ABSTRACT
Sustainable management of natural resources is critical to food security. The shrimp feed and fishery sector is expanding rapidly, necessitating the development of alternative sustainable components. Several factors necessitate the exploration of a new source of environmentally friendly and nutrient-rich fish feed ingredients. Microalgal biomass has the potential to support the growth of fish and shrimp aquaculture for global food security in the bio-economy. Algal biorefineries must valorize the whole crop to develop a viable microalgae-based economy. Microalgae have the potential to replace fish meal and fish oil in aquaculture and ensure sustainability standards. Microalgae biomasses provide essential amino acids, valuable triglycerides such as lipids, vitamins, and pigments, making them suitable as nutritional supplements in livestock feed formulations. Fish and microalgae have similar nutritional profiles, and digestibility is a critical aspect of the aquafeed formulation. A highly digestible feed reduces production costs, feed waste, and the risk of eutrophication. Due to low input costs, low carbon footprint, wastewater treatment benefits, and carbon credits from industrial CO2 conversion, microalgae-based fish and shrimp feeds have the potential to provide significant economic benefits. However, several challenges must be addressed before microalgal biomass and bioproducts may be used as fish feeds, including heavy metal bioaccumulation, poor algal biomass digestion, and antinutrient effects. Knowledge of biochemical composition is limited and diverse, and information on nutritional value is scattered or contradictory. This review article presents alternative approaches that could be used in aquaculture to make microalgal biomass a viable alternative to fish meal.
graphical abstract
1. Introduction
Aquaculture has become increasingly important for food security in the 21st century. By 2050, the global population is projected to increase from 7.6 to 9.8 billion, resulting in a 60 to 100% increase in food consumption [Citation1,Citation2]. Fish meals, which are high in protein, are an excellent source of nutrients for fish and shrimp in aquaculture systems [Citation3,Citation4]. However, volatility in the global market for fish meals has harmed long-term revenue and security in the aquaculture sector [Citation3,Citation5]. In recent years, the gradual exhaustion of marine fisheries resources has posed a serious problem for fish meal production [Citation6]. In China, for example, marine capture increased more than 20-fold from 0.6 million tons in 1950 to 13.6 million tons in 2011. Additionally, periodic closures and efforts to manage fishing capacity in the offshore ocean have significantly reduced fisheries productivity, thereby limiting fish meal production [Citation7]. Finally, industrialization and urbanization-related marine pollution contaminate fish products and affect the safety of fish meals [Citation8]. Aquaculture is one of the fastest-growing sectors of the food industry. In 2019, the aquaculture industry was expected to be valued at US$ 31.94 billion [Citation9]. The aquaculture industry is expected to grow at a rate of more than 7.1% between 2020 and 2027. Increased human consumption and commercial acceptability are now driving the growth of the aquaculture industry.
In recent years, several new species have been introduced into the industry. Aquaculture has grown faster than other major food industries in recent years due to increased fish consumption. It has been demonstrated that a combination of two microalgal species, Nannochloropsis oculata, and Schizochytrium sp., can be used to feed Nile tilapia (Oreochromis niloticus), the second-largest farmed fish in the world. This study demonstrated that microalgae-based feeds can enhance nutritional quality and fish growth metrics [Citation10]. In another study, Nannochloropsis sp. and Isochrysis sp. were used to substitute fish meals and fish oil in the diet of rainbow trout (Oncorhynchus mykiss), a key model species for salmonid aquaculture. Compared to fish meal and fish oil, Isochrysis sp. was found to significantly increase apparent digestibility coefficients for crude proteins, amino acids, lipids, and fatty acids [Citation11]. Several commercially available algae products, including Verdemin (Ulva ohnoi) and Rosamin (Entomoneis spp.), were evaluated as potential feed components for Atlantic Salmon (Salmo salar). Verdemin and Rosamin were found to have no significant effects on the growth or feeding efficiency of Atlantic Salmon at doses of 2.5 and 5.0%, respectively. However, a significant increase in long-chain omega-3 polyunsaturated fatty acids (n-3 LC-PUFA) was observed in fish fed 5% Rosamin [Citation12]. Therefore, a plant-based fish meal has become an increasingly popular option in recent years.
Improved fish nutrition can reduce feed waste, resulting in enhanced financial sustainability. A diet rich in omega-3 fatty acids, antioxidants, and prebiotics has been shown to increase the production, duration, and quality of farmed fish [Citation13]. Furthermore, supplementing animal feed with algae improves growth and weight gain, decreases feed consumption, increases immunological response, resistance to illness, antibacterial and antiviral activity, and enriches livestock products with bioactive components [Citation14]. Microalgae-related biotechnologies and bioproducts have been rapidly developed in recent years. Research in microalgae has previously focused on improving biomass harvesting efficiency and producing specific high-value compounds in algal cells [Citation15,Citation16]. The rapid growth of the algal bio-economy has been driven by significant advances in algal biotechnology that have turned algae into an efficient ‘cell factory’ for food production [Citation14]. The cost of microalgal feed remains higher than that of conventional feed. The cost of microalgal feed must come down to be competitive. Algal biotechnology is closely related to the growth of the algal bio-economy in terms of food and feed production. Algal biotechnology focuses on increasing algal productivity to reduce the cost of biomass production. Several recent biotechnological approaches have resulted in increased biomass production and accumulation of useful metabolites . These include bioreactor design, production of genetically modified strains, high-throughput screening, rapid sampling, and genetic and metabolic engineering. Microalgal biotechnology focuses on improving the production of carbohydrates, proteins, polyunsaturated fatty acids, pigments, and other valuable nutrients from microalgae through strain optimization, carbon flux alterations, stress condition modifications, and metabolic pathway prediction. In recent years, biotech and bioengineering techniques have enabled algae to become more efficient ‘cell factories’ for carbon sequestration and food production [Citation14,Citation17].
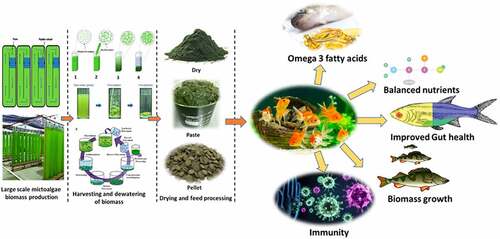
Microalgae biomass has been proposed as a high-value feed for fish and shrimp in sustainable aquaculture [Citation18,Citation19]. outlines the benefits and drawbacks of a variety of fish-based alternatives to traditional meals. The idea of employing microalgae biomass and bio-products as aquafeed for fish and shrimp growth is novel; nevertheless, various obstacles must be overcome before the concept can be successfully applied. Several issues need to be resolved, including potential safety issues, antinutritional factors (ANFs), limited digestibility, and others. There is a significant amount of cellulose in microalgal cells, which can affect the digestion of algal biomass in fish diets [Citation20]. Microalgae cells, which contain a range of negative charges, have been found to have a substantial potential for the adsorption and accumulation of heavy metals (HMs) [Citation21]. As a consequence of the food chain, these harmful components will concentrate on aquatic animals, endangering the health of humans who consume fish and shrimp. The majority of previous research focused on the benefits and downsides of microalgae biomass in aquafeed. Aquafeed accounts for at least 75%-90% of aquaculture<apos;>s operational expenses. New feed additives are needed, as traditional feed materials such as fish meal, fish oil, and soybean meal have become unsustainable. Aquafeed made from microalgae is not only ecologically beneficial, but with appropriate optimization, it may also be commercially feasible. Microalgae also have a nutritional profile that is comparable to that of many fish. The digestibility of the feed is an important issue to consider when formulating it. A highly digestible feed can help reduce production costs, waste, and the risk of eutrophication in the environment. This article discusses the digestibility of numerous microalgae in fish, as well as approaches to enhance microalgal digestibility. represents the technology process lineup for the production of beneficial meals derived from fish using the algae-based feed.
Figure 1. Technology process lineup for the production of beneficial fish-derived food by using an algae-based feed.
Table 1. Presents the benefits and drawbacks of an alternative fish diet
2. Microalgae as fish meal
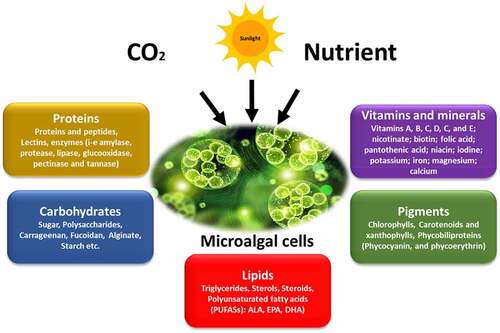
Microalgae are photosynthetic microorganisms that utilize atmospheric carbon dioxide (CO2) and sunlight energy to produce a variety of proteins, carbohydrates, lipids, minerals, vitamins, polyphenols, flavonoids, and carotenoids, as shown in . Microalgae-based products can be used in a variety of industries, including food and beverages, animal feed, cosmetics, chemicals, and biofuels. By 2028, the market for microalgae-based products is expected to grow from US$ 1,547.23 million in 2020 to US$ 2,811.10 million. It is expected to grow at a compound annual growth rate (CAGR) of 7.9% between 2021 and 2028 [Citation36]. To date, the microalgae sector has focused mainly on species that are associated with food and cosmetics. Microalgae species, including Spirulina sp., Dunaliella sp., Isochrysis sp., Pavlova sp., and others, are also used as larvae feed by fish hatcheries, although these are not normally grown on a large scale. However, in recent decades, microalgae have been explored as a possible bulk-feeding ingredient for fingerlings and adult fish [Citation37]. Microalgae have been suggested as a substitute for fish food for several reasons. Microalgae have the highest net biomass productivity compared to any terrestrial plant or animal [Citation38].
