-regulated protein level of anti-apoptotic Bcl-2 were observed through western blot analysis (vs si-NC; ). Overall, SHMT2 interference impeded the initiation of OSCC.
Figure 2. SHMT2 interference ameliorates the initiation of OSCC. (a-b) With the aid of RT-qPCR and western blot, the knockdown efficiency of SHMT2 was determined. (c-d) CCK-8 and colony formation assays appraised cell proliferation. (e-f) The relative apoptosis rate was estimated with the employment of TUNEL. (g-h) The expression of proteins linked to apoptosis was examined with the application of western blot. ***P < 0.001 vs. si-NC.
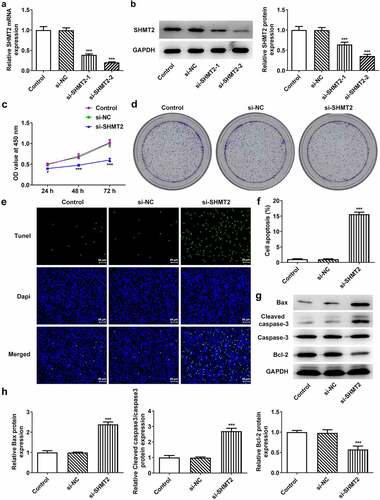
SHMT2 knockdown suppresses cell migration, invasion, and epithelial–mesenchymal transition (EMT) in OSCC
To verify the influence of SHMT2 on the metastasis of OSCC, the migration, invasion, and EMT of CAL-27 cells were studied in the following experiments. revealed that there has been a steep fall in the ability of CAL-27 cells to migrate and invade after SHMT2 knockdown. Meanwhile, migration markers MMP2 and MMP9 expression was also decreased rapidly due to SHMT2 depletion (). Not only that, what can be seen in was the increased expression of EMT biomarkers Vimentin and decreased E-cadherin expression in CAL-27 cells transfected with si-SHMT2. Thus, it can be concluded that SHMT2 knockdown was able to exert inhibitory effects on the migration, invasion, and EMT of OSCC cells.
Figure 3. SHMT2 deficiency suppresses the progression of OSCC. (a-b) Wound healing and transwell assays appraised cell migration and invasion. (c) Western blot analysis of MMP2 and MMP9 expression. (d) Western blot analysis of the expression of EMT-related factors. *P < 0.05, **P < 0.01, ***P < 0.001 vs. si-NC.
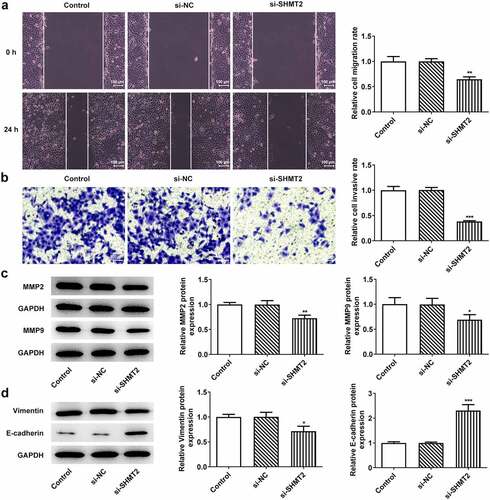
ILF2 binds to SHMT2 and ILF2 can be downregulated by SHMT2 silencing in OSCC cells
To explore the potential mechanisms of SHMT2 on the regulation of OSCC progression, MINT and BioGRID databases were used to predict the proteins that could interact with SHMT2. The specific interaction of SHMT2 and ILF2 was presented in . Further investigation of the TNMplot database revealed that the expression of ILF2 was increased in oral tumor tissue and was closely related to tumor metastasis (). The expression of ILF2 in CAL-27 cells was higher than that in HOK cells () while declined ILF2 was noticed in the cells transfected with si-SHMT2 in comparison with the si-NC group (). Co-IP experiment provided more evidence that SHMT2 and ILF2 bound to each other in CAL-27 cells (). Taken together, the results presented in this section implied that there was a close interaction between ILF2 and SHMT2.