Microalgae, unlike land-based plants, do not need fertile soil to grow. Microalgae can even grow in seawater or wastewater [Citation39]. Current land-use patterns do not require large-scale microalgae cultivation on non-arable land or non-potable water (or practices). Unlike insects and bacteria, microalgae have minimal nutritional requirements. In a biorefinery, microalgae might be utilized to produce fish feed [Citation40–42]. The promise of microalgae is based on its protein, lipid, carbohydrate, and pigment composition, which is ideal for fish health. Some economically relevant microalgal species have chemical compositions that are equivalent to feed components utilized in the aquafeed industry (). Methionine, for example, is abundant in microalgae, unlike plant-based components such as Chlorella and Chlamydomonas [Citation43]. The proportion of starch in microalgal species varies from 7 to 45% [Citation44]. Other microalgae contain less starch (30–49%) than Tetraselmis subcordiformis, C. rheinhardtii, and C. vulgaris [Citation44].
Table 2. Nutrient content of several microalgae species
Next-generation microalgae-based feeds have the potential to provide a sustainable source of aquaculture food. In addition to providing essential nutrients, microalgae are an essential food source for zooplankton and lower trophic fish, which in turn provide food for fish at higher trophic levels. Microalgae can contain up to 60% protein, 60% carbohydrates, or 70% oil, depending on the species of algae and its growing conditions. Secondary metabolites generated by microalgae, such as pigments, growth-promoting compounds, and hormones, have intrinsic antioxidants, antibacterial, anti-inflammatory, and immune-stimulant properties that benefit both marine and freshwater species [Citation19,Citation45–47]. Furthermore, numerous species can produce eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and colors (e.g., carotenoids) from the ground up, proving their adaptability. In the future, the cultivation of microalgae on non-arable land or along coastlines may greatly increase the global photosynthetic primary production by lowering water demand and recycling nutrients by using wastewater and seawater, as well as converting atmospheric CO2 into nutrient-dense feed and animal health products. In this sense, a circular aquaculture firm could arise as part of the larger circular bio-economy [Citation19,Citation48–50]. In addition, the contents of EPA and DHA in microalgal lipids are significantly higher and also less contaminated than fish oil. PUFA-rich microalgae include species of Schizochytrium, Crypthecodinium, Nannochloropsis, Isochrysis, Nitzschia, Diacronema, Porphyridium, and Desmodesmus, which produce EPA and DHA, respectively [Citation51,Citation52]. For example, Nannochloropsis sp. and Phaeodactylum tricornutum have about 39% EPA and 30% DHA of total omega-3 fatty acids, while Schizochytrium sp. and Thraustochytrium have about 40% DHA and 22% EPA, respectively [Citation53]. Furthermore, microalgae produce antioxidant pigments, and some microalgae contain vitamins and immunostimulants that are beneficial to aquatic organisms [Citation13,Citation54].
Microalgae have the potential to control food production and pollution by assimilating nutrients from water and wastewater [Citation55,Citation56]. Wet effluents from markets and slaughterhouses contain large amounts of nitrogen and phosphorus [Citation57]. Washing fruits and vegetables at public markets or slaughterhouses and washing poultry and fish produces wet market wastewater [Citation58], which has higher levels of nitrogen, phosphate, chemical oxygen demand, biological oxygen demand, fats, solid particles, oils, and greases than domestic wastewater [Citation59]. Algae biomass derived from nutrient recycling of wet market and slaughterhouse effluents can be used as a fish meal. A biorefinery strategy may result in resource-efficient, environmentally friendly value chains with a low carbon footprint through the co-production of bio-based and biodegradable products when algal generation systems are integrated into aquaculture operations (a biorefinery approach). This strategy may also benefit the aquaculture industry and the general public in other ways, such as healthier diets and ecosystem services [Citation48,Citation60].
2.1 Microalgae fatty acids
Lipids play a dynamic role in the formation of membranes and are important energy storage molecules. Although microalgae may have oil contents exceeding 60% by weight of dry biomass, the most common oil contents are in the range of 20–50% [Citation76–78]. Polyunsaturated fatty acids present in microalgal lipids include arachidonic acid (ARA) and DHA. Cryptothecodinium and Schizochytrium contain DHA, Phaeodactylum, Nitzschia, Isochrysis, and Diacronema contain EPA, and Cryptothecodinium and Schizochytrium contain ARA. Microalgal species can produce EPA concentrations ranging from 7 to 34% fatty acids. These fatty acids are rare and difficult to synthesize in a lab. These components are currently derived from fish oil and are restricted in vegetable oils, including palm, soybean, rapeseed, and canola, in aquafeed [Citation79]. Microalgae may produce high amounts of lipids and have a nutritionally advantageous fatty acid composition when grown under stress conditions. Polyunsaturated fatty acids (PUFAs), such as EPA (20:5, −3), and DHA (22:6, −3) are the important constituents of microalgal lipids. Microalgae species, such as S. limacinum, P. tricornutum, and Nannochloropsis sp., comprise 30–40% of ω-3 fatty acids in their total content [Citation53]. LC-PUFAs such as ω-3 and 6 must be consumed regularly because humans and many animals cannot synthesize them [Citation80]. PUFAs are also necessary ingredients for fish growth because they cannot be formed from saturated and monounsaturated fatty acids [Citation81]. Fish have high tropical levels in aquatic environments due to their ability to metabolize PUFA to produce LC-PUFA, which strongly contributes to tropical upgrading. It is critical to understand how farmed fish create and store LC-PUFAs to substitute terrestrial plant lipids for fish oil in commercial aquafeeds. Through enhanced synthesis and selective use of dietary fatty acids, it is possible to increase the body<apos;>s storage of LC-PUFA. Data synthesis was performed to determine optimal fatty acid levels that improve the generation and storage of omega-3 polyunsaturated fatty acids in edible portions of salmonids [Citation82].
Microalgae improved the fatty acid profile of fish and shrimp by improving the ω-3/ ω-6 ratio, increasing PUFA content, and enriching long-chain PUFAs [Citation83,Citation84]. Aquatic animals have higher nutritional value when their fatty acid profile is improved, which benefits consumers. Additionally, supplementing aquatic animals’ diets with microalgal PUFAs can increase their growth and immunity It has been reported that Nile tilapia can digest Schizochytrium sp. lipids with 98% efficiency [Citation85]. Lipid digestibility in juvenile European seabass (Dicentrarchus labrax), Nile tilapia with C. vulgaris, Schizochytrium sp. Spirulina sp., and Chlorella sp., and African catfish (Clarus gariepinus) with C. vulgaris and S. maxima has been shown to be more than 80% [Citation85–87]. Lower digestibility has been noted under certain conditions, such as for N. gaditana in juvenile African catfish [Citation88]. A microalgae-based feed might also improve fish survival due to its functional properties, including probiotics, prebiotics, immunostimulants, antivirals, antibacterials, etc. Bacteria or microbes that are probiotics are believed to contribute to a healthy gut when consumed. For example, microalgae are probiotics for fish. The microbiome in the colon digests algal cells and generates probiotics that inhibit the growth of infectious agents [Citation89,Citation90]. Consumption of Tetraselmis suecica live cells significantly reduced the number of harmful bacteria in the stomach of white shrimp (Fenneropenaeus indicus) as compared to a control group [Citation91]. The addition of Schizochytrium sp. meal to the diet at 1.2% significantly improved Nile tilapia health [Citation92]. Previous studies have found that Spirulina can induce non-specific immune responses against infections in a variety of fish [Citation93,Citation94]. When S. Platensis is used at a 10% concentration, it has been proven to dramatically increase the production of white blood cells, red blood cells, hemoglobin, albumin, and total protein in rainbow trout [Citation95].