Figure 4. ILF2 binds to SHMT2 and can be downregulated by SHMT2 silencing in OSCC cells. (a-b) The interaction between SHMT2 and ILF2 was found through the MINT and BioGRID databases. (c) ILF2 level in oral tumor tissue was analyzed using TNMplot database. (d-e) RT-qPCR and western blot analysis of ILF2 expression. **P < 0.01, ***P < 0.001 vs. HOK. (f) ILF2 expression in SHMT2-knockdown CAL-27 cells was detected with the use of western blot. ***P < 0.001 vs. si-NC. (g) The interaction of SHMT2 and ILF2 was identified using Co-IP.
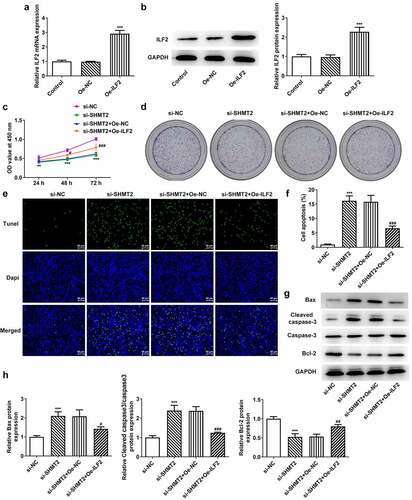
ILF2 upregulation reverses the impacts of SHMT2 knockdown on the proliferation and apoptosis of OSCC cells
SHMT2 knockdown has been shown to reduce ILF2 expression in the above results. Therefore, this set of experiments sought to confirm whether ILF2 overexpression in turn hinders the influence of SHMT2 interference on the proliferation and apoptosis of OSCC cells. presented that there has been a steep upregulation in the expression of ILF2 after CAL-27 cells transfected with Oe-ILF2. set up that Oe-ILF2 induced an elevated cell viability in CAL-27 with transfection of si-SHMT2. A similarly elevated level of colony formation was observed in cells co-transfected with si-SHMT2 and Oe-ILF2 (). Additionally, a decreased rate of apoptosis was also observed when ILF2 was up-regulated (), accompanied by reduced Bax and cleaved caspase-3 expression and increased Bcl-2 expression in CAL-27 transfected with si-SHMT2 (). In view of these evidences, the upregulation of ILF2 counteracted the impacts of SHMT2 knockdown on the occurrence of OSCC.
Figure 5. ILF2 upregulation reverses the impacts of SHMT2 knockdown on the occurrence of OSCC. (a-b) RT-qPCR and western blot analysis of the overexpression efficiency of ILF2. ***P < 0.001 vs. Oe-NC. (c) CCK-8 assay appraised cell viability in CAL-27 cells transfected with si-SHMT2 and Oe-ILF2. (d) Cell proliferation was identified utilizing colony formation assay in CAL-27 cells transfected with si-SHMT2 and Oe-ILF2. (e-f) TUNEL assay appraised cell apoptosis. (g-h) Western blot analysis of the expression of apoptosis-related factors. **P < 0.01, ***P < 0.001 vs. si-NC; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. si-SHMT2+ Oe-NC.
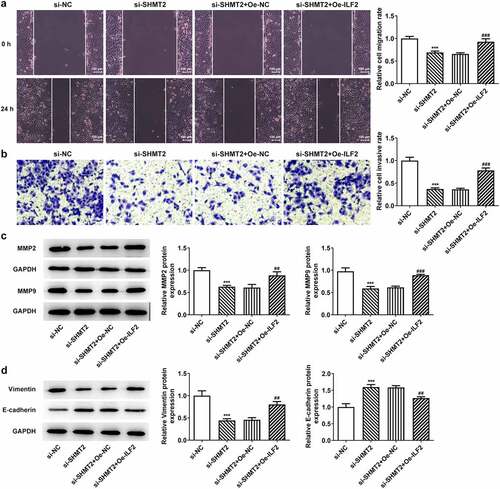
ILF2 upregulation reverses the impacts of SHMT2 knockdown on the migration, invasion, and EMT of OSCC cells
This section was to investigate the effects of ILF2 upregulation on the invasion, migration, and EMT process of CAL-27 cells with SHMT2 silencing. Details in presented that the CAL-27 cells co-transfected with si-SHMT2 and Oe-ILF2 showed a higher migration and invasion abilities compared with the si-SHMT2+ Oe-NC group. Accordingly, the expression of MMP2 and MMP9 was also elevated in the si-SHMT2+ Oe-ILF2 group (). Furthermore, Oe-ILF2 also enhanced the expression of the EMT-associated protein Vimentin and lowered the expression of E-cadherin in CAL-27 with the transfection of si-SHMT2 (). These findings clearly indicated that overexpression of ILF2 offset the effects of si-SHMT2 on OSCC cell migration, invasion, and EMT.
Figure 6. ILF2 elevation restores the impacts of SHMT2 knockdown on OSCC cell migration, invasion and EMT. (a-b) Wound healing and transwell assays appraised cell migration and invasion. (c) Western blot analysis of MMP2 and MMP9 expression. (d) Western blot analysis of the expression of EMT-related factors. ***P < 0.001 vs. si-NC; ##P < 0.01, ###P < 0.001 vs. si-SHMT2+ Oe-NC.
Discussion
OSCC is a powerful and aggressive head and neck tumor with frequent metastases and recurrences [Citation25]. Although several studies have highlighted the importance of SHMT2 in a variety of tumors, whether SHMT2 works in OSCC has not been elucidated. Determining the contribution of SHMT2 in OSCC will facilitate the development of new therapies with higher specificity.
SHMT2 has shown prognostic and therapeutic value for many cancers, such as hepatic carcinoma, breast cancer, glioma, and thyroid cancer [Citation26–29]. To our knowledge, SHMT2 is a key metabolic enzyme in the synthesis of glycine from serine and up-regulation of SHMT2 predicted a poor prognosis of OSCC patients [Citation12]. However, the function of SHMT2 in OSCC is uncertain. A few observations were made in the current study which will further our understanding of OSCC tumor progression. First, the up-regulation of SHMT2 expression in OSCC cells was discovered, which was in conformity to previous studies. It is well known that the transition from normal oral epithelial cells to malignancy is typically marked by cell proliferation [Citation30]. SHMT2 knockdown was shown to reduce or stop the proliferation of transformed cells and was directly associated with a glycine-dependent cell proliferation rate [Citation31]. Emerging evidence supports the notion that SHMT2 is a key factor that controls colorectal cancer cell growth, and SHMT2 knockdown impaired the proliferation of colorectal cancer in vitro and in vivo [Citation32]. Therefore, we knocked down SHMT2 gene in CAL-27 cells and found that this resulted in a decrease in cell viability and a slowdown in colony formation. Moreover, as a key metabolic enzyme, SHMT2 carries out the conversion of serine and glycine in mitochondria which is known to mediate a variety of biological cellular processes [Citation33,Citation34]. These findings stimulated us to explore the impact of SHMT2 knockdown on apoptosis. It has been shown that overexpression of Bcl-2 strongly predicted a worse prognosis of OSCC patients [Citation35]. Our study found that knockdown of SHMT2 promoted apoptosis through upregulation of Bax and cleaved caspase3 expression and downregulation of Bcl-2 level, consistent with the role of SHMT2 on apoptosis in bladder cancer [Citation36]. These findings have identified SHMT2 as a pivotal participant in cell viability and apoptosis in OSCC.