LC-PUFAs are beneficial to animals and humans as they provide biologically active compounds such as prostaglandins and thromboxanes, which are crucial for the formation of cholesterol and triglycerides in the blood, as well as the prevention of certain diseases such as arthritis and rheumatoid arthritis [Citation96]. DHA (22:6) and EPA (20:5), two of the most beneficial LC-PUFAs, stand out for their many health benefits. DHA improves brain health, which maintains neurons, boosts short- and long-term memory, and aids in the treatment of brain disorders such as memory loss and cognitive decline. By reducing oxidative stress and plasma triglycerides, as well as providing benefits for the treatment of inflammation, arrhythmia, and cardiovascular disease [Citation97,Citation98]. Microalgae also produce a wide range of fatty acids that are useful for the food and feed industry, including gamma-linolenic acid (GLA), linoleic acid (LA), alpha-linolenic acid (ALA), and ARA [Citation99]. Wound healing and regeneration, as well as the eradication of invading microorganisms, may be aided by these essential fatty acids [Citation100]. The lipid content of C. vulgaris has been reported to be 35–40% by weight, with a content of 27% linolenic and 24% linoleic acid [Citation101]. Spirulina sp. has been promoted as an inexpensive source of GLA [Citation100]. Recent research has indicated that microalgae such as Chlorella sp. and Schizochytrium sp. are more attractive than other autotrophic species due to their nutritional qualities and ability to be effectively consumed by aquaculture species. Thanks to the well-developed technology for mass cultivation of Schizochytrium and Chlorella sp. in aquafeeds, the use of fish oil can be significantly reduced in the future. To achieve sustainable substitution of fish oil in aquaculture, research must focus on the use of microalgal species.
2.2 Proteins and amino acids derived from microalgae
Proteins have been recognized as the building components that are responsible for individual growth. Proteins are constructed from peptide bonds that link amino acid units [Citation102]. In terms of quality and amino acid composition, microalgae protein is a great alternative to fish meals. The protein concentration of algae has been reported to range from 40 to 60 wt/wt % [Citation103]. Another study discovered that C. vulgaris has between 51 and 58% protein, while Spirulina sp. comprises between 60% and 71%. Additionally, Arthrosphira platensis has a protein content of 70% by weight [Citation104]. Protein is so abundant in some microalgae species that it accounts for more than half of their biomass. Most Spirulina strains, as well as a few Chlorella and Nannochloropsis strains, have a protein content of 40 to 65% [Citation105]. Microalgae can synthesize all amino acid molecules; therefore, algae-derived amino acids are preferred over other protein-rich foods [Citation106]. Microalgae can synthesize several protein compounds faster than traditional protein sources. summarizes the current research on microalgal biomass used as a replacement for fishmeal and fish oil.
Table 3. Microalgal biomass as an alternative or supplement to fishmeal and fish oil
Kim et al. [Citation107] discovered that parrotfish (Oplegnathus fasciatus) fed 5% Arthrospira had significantly higher weight, protein efficiency, and feed consumption compared to the control group fed fish meal. Fish need meals with 30% to 55% crude protein and amino acids tailored to their individual nutritional needs to achieve maximum growth [Citation108]. Sørensen et al. [Citation109], demonstrated that Phaeodactylum tricornutum can replace up to 6% of fish meal in the diet of Atlantic salmon (Salmo salar) without affecting digestibility, utilization, or growth performance. Protein digestibility of microalgae varies from 50% to 94% in different fish species. Protein digestibility of rainbow trout, Nile tilapia, European seabass, and African catfish has been reported to exceed 80% [Citation110]. In African catfish and Nile tilapia, bead milling improved N. gaditana protein digestibility by 16 and 17 %, respectively [Citation86]. Enzymatically processed microalgae digest protein 6% faster than whole-cell Nannochloropsis oceanica [Citation40]. Protein digestion was enhanced in a diet containing Schizochytrium sp. when organic minerals were added [Citation87]. N. oceanica and C. vulgaris provided amino acids with higher digestibility than 90% for European seabass and Atlantic salmon [Citation87,Citation111]. Tetraselmis sp. exhibited significantly lower mino acid digestibility than juvenile European seabass. Pretreatment would break down larger proteins into peptides and individual amino acids, which increases amino acid digestibility [Citation110].
Threonine, isoleucine, lysine, leucine, methionine, valine, and histidine are essential amino acids that the body cannot produce itself. Therefore, it is important to consume them through foods that contain EAAs, such as tofu, eggs, and fish [Citation112]. Vegans and vegetarians have few options since the majority of plant-based proteins do not meet the EAA profile. To overcome this problem, an alternative source with a balanced protein profile and low cost is required [Citation113]. Protein digestibility of the microalgal protein (S. platensis protein concentration) ranges from 87.5 to 97.8% [Citation114]. However, certain algae (510–710 g/kg) have more protein than eggs or soybeans (132–370 g/kg) and have fairly comparable EAAs [Citation115]. Due to their high content of EAA, microalgae are considered one of the best vegan protein sources. It is well known that microalgae contain EAAs and non-NEAAs, both of which have health benefits [Citation113]. NEAAs include amino acids, proline, arginine, glutamic acid, glycine, aspartic acid, tyrosine, cysteine, serine, and glutamic acid are a few examples. The amino acid profile of C. vulgaris and H. pluvialis, the proportion of NEAAs is around 51% and 49%, respectively [Citation116]. A healthy immune system is influenced by these chemicals, as well as gene expression, antioxidant responses, and cell signaling [Citation117]. Many studies indicate that the amount of microalgal meal that should be added to aquafeed varies depending on the type of algae used and the aquaculture species being fed. It would be beneficial to study the growth capacity of microalgae and identify the variables that influence their effectiveness.
2.3 Microalgae-based pigments
The color of microalgae is one of its most distinguishing properties, which is determined by pigments. Microalgae pigments are critical for their nutritional performance in aquaculture. In addition to chlorophyll, microalgae include carotenoids and phycobiliproteins. The Nannochloropsis genus contains pigments such as chlorophyll and astaxanthin. Photosynthesis in algae is facilitated by pigments, which are brightly colored chemical compounds. Carotenoids, chlorophylls, and phycobilin are the three primary types of microalgal photosynthetic pigments [Citation127,Citation128]. Microalgae pigments are eye-catching natural colors that include high-value components with health-promoting qualities that include antioxidants, vitamin precursors, neuroprotective, and immunological boosters [Citation129]. These pigments may address the increased demand for natural colors due to health concerns about the adverse effects of synthetic pigments [Citation127,Citation130]. Aquaculture uses a high concentration of carotenoids, such as β-carotene and astaxanthin, due to their vibrant color and antioxidant effects. These molecules have the potential to improve the quality and value of farmed fish, such as salmon and Asian tiger shrimp (Penaeus monodon) [Citation131]. Phytochemicals such as astaxanthin and β-carotene are abundantly generated by the microalgae Haematococcus pluvialis and Dunaliella salina (3–7% wt/wt) in natural abundance [Citation132,Citation133]. shows the pigment compositions of numerous algae species, as well as the health benefits associated with them.
Table 4. Algal pigment compositions and their health benefits
Microalgal biomass has been shown to affect fish pigmentation. H. pluvialis is the most commonly used microalgae specifically for color enhancement. In the aquaculture industry, whole cells and extracts of H. pluvialis extracts are used as feed additives (1.5–1.7%) [Citation134]. Several algae species are used as pigments in fish feed. The Haematococcus produces Astaxanthin, which gives salmon its pink hue [Citation134]. Additionally, Spirulina contains additional carotenoids that ornamental koi and other fish can convert to astaxanthin and other brightly colored pigments [Citation135]. Phaeodactylum tricornutum produces large quantities of fucoxanthin, which has been shown to contribute to the golden yellow coloration of gilthead seabreams [Citation136]. Carotenoids are found in a wide variety of products, including natural feed colors, food supplements, vitamin supplements, and health foods. The high concentration of carotenoids in D. saline makes it the most popular species for large-scale production (up to 14% dry weight) [Citation137]. Microalgae strains that are commercially feasible for pigment synthesis must meet a series of criteria, including improved nutritional components, non-toxicity, and the presence of digestible cell walls for nutrient absorption [Citation129]. Phycobiliproteins, β-carotene, and astaxanthin are used mainly as colorants, pharmaceuticals, aquaculture, and nutraceuticals. Chlorococcum sp. (Astaxanthin, lutein, β-carotene), D. salina (β-carotene, zeaxanthin, chlorophylls a, b), H. pluvialis (astaxanthin, canthaxanthin, lutein), Spirulina sp. (β-carotene, zeaxanthin, phycocyanin, allophycocyanin), Porphyridium sp. (phycoerythrin) [Citation138–143].
Natural feed pigments, feed additives, nutrients, and health food products are commonly made from carotenoids. Carotenoids found in abundance in D. salina make it the species most often exploited for large-scale production [Citation144]. Carotenes from Dunaliella species were shown to improve the health of L. vannamei shrimp given high-carotene diets. Coloration and market acceptability can be achieved by supplementing Red tilapia diets with A. platensis, a source of pigmentation [Citation145,Citation146]. According to these studies, low amounts of Arthrospira or other microalgae can enhance the color and flavor of numerous fish species, such as tilapia. Therefore, additional studies are required to evaluate the impact of various microalgal pigments on commercial aquaculture.
2.4 Microalgae-based vitamins
Microalgae are high in vitamins, and vitamin B has been shown to function as a cofactor for mitochondrial enzymes, reducing oxidative breakdown and improving metabolism [Citation147,Citation148]. Microalgae, including Spirulina sp., have more Vitamin B12 (127–244 g/g) than plant or animal-based foods. This vitamin helps prevent megaloblastic anemia, which causes fatigue and weakness [Citation149]. Another study found a significant amount of vitamin E (3.7 mg/g) in the Euglena gracilis microalgae. Vitamin E has been reported to reduce the risk of cancer, eye disease, heart disease, and other diseases [Citation150]. A high amount of vitamin C content (3.44 mg/g) has been found in the Eisenia arborea brown microalgae, which is equivalent to that of mandarin oranges [Citation151]. This antioxidant vitamin is necessary for immune system function, tissue formation, and repair [Citation152].
2.5 Carbohydrates derived from microalgae
Microalgae are rich in carbohydrates, and polysaccharides are readily found in both their cytoplasm and chloroplast [Citation153]. Microalgae carbohydrates are used for several reasons, including energy storage and structural components in cell walls [Citation154]. Due to the high photoconversion efficiency, macroalgae such as P. cruentum contain carbohydrates (40–57 wt% dry weight), Prymnesium parvum (30–33 wt% dry weight), and S. quadricauda (21–52 wt% dry weight) [Citation155,Citation156]. For each species of the algal genus, there is a distinct variation in glucose metabolism and content [Citation156,Citation157]. Algae with high carbohydrate yield and sugar content are suitable for human consumption. The culture system and environmental conditions can impact algal production and glucose content [Citation153]. Microalgae contain about 10–25% carbohydrates and their amount varies with culture age and growth conditions [Citation158]. A variety of starches, cellulose, sugars and other polysaccharides are found in microalgae. Bacteria and fungi naturally produce the polysaccharide β-glucan, which is made of D-glucose. These polysaccharides can be found in large quantities in the Chlorella sp., microalgae [Citation78,Citation159]. displays the monosaccharide compositions of several microalgae.
Table 5. Algal monosaccharide compositions
Microalgal carbohydrates are digestible according to the type and quantity of carbohydrates in biomass, as well as the kind of fish that consumes microalgal carbohydrates [Citation160]. The carbohydrate digestibility of microalgal species varies between 22% and 83%, depending on fish species [Citation110]. S. maxima and C. vulgaris were found to have a higher carbohydrate digestibility (greater than 70%) in Nile tilapia [Citation86]. Microalgae contain starch-like carbohydrates that can be easily digested. In vitro research revealed that C. sorokiniana, Klamath, and N. sphaeroides showed greater carbohydrate digestibility [Citation161]. Studies conducted on Nile tilapia, African catfish, and in vitro studies have shown that C. vulgaris has a higher carbohydrate digestibility value than other algal species [Citation86]. Certain microalgal species, such as Spirulina sp., Chlorella sp., and Schizochytrium sp., have been demonstrated to have excellent fiber digestibility [Citation162]. Isochrysis sp. and Nannochloropsis sp. fiber digestibility in rainbow trout was determined to be 96% and 38%, respectively [Citation11]. Even though Isochrysis sp. contained more fiber than Nannochloropsis sp., the latter had a higher fiber digestibility, suggesting that the type of fiber (soluble or insoluble) is important for digestion. Compared to other nutrients, starch is a readily digestible food for fish and crustaceans [Citation163].
The antiviral and antibacterial properties of β-glucan have been shown in humans, and it has also been shown in fish to have high antibacterial and immune-stimulating properties [Citation164]. Furthermore, the types of carbon sources used and the metabolic mechanism employed are other important variables that affect the sugar concentration in microalgae [Citation115]. The use of light has been shown to influence both algal growth and biomass composition during algal culture since light is an important energy source for photosynthetic activity [Citation78,Citation153]. For algal cultivation, the light intensity is normally between 200 and 400 mol photons m2/s. Nutritional restriction may be used to alter the metabolic process of microalgae and cause glucose accumulation [Citation44].
3. Nutrients digestibility
It is important to understand how digestible feed components are used to calculate their nutritional value. Microalgal products are assessed for their nutritional digestibility before they can be used in aquafeeds. Many different types of microalgal organisms, from prokaryotes to eukaryotes, contribute to the wide variety. There has also been evidence of intra-species diversity in metabolic profiles. The nutritional and energy digestibility of various microalgal species varies due to differences in chemical composition and physical structure. Fish digestion of microalgae is influenced by the composition and rigidity of the cell [Citation161]. In the prokaryotic (cyanobacterial) microalgae, peptidoglycan layers are present in the cell wall, while in eukaryotic microalgae, cellulosic layers occur [Citation86,Citation170,Citation171]. Fish prefer microalgae with peptidoglycan (murein) layered cell walls over cellulose layered microalgae [Citation86]. The rigidity of the cell wall also affects digestion. Thick-walled microalgae are less digestible than species with thin or no cell walls. Desmodesmus, Nannochloropsis, Haemotococcus and Chlorella, microalgae are with thick cell walls, while I. galbana, Porphyridium cruentum, and D. salina are with thin cell walls [Citation85,Citation110,Citation172].
However, certain proteins may still alter the digestion of microalgae. Unwanted trypsin inhibitor, an enzyme that inhibits proteolytic enzymes, may cause poor digestion of Nannochloropsis sp. Some marine microalgae include lipase inhibitors that may affect lipid digestion [Citation87,Citation173,Citation174]. Microalgae are made up of non-starch polysaccharides and fibers, making them difficult to digest [Citation11]. Polysaccharides that are not made of starch, such as cellulose, gums, pectins, and hemicelluloses, are usually difficult to break down [Citation170,Citation175,Citation176]. Digestive enzymes are absent in certain fish species, such as Nile tilapia, which cannot break down the beta glycosidic bonds found in non-starch polysaccharides [Citation177]. Undigested carbohydrates are transported quickly by the digestive tract, absorb proteins, and reduce protein digestibility [Citation178,Citation179]. Fiber concentrations were shown to have a negative relationship with organic matter, protein, and carbohydrate digestibility [Citation147,Citation161]. This hypothesis has not been supported by any other study. Therefore, more research is needed to link fiber content with nutrient digestion [Citation161].
In rainbow trout, Isochrysis sp. absorption of nutrients was shown to be superior to Nannochloropsis sp [Citation11]. Fiber and other anti-nutrients also reduce proteolytic and amylase activity and digestibility [Citation180,Citation181]. Other variables can impact the digestion of a microalgae diet. Exopolysaccharides can form stable complexes with proteins, inhibiting proteolysis [Citation161]. Exopolysaccharides are exopolysaccharides that are released or remain attached to cells in an algal culture [Citation161]. Phenolic chemicals found in plants and seaweed can precipitate proteins. Although microalgae have a modest phenolic content (0–20 mg GA/g-DW), plant phenolic substances in the diet could impact microalgal protein digestion [Citation182–184]. The digestibility of amino acids may be impaired. Furthermore, differences in physiology between fish species cause different digestibilities for the same microalgae [Citation86]. Fish species vary in their digestive enzyme profiles, in addition to their physiology. Only a few fish, such as the Rohu (Labeo rohita), have the enzymes required to break down cellulose [Citation185].
3.1 Bioaccumulation of heavy metals
During cultivation, microalgae cells can absorb and accumulate substantial amounts of heavy metals (HMs) [Citation78,Citation186–188]. Several studies have examined the bioaccumulation of HMs in microalgal biomass due to the existence of negatively charged functional groups on the surface of microalgal cells [Citation189–191]. HMs absorbed by microalgae include predominantly arsenic (As), cadmium (Cd), copper (Cu), chromium (Cr), lead (Pb), mercury (Hg), nickel (Ni), and lanthanum (La). In fact, both living and non-living microalgae are effective in absorbing HMs from the environment and water [Citation21,Citation191]. Non-living microalgae can accumulate Cd, Cr, Hg, and Pb at 59, 98, 36, and 131 mg/g, respectively. Non-living algal biomass has a strong capacity to adsorb HMs primarily due to their surface functional groups [Citation192].
When microalgal biomass containing concentrated HMs is fed to fish, harmful compounds can migrate up the food chain. Certain strategies can be employed to avoid bioaccumulation of HMs in the food chain when microalgae are used as aquafeed. The amount of HMs in the culture media should be monitored regularly during microalgae culture. If HMs concentrations exceed the EPA<apos;>s guidelines, the culture medium must be treated or relieved. This method may avoid the bio-accumulation of HMs in the food chain. Before using microalgae as fish food, it is possible to eliminate HMs from them. HMs desorption may occur through pH-induced desorption or metal-chelating agent treatment. The pH of algal cells must be lowered to the isoelectric points of functional groups on their surfaces to neutralize their surface charge [Citation193]. Heavy metals adsorbed on the surface of algae might be combined with metal chelating agents to facilitate their desorption. HMs have been successfully desorbed from microalgae biomass using these two approaches [Citation193–196]. When HMs are attached to the surface of algal cells rather than within algal cells, desorption techniques may be used to remove them from the biomass of microalgae. In contrast to the accumulation of HMs inside algal cells, microalgal cells can be treated by desorption to remove HMs adhering to the surface. Therefore, after desorption, HMs content in algal cells should be evaluated to determine whether microalgae can serve as aquafeed.