It is well recognized that cell invasion and migration in OSCC determines the prognostic ability of the patient [Citation37,Citation38]. A growing body of researches have exposed that SHMT2 contribute to tumor migration and invasion [Citation39–41]. SHMT2 inhibition weakened cell migration and invasion in human malignancies [Citation7,Citation42,Citation43]. Indeed, we observed decreased migratory and invasive capacities in SHMT2-knockdown CAL-27 cells, accompanied by similarly decreased expression of migration-associated MMP2 and MMP9. Taken together, we demonstrated that SHMT2 is an important factor driving tumor metastasis and invasion in OSCC. Likewise, EMT event, which is chiefly marked by the decrease of E-cadherin and the increase of vimentin, occupies an important position in tumor metastasis [Citation4,Citation44]. It was reported that SHMT2 affects the EMT process in OSCC by modulating the classical marker of EMT [Citation12]. In this study, it was noted that SHMT2 deficiency also lead to prominent inhibition of invasion, metastasis, and EMT in OSCC.
It has been proposed that ILF2 can activate STAT3- and JNK-signaling pathways [Citation45]. Meanwhile, SHMT2 is a downstream of STAT3 [Citation46]. The above study implies a possible relationship between SHMT2 and ILF2. In our study, the interaction of SHMT2 with ILF2 has been predicted through databases and confirmed by immunoprecipitation experiments. Recently, a great deal of studies have indicated that ILF2 was upregulated in numerous cancers, such as non-small cell lung cancer, gastric cancer and pancreatic carcinoma [Citation16,Citation19,Citation47]. And high expression of ILF2, which was observed in glioma, cervical cancer, esophageal squamous cell cancer, and bladder cancer, was significantly related to the poor prognosis of these malignant tumors [Citation15,Citation17,Citation36,Citation48]. Consistent with aforementioned studies, the upregulation of ILF2 was also we observed in CAL-27 cells in our experiments. Subsequently, our experiments were performed to verify the effect of ILF2 upregulation on the progression of SHMT2 knockout OSCC cells by constructing ILF2 overexpression plasmids. It is documented that upregulation of ILF2 facilitates the initiation of cancers by regulating cell cycle processes [Citation15,Citation17,Citation49]. The carcinogenic role of ILF2 is partially achieved by inducing the up-regulation of Bcl-2 and by inhibiting pro-apoptotic Bax expression [Citation49]. The reduced cell viability and increased apoptosis rates observed in our experiments following SHMT2 knockdown were partially reversed by ILF2 overexpression. Additionally, upregulation of ILF2 was also testified to exacerbate cell invasion and metastasis in melanoma and to regulate EMT-related genes in pancreatic cancer PANC-1 cells [Citation47,Citation50]. In our experiments, ILF2 served as a promoter in the invasion, migration, and EMT process in SHMT2-knockdown CAL-27 cells. However, the use of only one OSCC cell line to clarify the effects of SHMT2 and ILF2 in OSCC is a potential limitation of the present study. Subsequent experiments will incorporate more typical OSCC cell lines to support this conclusion.
Conclusion
To sum up, we have identified a novel role for SHMT2-ILF2 in promoting OSCC progression. We have also demonstrated that SHMT2 promotes the progression of OSCC by binding to ILF2. These findings provide new therapeutic opportunities for the development of anti-OSCC oncology drugs.
Data Availability of statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lindemann A, Takahashi H, Patel AA, et al. Targeting the DNA damage response in OSCC with TP53 mutations. J Dent Res. 2018;97:635–644.
- Sasahira T, Bosserhoff AK, Kirita T. The importance of melanoma inhibitory activity gene family in the tumor progression of oral cancer. Pathol Int. 2018;68:278–286.
- Gonzalez-Garcia R, Naval-Gias L, Rodriguez-Campo FJ, et al. Contralateral lymph neck node metastasis of squamous cell carcinoma of the oral cavity: a retrospective analytic study in 315 patients. J Oral Maxillofac Surg. 2008;66:1390–1398.
- Sasahira T, Kirita T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int J Mol Sci. 2018;19:2413.
- Bugshan A, Farooq I. Oral squamous cell carcinoma: metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Res 9: 229, 2020.
- Di Salvo ML, Contestabile R, Paiardini A, et al. Glycine consumption and mitochondrial serine hydroxymethyltransferase in cancer cells: the heme connection. Med Hypotheses. 2013;80:633–636.
- Liu C, Wang L, Liu X, et al. Cytoplasmic SHMT2 drives the progression and metastasis of colorectal cancer by inhibiting beta-catenin degradation. Theranostics. 2021;11:2966–2986.
- Lee GY, Haverty PM, Li L, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74:3114–3126.
- Ding J, Li T, Wang X, et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 2013;18:896–907.
- Anderson DD, Quintero CM, Stover PJ. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc Natl Acad Sci U S A. 2011;108:15163–15168.
- Nguyen TH, Vemu PL, Hoy GE, et al. Serine hydroxymethyltransferase 2 expression promotes tumorigenesis in rhabdomyosarcoma with 12q13-q14 amplification. J Clin Invest. 2021;131.
- Wu ZZ, Wang S, Yang QC, et al. Increased expression of SHMT2 is associated with poor prognosis and advanced pathological grade in oral squamous cell carcinoma. Front Oncol. 2020;10:588530.
- Du H, Le Y, Sun F, et al. ILF2 directly binds and stabilizes creb to stimulate malignant phenotypes of liver cancer cells. Anal Cell Pathol (Amst). 2019;1575031. DOI:10.1155/2019/1575031
- Marcoulatos P, Avgerinos E, Tsantzalos DV, et al. Mapping interleukin enhancer binding factor 3 gene (ILF3) to human chromosome 19 (19q11-qter and 19p11-p13.1) by polymerase chain reaction amplification of human-rodent somatic cell hybrid DNA templates. J Interferon Cytokine Res. 1998;18:351–355.
- Huang Q, He X, Qiu X, et al. Expression of NF45 correlates with malignant grade in gliomas and plays a pivotal role in tumor growth. Tumour Biol. 2014;35:10149–10157.
- Ni T, Mao G, Xue Q, et al. Upregulated expression of ILF2 in non-small cell lung cancer is associated with tumor cell proliferation and poor prognosis. J Mol Histol. 2015;46:325–335.
- Ni S, Zhu J, Zhang J, et al. Expression and clinical role of NF45 as a novel cell cycle protein in esophageal squamous cell carcinoma (ESCC). Tumour Biol. 2015;36:747–756.
- Haskins WE, Eedala S, Jadhav YL, et al. Insights on neoplastic stem cells from gel-based proteomics of childhood germ cell tumors. Pediatr Blood Cancer. 2012;58:722–728.
- Yin ZH, Jiang XW, Shi WB, et al. Expression and clinical significance of ILF2 in gastric cancer. Dis Markers. 2017;2017:4387081.
- Liu Y, Yin C, Deng MM, et al. High expression of SHMT2 is correlated with tumor progression and predicts poor prognosis in gastrointestinal tumors. Eur Rev Med Pharmacol Sci. 2019;23:9379–9392.
- Calderone A, Iannuccelli M, Peluso D, et al. Using the MINT database to search protein interactions. Curr Protoc Bioinformatics. 2020;69:e93.
- Oughtred R, Stark C, Breitkreutz BJ, et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019;47:D529–D541.
- Bartha A, Gyorffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci 2021;22:2622.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408.
- Bhattacharya A, Roy R, Snijders AM, et al. Two distinct routes to oral cancer differing in genome instability and risk for cervical node metastasis. Clin Cancer Res. 2011;17:7024–7034.
- Bernhardt S, Bayerlova M, Vetter M, et al. Proteomic profiling of breast cancer metabolism identifies SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res. 2017;19:112.
- Woo CC, Chen WC, Teo XQ, et al. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget. 2016;7:53005–53017.
- Wu M, Wanggou S, Li X, et al. Overexpression of mitochondrial serine hydroxyl-methyltransferase 2 is associated with poor prognosis and promotes cell proliferation and invasion in gliomas. Onco Targets Ther. 2017;10:3781–3788.