3.2 Deficiency in digestibility
Since microalgae have a high starch concentration, replacing the biomass of microalgae with a fish meal reduces digestibility. Furthermore, aquatic animals must dissolve the cellulose-rich cell walls of algae before consuming their nutritional components. For these two challenges, meals supplemented with microalgae- in fish and shrimp culture are poorly absorbed [Citation197]. Dietary wheat starch levels of 20% in juvenile largemouth bass (Micropterus salmoides) not only impeded weight gain but also produced oxidative stress and impaired innate immunity. Fish offered 5% and 10% starch diets showed significant weight gain, growth rate, protein efficiency ratio, and feed conversion ratio when compared to fish-fed 20% starch diets [Citation198]. It is generally recognized that omnivore fish digest carbohydrates are better than carnivorous fish, although they exhibit significant anatomical and physiological differences in their digestive systems [Citation198,Citation199]. Another study found that omnivorous fish have various digestive capacities [Citation20]. Rather than raw biomass, defatted microalgae biomass is often used as a supplement to fish feed in the fishing sector. After oil extraction, the starch content in the biomass could be increased. The microalgae biomass must be effectively managed to avoid an excessive starch content. The apparent digestibility coefficients (ADCs) of macronutrients, amino acids, and fatty acids were studied in freshwater (Arthrospira, Chlorella) and marine microalgal (Schizochytrium) components in Nile tilapia [Citation85]. Compared to chlorella, Arthrospira exhibited significantly higher ADCs of crude protein and all EAAs (86%), which corresponded well with the reported values for fishmeal and plant feeds. Schizochytrium had the highest DHA content, as well as the highest ADCs for lipids (total PUFA 98%), ω-3 (98%), and ω-6 (92.4%), as well as the maximum digestibility of DHA. Spirulina and Schizochytrium were shown to be effective protein substitutes for tilapia diets, while Schizochytrium was discovered to be a good LC-PUFA supplement [Citation200].
For microalgal cells to be preserved, they usually need to be spray-dried after they have been removed from the growing reactor. Furthermore, microalgal biomass is dried in a variety of ways, such as sun drying, drum drying, and oven drying. However, the microalgae cell wall remains intact in these circumstances, indicating that digestion is restricted [Citation87,Citation201,Citation202]. A cellulose-rich cell wall causes poor digestion of microalgae-supplemented fish diets [Citation203]. The cell wall protects the algal cell and its intracellular components during growth. It is necessary to disrupt the cellulose structure of the cell wall in order to access the intracellular contents. Due to a lack of intestinal flora, several species of fish are unable to digest non-starch carbohydrates effectively [Citation204,Citation205]. Therefore, cellulose cannot be digested by fish, and the structure of the cell walls of microalgae cannot be effectively dissolved by many species of fish. As a consequence, aquatic organisms will not be able to consume nutrients found in algal cells. To improve the digestibility of microalgal biomass for use in fish/shrimp aquaculture, certain pretreatment techniques could be applied. Microalgae, for starters, contain starch, which may be separated before using algal biomass as aquafeed. Second, destroying the cell wall may cause the nutritional components of the microalgae to be released [Citation206]. Enzymatic digestion and physical treatment have been shown to destroy microalgae cell walls effectively [Citation206,Citation207]. Microalgae cell walls are made up of pectin, hemicellulose, cellulose, and glycoproteins. In algae, enzymes such as glucosidase, cellulase, hemicellulase, xylanase, and exoglucanase break down the cell wall [Citation206].
To improve nutrient digestibility, biomass can be pretreated/processed using pasteurization, freeze-drying, bead milling, high-pressure homogenization, pulse electric field, microwave, chemical, and enzymatic treatments [Citation208]. During biomass processing or pretreatment, the rigid cell wall of algae is broken down, releasing internal nutrients that can be digested and absorbed by fish. According to studies, the processing of specific microalgae is linked to improved digestibility in certain fish [Citation87,Citation209,Citation210]. Physical treatment processes such as sonication, the beating of beads, and freezing can be used to damage the microalgal cell wall. The cost of the aforementioned methodologies should be used to determine their viability in a real-world application [Citation4]. The grinding of Tetraselmis sp. beads, for example, increased protein digestibility in European seabass by 20% compared to untreated cells [Citation87]. Pretreatment with bead milled microalgae rather than whole biomass increased the digestibility of amino acids such as phenylalanine and aspartic acid in European seabass, but not the digestibility of essential amino acids [Citation87]. Nannochloropsis and Chlorella cell walls were broken by bead milling for 10 minutes, which could release nutrients and improve digestibility [Citation86,Citation209,Citation211]. For European seabass, enzyme processing improved the protein digestion of Nannochloropsis sp. and Chlorella sp., as well as energy digestion of Nannochloropsis sp. Tetraselmis sp., and Chlorella sp., by 14%, 11%, and 40%, respectively [Citation87]. Due to the fact that the degree of cell rupture differs between species, nutritional accessibility can vary despite equal processing conditions [Citation212].
3.3 Anti-nutritional factors
Anti-nutritional factors (ANFs) are biological components that affect gastrointestinal and metabolic function in animals and humans. These ANFs were detected in vegetable soybean and peanut proteins together with phytic, lectin, and tannic acids. Due to the increased nature of ANFs, protein meals or peanut meals cannot be used in aquafeed [Citation213]. Microalgae have been shown to contain a variety of ANFs, such as Tetradesmus obliquus, Kirchneriella lunaris, and Pseudokirchneriella subcapitata [Citation214,Citation215]. Previous studies indicate that the tannic acid levels of S. maxima (6.86 mg/g) and C. vulgaris (1.44 mg/g) were comparable [Citation216]. High absorption of microalgae may negatively affect fish growth because of antinutritional components in microalgae. ANF-supplemented aquafeed reduced liver enzyme activities and reduced intestinal brush border enzymes in juvenile Japanese seabass (Lateolabrax japonicus) [Citation217].
It would be beneficial to discover and study anti-nutritional components in microalgae to increase their incorporation into fish diets. A pretreatment strategy for microalgae should also include the scavenging of anti-nutritional factors. Many studies have demonstrated effective ways to eliminate the anti-nutritional components from soybean meals [Citation218,Citation219]. Antinutritional chemicals may be removed by specific microorganisms through their activities. When Aspergillus sojae and/or Aspergillus ficuum are used in conjunction with solid-state fermentation, the phytic acid was reduced by 53.27% to 73.16%. Enzymes produced from microorganisms may also be used to remove certain anti-nutritional substances [Citation220]. Another study found a maximum tannin removal rate of 73%. If antinutritional components are identified in microalgae, different physical treatment methods, including extraction, frying, blanching, and soaking, may be utilized to minimize their concentration. The pretreatment treatments described above have been shown to significantly decrease ANFs in a variety of vegetable proteins [Citation221–224]. For example, extrusion cooking with optimal barrel temperature, extruder speed, and moisture content removed 61.25 % of tannin in linseed meals [Citation224]. Eventually, these technologies may be utilized to pre-retreat microalgae to eliminate anti-nutritional components and surge algal biomass assimilation into fish diets, which would be beneficial in the long run.
3.4 Challenges in microalgae biomass harvesting and processing
Most aquafeed substitutes microalgae biomass for fish meal. Microalgae cultivated in a culture medium should be harvested and dried, and the moisture adjusted before being used to make algae biomass-supplemented fish feed pellets. Centrifugation, sedimentation, and filtration are all processes used to collect suspended algae cells in a culture medium. The collection of algae biomass accounts for 30% of microalgae production costs. In addition to that, flocculation harvesting incorporates aluminum into biomass, making microalgae-based aquafeed based on microalgae undesirable [Citation225]. Drying wet microalgae (with a moisture content of 70–90 %) is required before the algae can be delivered to the feed factory. This is an energy-intensive, time-consuming, and costly technique [Citation226]. Managing the moisture content of dehydrated microalgae biomass is important throughout the pelletization process.
Certain alternatives have been proposed and thoroughly explored to overcome the aforementioned concerns. The development of more cost-effective biomass harvesting methods is an essential first step. Co-cultivation of a filamentous fungus with microalgae previously resulted in fungal-algal pellets [Citation15,Citation227]. As a consequence, biomass collection has become a passive process that does not require human interaction. Several fungal strains, including Mucor circinelloides and Aspergillus oryzae, produce high-value components when cultured with microalgae, which can improve the nutritional content of biomass [Citation228,Citation229]. Microalgae immobilization techniques have also been implemented to minimize collection costs. After growing in the substratum, a biofilm<apos;>s biomass may be scraped off using scrapers. Immobilized microalgae are more economical to harvest than suspended microalgae [Citation230]. Microalgae can be used to make an eco-friendly closed or semi-closed food chain aquaculture system. Zooplankton is the main food source for fish and shrimp in this environment. In addition to breaking down animal waste, microalgae can also transform it into valuable components [Citation15,Citation231]. Therefore, harvesting and drying can be avoided, and microalgae can be used to improve aquaculture water quality. Therefore, this unique approach to microalgae aquaculture is more economically feasible while still addressing the objectives of a circular economy.