- Jin M, Lee WK, You MH, et al. SHMT2 expression as a diagnostic and prognostic marker for thyroid cancer. Endocr Connect. 2021;10:630–636.
- Dwivedi N, Chandra S, Kashyap B, et al. Suprabasal expression of Ki-67 as a marker for the severity of oral epithelial dysplasia and oral squamous cell carcinoma. Contemp Clin Dent. 2013;4:7–12.
- Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044.
- Cui X, Cui Y, Du T, et al. SHMT2 drives the progression of colorectal cancer by regulating UHRF1 expression. Can J Gastroenterol Hepatol. 2022;2022:3758697.
- Wei Z, Song J, Wang G, et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun. 2018;9:4468.
- Dijk SN, Protasoni M, Elpidorou M, et al. Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: the effects of doxycycline and gemcitabine. Sci Rep. 2020;10:4363.
- Kato K, Kawashiri S, Yoshizawa K, et al. Apoptosis-associated markers and clinical outcome in human oral squamous cell carcinomas. J Oral Pathol Med. 2008;37:364–371.
- Zhang P, Yang Q. Overexpression of SHMT2 predicts a poor prognosis and promotes tumor cell growth in bladder cancer. Front Genet. 2021;12:682856.
- Carnielli CM, Macedo CCS, De Rossi T, et al. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat Commun. 2018;9:3598.
- Wang L, Ge S, Zhou F. MicroRNA-487a-3p inhibits the growth and invasiveness of oral squamous cell carcinoma by targeting PPM1A. Bioengineered. 2021;12:937–947.
- Miyo M, Konno M, Colvin H, et al. The importance of mitochondrial folate enzymes in human colorectal cancer. Oncol Rep. 2017;37:417–425.
- Ning S, Ma S, Saleh AQ, et al. SHMT2 overexpression predicts poor prognosis in intrahepatic cholangiocarcinoma. Gastroenterol Res Pract. 2018;2018:4369253.
- Noguchi K, Konno M, Koseki J, et al. The mitochondrial one-carbon metabolic pathway is associated with patient survival in pancreatic cancer. Oncol Lett. 2018;16:1827–1834.
- Ji L, Tang Y, Pang X, et al. Increased expression of serine hydroxymethyltransferase 2 (SHMT2) is a negative prognostic marker in patients with hepatocellular carcinoma and is associated with proliferation of hepg2 cells. Med Sci Monit. 2019;25:5823–5832.
- Qi C, Qin X, Zhou Z, et al. Circ_0072995 promotes cell carcinogenesis via up-regulating miR-149-5p-mediated SHMT2 in breast cancer. Cancer Manag Res. 2020;12:11169–11181.
- Sasahira T, Kirita T, Kuniyasu H. Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol. 2014;19:431–436.
- Jia Y, Li X, Nan A, et al. Circular RNA 406961 interacts with ILF2 to regulate PM2.5-induced inflammatory responses in human bronchial epithelial cells via activation of STAT3/JNK pathways. Environ Int. 2020;141:105755.
- Marrocco I, Altieri F, Rubini E, et al. Shmt2: a stat3 signaling new player in prostate cancer energy metabolism. Cells 2019;8:1048.
- Bi Y, Shen W, Min M, et al. MicroRNA-7 functions as a tumor-suppressor gene by regulating ILF2 in pancreatic carcinoma. Int J Mol Med. 2017;39:900–906.
- Shamanna RA, Hoque M, Pe’ery T, et al. Induction of p53, p21 and apoptosis by silencing the NF90/NF45 complex in human papilloma virus-transformed cervical carcinoma cells. Oncogene. 2013;32:5176–5185.
- Cheng S, Jiang X, Ding C, et al. Expression and critical role of interleukin enhancer binding factor 2 in hepatocellular carcinoma. Int J Mol Sci. 2016;17:1373.
- Zhang X, Bustos MA, Gross R, et al. Interleukin enhancer-binding factor 2 promotes cell proliferation and DNA damage response in metastatic melanoma. Clin Transl Med. 2021;11:e608.