4. Economic and environmental feasibility
To develop algae-based products as a possible source of food or feed for humans and animals, their economic feasibility and long-term viability must be improved. The Techno-economic assessment (TEA) and life cycle assessment (LCA) methods can analyze both the manufacturing route and the technical process of R&D activities to achieve commercial and environmental viability [Citation232]. Based on six potential alternative sites, a techno-economic study was conducted on the entire life cycle of a 100-hectare microalgal production plant. Due to improved photosynthetic efficiency and photobioreactors with shorter light paths, it has been determined that the cost of algal production in Spain is approximated at 3.4 euros per kilogram of dry biomass with a predicted decrease to 0.5 euros per kilogram in ten years. The production of high-value metabolites (such as pigments) could generate 657 million euros in profits over the next 15 years (or more) [Citation233].
Biotechnology can be used to create bio-based products, bioenergy, food, and feed while improving ethical and environmental sustainability, optimizing production processes, and lowering costs. Furthermore, to generate biomass, certain microalgal species have been shown to thrive in complex organic waste streams (digestate, wastewater, etc.) and to remove nutrient pollutants (P, N, and other toxins) [Citation234–237]. As a consequence, integrated biorefineries based on microalgal biotechnology could recover some agricultural by-products while reducing disposal costs. Compared to monoculture cultivation, heterotrophic cultivation is cost-effective since it takes less land and investment, consumes more energy and carbon, and has a lower cost of downstream processing [Citation237,Citation238]. Microalgae protein has a significant environmental impact because the drying process is so energy-intensive. In contrast to beef and pork, the LCA in autotrophic and heterotrophic algae cultures resulted in more ecologically sustainable products derived from heterotrophic cultivation [Citation239]. When using hydrolyzed food waste to obtain carbon in microalgal production, the environmental benefit could be 4.5 times greater, making it much more eco-friendly among protein sources.
5. Current concerns with microalgae in aquafeed
The high cost of microalgae production continues to be a barrier to aquaculture. Microalgae have the potential to be used economically by cutting the costs of production and distribution [Citation85,Citation240]. Microalgae are difficult to dry and pelletize, and incorrect drying can alter their nutritional and physical qualities, which in turn reduces their use as feed. Some microalgae (such as Chlorella) have thick cell walls that hinder nutrient uptake. Some microalgae (e.g., D. tertiolecta) have extracellular polysaccharides that might interfere with nutritional absorption. Poor digestibility and significant salt buildup in marine microalgae species used as fish feed might cause problems. Algae can only provide 10–15% of dietary protein requirements in test diets without affecting development or food consumption. Several microalgae have a high carbohydrate and low protein content because their tough cell walls inhibit the digestion of fats and proteins [Citation163].
Due to the high concentrations of trace elements and toxins found in microalgae biomass, it is not recommended for use in aquafeeds. Protein left behind after fat is extracted for the generation of biofuels is often proposed for use in animal feed [Citation241]. It is possible that microalgae used to produce biofuels are not suitable for feeding and that the demand for low-cost fuel production could result in toxic protein residues [Citation242,Citation243]. It is preferable to first utilize algal biomass for higher-value products, such as aquafeed, and then use any remaining chemicals for biofuel production. This means a high-value product-first philosophy [Citation244,Citation245]. The lack of substantial amounts of microalgal biomass may hinder the growth of the aquafeed sector. Massive amounts of microalgal biomass for the aquafeed industry require successful large-scale algal growth of commercially relevant microalgae species [Citation246].
6. Perspective and future direction
The cell walls of microalgae vary in content and structure. This emphasizes the need to screen for commercial strains that can be readily handled for cell disruption. Only a few microalgal species have been digestible tested. Several microalgae and cyanobacteria species, including Anabaena sp. and Nostoc sp., have not been tested for digestibility by fish or other aquatic species. D. salina, for example, does not have a cell wall, which can improve digestion of cellular metabolites. It is also critical to explore the impact of environmental variables and stress on the chemical composition of microalgae. For example, nitrogen deficiency could cause a buildup of PUFA and starch, which fish can consume. The metabolic response of microalgae to stress, such as nitrogen deficiency, differs between species. In certain strains, nitrogen deficiency lowers protein levels while increasing carbohydrate levels [Citation247]. Optimal stress management and species selection are required to achieve a desirable biochemical profile and digestibility.
In addition to the availability of microalgal biomass at a reasonable price, microalgal and aquafeed producers must address substantial variability in the proximate composition, digestibility, and growth conditions. Aquaculture requires a diverse range of microalgal species. Microalgal species with better nutritional or growth properties may be more efficient. The nutritional value of various microalgae should be investigated. This means that the cell wall must be broken down to make the algal elements available to digestive enzymes [Citation147,Citation211,Citation248]. However, further processing stages may increase costs. To improve fish health, microalgal material must be tested for harmful chemicals prior to commercialization. In addition to nutritional content and digestibility, the processing requirements of industrial production lines for compound feed must also be considered. The composition of extruded fish feed is acknowledged to be one of the most critical elements that impact physical quality [Citation249]. Microalgae were examined in a recent study to physically determine their effects on extruded fish feed, but further research is necessary [Citation208].
Due to the explosive growth of the aquaculture industry, it is important to study the digestibility of microalgae. So far, digestibility has been examined only for salmonids, tilapia, seabass, and African catfish. During feed screening trials, microalgae should be evaluated for anti-nutritional factors, such as digestion enzyme inhibitors (e.g., caulerpenyene, a terpene) and nutrient bioavailability factors. Studies on macronutrients, such as lipids, carbohydrates, protein, and energy, have previously focused on microalgae. However, specific classes of these macromolecules have been shown to impact digestion. Therefore, it is essential to determine the digestibility and composition of microalgae for certain species of fish.
The energy-intensive harvesting stage of microalgae biomass results in high production costs. Microalgal biorefinery approaches might reduce microalgal feed component manufacturing costs. This approach extracts high-value biochemical components from biomass, such as lipids and carotenoids, which are then used in fish and animal feed. Supercritical fluid extraction and organic solvent extraction in combination are effective for defatting biomass. Although defatted biomass from the aforementioned biorefinery processes has been studied, no reports on saponified biomass have been published. The saponification-based biorefinery produces carotenoids and other value-added microalgae products. The residual biomass may be used as fish feed. Therefore, the residual biomass of biorefinery methods must be examined for digestion in fish.
7. Conclusions
Due to the depletion and pollution of marine resources, aquaculture is increasingly turning to microalgae as a substitute for fishmeal. Astaxanthin, polyunsaturated fatty acids (PUFAs), and phycocyanin are just a few of the high-quality components found in microalgae. There is little doubt that these high-quality constituents can improve an animal<apos;>s immunological response, increase its survival rate, and help it gain weight. The biosynthesis of astaxanthin, PUFAs, and phycocyanin in microalgal cells is technically feasible to produce high-quality biochemical products. Thanks to significant bioengineering technology advances, microalgal biomass and bioproducts such as aquafeed are no longer just a theory. In addition, the culture medium should be regulated to avoid the accumulation of HMs in the biomass. To make microalgae suitable for aquafeeds, the cell wall of the algae must be broken down to release intracellular nutrients and scavenge nutrient-hostile chemicals. A co-culture of microalgae with filamentous fungi is being developed to facilitate harvesting and minimize microalgal biomass costs. Eco-friendly aquaculture systems based on microalgae, zooplankton, and fish/shrimp are also gaining importance in the circular bio-economy. A critical analysis revealed that bioengineering tools and strategies could significantly improve the development of aquaculture-based food production. Additionally, the study also provides new insights into the challenges and potential breakthroughs regarding microalgae biomass production and bioproducts as sustainable ingredients in the future.
Acknowledgements
We would like to express our gratitude to Khalifa University, Abu Dhabi, United Arab Emirates, for their assistance via the grant CIRA-2018-27.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grafton RQ, Williams J, Jiang Q. Food and water gaps to 2050: preliminary results from the global food and water system (GFWS) platform. Food Sec. 2015;7(2):209–220.
- Tilman D, Balzer C, Hill J, et al. Global food demand and the sustainable intensification of agriculture. Proc Nat Acad Sci. 2011;108(50):20260–20264. DOI:10.1073/pnas.1116437108.
- Olsen RL, Hasan MR. A limited supply of fishmeal: impact on future increases in global aquaculture production. Trends Food SciTechnol. 2012;27(2):120–128.
- Chen F, Leng Y, Lu Q, et al. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021;60:102541.
- Jannathulla R, Rajaram V, Kalanjiam R, et al. Fishmeal availability in the scenarios of climate change: inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquacult Res. 2019;50(12):3493–3506. DOI:10.1111/are.14324.
- Zhao S, Lü B, Li R, et al. A preliminary analysis of fishery resource exhaustion in the context of biodiversity decline. Sci China Earth Sci. 2016;59(2):223–235. DOI:10.1007/s11430-015-5193-4.
- Shen G, Heino M. An overview of marine fisheries management in China. Marine Policy. 2014;44:265–272.
- Hanachi P, Karbalaei S, Walker TR, et al. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ Sci Pollut Res. 2019;26(23):23777–23787. DOI:10.1007/s11356-019-05637-6.
- Marketwatch, Aquaculture Market Size. 2020. https://www.marketwatch.com/ (accesed 27 August 2021). 2020.
- Sarker PK, Kapuscinski AR, McKuin B, et al. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci Rep. 2020;10(1):19328. DOI:10.1038/s41598-020-75289-x.
- Sarker PK, Kapuscinski AR, Vandenberg GW, Proulx, E,Sitek AJ, Thomsen L, et al. Towards sustainable and ocean-friendly aquafeeds: evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elem Sci Anth. 2020;8(5). DOI:10.1525/elementa.404.
- Norambuena F, Hermon K, Skrzypczyk V, et al. Algae in fish feed: performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS One. 2015;10(4):e0124042. DOI:10.1371/journal.pone.0124042.
- Nagappan S, Das P, AbdulQuadir M, et al. Potential of microalgae as a sustainable feed ingredient for aquaculture. J Biotechnol. 2021;341:1–20.
- Kusmayadi A, Leong YK, Yen H-W, et al. Microalgae as sustainable food and feed sources for animals and humans – biotechnological and environmental aspects. Chemosphere. 2021;271:129800.
- Li H, Chen S, Liao K, et al. Microalgae biotechnology as a promising pathway to ecofriendly aquaculture: a state‐of‐the‐art review. J Chem Technol Biot. 2021;96(4):837–852. DOI:10.1002/jctb.6624.
- Lu Q, Li H, Zou Y, et al. Astaxanthin as a microalgal metabolite for aquaculture: a review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021;54:102178.
- Beal CM, Gerber LN, Sills DL, et al. Algal biofuel production for fuels and feed in a 100-ha facility: a comprehensive techno-economic analysis and life cycle assessment. Algal Res. 2015;10:266–279.
- Camacho-Rodríguez J, Macías-Sánchez MD, Cerón-García MC, et al. Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: nutrient stability under different storage conditions. J Appl Phycol. 2018;30(2):1049–1059. DOI:10.1007/s10811-017-1281-5.
- Ahmad A, Banat F, Alsafar H, et al. Recent breakthroughs in integrated biomolecular and biotechnological approaches for enhanced lipid and carotenoid production from microalgae. Phytochem Rev. 2022. DOI:10.1007/s11101-022-09804-5.
- Gominho-Rosa MDC, Rodrigues APO, Mattioni B, et al. Comparison between the omnivorous jundiá catfish (Rhamdia quelen) and Nile tilapia (Oreochromis niloticus) on the utilization of dietary starch sources: digestibility, enzyme activity and starch microstructure. Aquaculture. 2015;435:92–99.
- Pavithra KG, Kumar PS, Jaikumar V, et al. Microalgae for biofuel production and removal of heavy metals: a review. Environ Chem Lett 18. 2020;1905–1923. DOI:10.1007/s10311-020-01046-1.
- Nidhina N, Muthukumar S. Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem. 2015;173:920–926.
- Ullah Z, Ahmed G, Nisa MU, et al. Standardized ileal amino acid digestibility of commonly used feed ingredients in growing broilers. Asian-Australas J Anim Sci. 2016;29(9):1322. DOI:10.5713/ajas.15.0703.
- García-Vaquero M, Hayes M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev Int. 2016;32(1):15–45.
- Blomqvist J, Pickova J, Tilami SK, et al. Oleaginous yeast as a component in fish feed. Sci Rep. 2018;8(1):1–8. DOI:10.1038/s41598-018-34232-x.
- Marques A, Dhont J, Sorgeloos P, et al. Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically grown brine shrimp Artemia franciscana. J Exp Mar Biol Ecol. 2004;312(1):115–136. DOI:10.1016/j.jembe.2004.06.008.
- Bosch G, Zhang S, Oonincx DG, Hendriks WH, et al. Protein quality of insects as potential ingredients for dog and cat foods. J Nutr Sci. 2014;3. DOI:10.1017/jns.2014.23.
- Aladetohun N, Sogbesan O. Utilization of blood meal as a protein ingredient from animal waste product in the diet of Oreochromis niloticus. Int J Fish Aquaculture. 2013;5(9):234–237.
- Hussain SM, Afzal M, Salim M, Javid A, Khichi TAA, Hussain M, Raza SA, et al. Apparent digestibility of fish meal, blood meal and meat meal for Labeo rohita fingerlings. Journal of Animal and Plant Sciences. 2011;21(4):807–811.
- Grazziotin A, Pimentel FA, Jong EVD, et al. Poultry feather hydrolysate as a protein source for growing rats. Braz J Vet Res Anim Sci. 2008;45(1):61–67. DOI:10.11606/S1413-95962008000700008.
- Yu R, Cao H, Huang Y, Peng M, Kajbaf K, Kumar V, Tao Z, Yang G, Wen C, et al. The effects of partial replacement of fishmeal protein by hydrolysed feather meal protein in the diet with high inclusion of plant protein on growth performance, fillet quality and physiological parameters of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture research. 2020;51(2):636–647. DOI:10.1111/are.14411.
- Draganovic V, van der Goot A, Boom R, Jonkers J, et al. Wheat gluten in extruded fish feed: effects on morphology and on physical and functional properties. Aquaculture Nutr. 2013;19(6):845–859. DOI:10.1111/anu.12029.
- Sørensen M, Morken T, Kosanovic M, et al. Pea and wheat starch possess different processing characteristics and affect physical quality and viscosity of extruded feed for Atlantic salmon. Aquaculture Nutr. 2011;17(2):e326–e336. DOI:10.1111/j.1365-2095.2010.00767.x.
- Katiyar R, Arora A. Health promoting functional lipids from microalgae pool: a review. Algal Res. 2020;46:101800.
- Madeira MS, Cardoso C, Lopes PA, et al. Microalgae as feed ingredients for livestock production and meat quality: a review. Livestock Sci. 2017;205:111–121.
- Globenewswire, Microalgae-based products market forecast to 2028. https://www.globenewswire.com/ (accesed 27 August 2021). 2021.
- Hodar A, et al. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: a review. J Exp Zool India. 2020;23(1):13–21.
- Rizwan M, Mujtaba G, Memon SA, et al. Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sust Energ Rev. 2018;92:394–404.
- Li K, Liu Q, Fang F, et al. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol. 2019;291:121934.
- Arun J, Gopinath KP, SundarRajan P, et al. A conceptual review on microalgae biorefinery through thermochemical and biological pathways: bio-circular approach on carbon capture and wastewater treatment. Bioresour Technol Rep. 2020;11:100477.
- Nagappan S, Nakkeeran E. Biorefinery: a concept for co-producing biofuel with value-added products. Environ Biotechnol. 2020;2(45):23.
- Imamoglu E. Simulation design for microalgal protein optimization. Bioengineered. 2015;6(6):342–346.
- Wan AH, Davies SJ, Soler-Vila A, Fitzgerald R, Johnson MP, et al. Macroalgae as a sustainable aquafeed ingredient. Revi Aquacult. 2019;11(3):458–492.
- Dragone G, Fernandes BD, Abreu AP, et al. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy. 2011;88(10):3331–3335. DOI:10.1016/j.apenergy.2011.03.012.
- Draaisma RB, Wijffels RH, (Ellen) Slegers PM, et al. Food commodities from microalgae. Curr Opin Biotechnol. 2013;24(2):169–177. DOI:10.1016/j.copbio.2012.09.012.
- García-Chavarría M, Lara-Flores M. The use of carotenoid in aquaculture. Res J Fisher Hydrobiol. 2013;8(2):38–49.
- Michalak I, Chojnacka K. Algae as production systems of bioactive compounds. Eng Life Sci. 2015;15(2):160–176.
- Carus M, Dammer L. The circular bioeconomy—concepts, opportunities, and limitations. Ind Biotechnol. 2018;14(2):83–91.
- Koyande AK, Show P-L, Guo R, et al. Bio-processing of algal bio-refinery: a review on current advances and future perspectives. Bioengineered. 2019;10(1):574–592. DOI:10.1080/21655979.2019.1679697.
- Ahmad A, Bhat AH, Buang A, et al. Biotechnological application of microalgae for integrated palm oil mill effluent (POME) remediation: a review. Int J Environ Sci Technol. 2019;16(3):1763–1788.
- Lu Q, Li H, Xiao Y, Liu H, et al. A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res. 2021;55:102281.
- Nagappan S, Kumar Verma S. Co-production of biodiesel and alpha-linolenic acid (omega-3 fatty acid) from microalgae, Desmodesmus sp. MCC34. Energy Sources Part A. 2018;40(24):2933–2940.
- Adarme-Vega TC, Lim DKY, Timmins M, et al. Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact. 2012;11(1):1–10. DOI:10.1186/1475-2859-11-96.
- Prabha SP, Nagappan S, Rathna R, Viveka R, Nakkeeran E, et al. Blue biotechnology: avision for future marine biorefineries. In refining biomass residues for sustainable energy and bioproducts. Elsevier;2020. p. 463–480.
- Ramaraj R, Tsai DD-W, Chen PH. Detention time study of algal biomass production with natural water medium. Chiang Mai J Sci. 2015;42(3):549–559.
- Baharuddin N, Aziz NS, Sohif HN, Basiran MN, et al. Marine microalgae flocculation using plant: the case of Nannochloropsis oculata and Moringa oleifera. Pak J Bot. 2016;48(2):831–840.
- Wan Mohamad Apandi WA, Matias Peralta HM. Removal of nutrients and selected heavy metals in wet market wastewater by using microalgae Scenedesmus sp. in applied mechanics and materials. Vol. 773, Trans Tech Publications, Ltd; 2015. p. 1210–1214.
- Jais N, Mohamed RMSR, Al-Gheethi AA, et al. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol Envir. 2017;19(1):37–52. DOI:10.1007/s10098-016-1235-7.
- Maizatul AY, Radin Mohamed RMS, Al-Gheethi AA, et al. An overview of the utilisation of microalgae biomass derived from nutrient recycling of wet market wastewater and slaughterhouse wastewater. Int Aqua Res. 2017;9(3):177–193. DOI:10.1007/s40071-017-0168-z.
- Yarnold J, Karan H, Oey M, et al. Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci. 2019;24(10):959–970. DOI:10.1016/j.tplants.2019.06.005.
- Ahmed RA, He M, Aftab RA, et al. Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep. 2017;7(1):1–10. DOI:10.1038/s41598-017-07540-x.
- Gomes D. Extraction and characterization of microalgae proteins from the extremophile Dunaliella. Lisboa: Técn; 2017. p. 1.
- Sydney EB, Sturm W, de Carvalho JC, et al. Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol. 2010;101(15):5892–5896. DOI:10.1016/j.biortech.2010.02.088.
- Weiss TL, Johnston JS, Fujisawa K, et al. Genome size and phylogenetic analysis of the A and L races of Botryococcus braunii. J Appl Phycol. 2011;23(5):833–839. DOI:10.1007/s10811-010-9586-7.
- D’Alessandro EB, Antoniosi Filho NR. Concepts and studies on lipid and pigments of microalgae: a review. Renew Sust Energ Rev. 2016;58:832–841.
- Shuba ES, Kifle D. Microalgae to biofuels:‘promising’alternative and renewable energy, review. Renew Sust Energ Rev. 2018;81:743–755.
- Zullaikah S, Jessinia MCP, Yasmin M, Rachimoellah M, Wu DW, et al. Lipids extraction from wet and unbroken microalgae Chlorella vulgaris using subcritical water. In: Materials science forum. Vol. 964, Trans Tech Publications Ltd; 2019. p. 103–108.
- Becker EW. Microalgae: biotechnology and microbiology. Vol. 10, Cambridge University Press; 1994.
- Wolkers H, Barbosa, M, Kleinegris, D, Bosma, R, Wijffels, RH, et al. Microalgae: the green gold of the future. Wageningen UR; 2011.
- Tandon P, Jin Q. Microalgae culture enhancement through key microbial approaches. Renew Sust Energ Rev. 2017;80:1089–1099.
- Mata TM, Rojas-Solórzano LR, Finol E, et al. Lipid content and productivity of Arthrospira platensis and Chlorella vulgaris under mixotrophic conditions and salinity stress. Chem Eng Trans. 2016;49:73–78.
- Fuentes MR, Fernández GA, Pérez JS, Guerrero JG, et al. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000;70(3):345–353. DOI:10.1016/S0308-8146(00)00101-1.
- Tibbetts SM, Bjornsson WJ, McGinn PJ. Biochemical composition and amino acid profiles of Nannochloropsis granulata algal biomass before and after supercritical fluid CO2 extraction at two processing temperatures. Anim Feed Sci Technol. 2015;204:62–71.
- Tibbetts SM, J. Melanson R, C. Park K, et al. Nutritional evaluation of whole and lipid-extracted biomass of the microalga Scenedesmus sp. AMDD isolated in Saskatchewan, Canada for animal feeds: proximate, amino acid, fatty acid, carotenoid and elemental composition. Curr Biotechnol. 2015;4(4):530–546. DOI:10.2174/2211550104666150827201854.
- Tibbetts SM, Whitney CG, MacPherson MJ, et al. Biochemical characterization of microalgal biomass from freshwater species isolated in Alberta, Canada for animal feed applications. Algal Res. 2015;11:435–447.
- Guschina IA, Harwood JL Algal lipids and their metabolism. In: Borowitzka M, Moheimani N editors. Algae for biofuels and energy. Springer; 2013. p. 17–36. DOI:10.1007/978-94-007-5479-9_2.
- Cheah WY, Show PL, Yap YJ, et al. Enhancing microalga Chlorella sorokiniana CY-1 biomass and lipid production in palm oil mill effluent (POME) using novel-designed photobioreactor. Bioengineered. 2020;11(1):61–69. DOI:10.1080/21655979.2019.1704536.
- Ahmad A, Banat F, Alsafar H, Hasan SW, et al. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. SciTotal Environ. 2022;806:150585.
- Brown MR. Nutritional value and use of microalgae in aquaculture. Avances en Nutrición Acuicola VI Memorias del VI Simposium Internacional de Nutrición Acuícola. 2002;3:281–292.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379.
- Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fisher Sci. 2003;11(2):107–184.
- Colombo SM, Parrish CC, Wijekoon MP. Optimizing long chain-polyunsaturated fatty acid synthesis in salmonids by balancing dietary inputs. PloS one. 2018;13(10):e0205347.
- Chen W, Wang Y, Han D, et al. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquaculture Nutr. 2019;25(5):1145–1155. DOI:10.1111/anu.12930.
- Ju Z, Forster I, Dominy W. Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture. 2009;292(3–4):237–243.
- Sarker P, Gamble MM, Kelson S, et al. Nile tilapia (Oreochromis niloticus) show high digestibility of lipid and fatty acids from marine Schizochytrium sp. and of protein and essential amino acids from freshwater Spirulina sp. feed ingredients. Aquaculture Nutr. 2016;22(1):109–119. DOI:10.1111/anu.12230.
- Teuling E, Schrama JW, Gruppen H, et al. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture. 2017;479:490–500.
- Batista S, Pintado M, Marques A, et al. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J Appl Phycol. 2020;32(5):3429–3446. DOI:10.1007/s10811-020-02185-2.
- Agboola JO, Teuling E, Wierenga PA, et al. Cell wall disruption: an effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquaculture Nutr. 2019;25(4):783–797. DOI:10.1111/anu.12896.
- Ghanbari M, Kneifel W, Domig KJ. A new view of the fish gut microbiome: advances from next-generation sequencing. Aquaculture. 2015;448:464–475.
- Nayak SK. Role of gastrointestinal microbiota in fish. Aquacult Res. 2010;41(11):1553–1573.
- Regunathan C, Wesley S. Control of vibrio spp. in shrimp hatcheries using the green algae Tetraselmis suecica. Asian Fisher Sci. 2004;17(1/2):147–158.
- Souza FPD, Lima ECSD, Urrea-Rojas AM, et al. Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of Nile tilapia in net cages. PLoS One. 2020;15(1):e0226977. DOI:10.1371/journal.pone.0226977.
- Cao S, Zhang P, Zou T, et al. Replacement of fishmeal by spirulina Arthrospira platensis affects growth, immune related-gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018;79:265–273.
- Sheikhzadeh N, Mousavi S, Hamidian G, et al. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture. 2019;510:1–8.
- Yeganeh S, Teimouri M, Amirkolaie AK. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res Vet Sci. 2015;101:84–88.
- Sousa I, Gouveia L, Batista AP, Raymundo A, Bandarra NM, et al. Microalgae in novel food products. Food Chem Res Dev. 2008;75–112.
- Henry EC. Handbook of microalgal culture: biotechnology and applied phycology. Wiley Online Library; 2004.
- Richmond A, editors. Handbook of microalgal culture: applied phycology and biotechnology. John Wiley & Sons; 2008.
- Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65(6):635–648.
- Choopani A, Poorsoltan M, Fazilati M, Latifi AM, Salavati H, et al. Spirulina: a source of gamma-linoleic acid and its applications. J Appl Biotechnol Rep. 2016;3(4):483–488.
- Freitas HR. Chlorella vulgaris as a source of essential fatty acids and micronutrients: a brief commentary. Open Plant Sci J. 2017;10(1):92–99.
- Soletto D, Binaghi L, Lodi A, et al. Batch and fed-batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture. 2005;243(1–4):217–224. DOI:10.1016/j.aquaculture.2004.10.005.
- Bleakley S, Hayes M. Algal proteins: extraction, application, and challenges concerning production. Foods. 2017;6(5):33.
- Christaki E, Florou-Paneri P, Bonos E. Microalgae: a novel ingredient in nutrition. Int J Food Sci Nutr. 2011;62(8):794–799.
- da Silva Vaz B, Moreira JB, Morais MGD, et al. Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci. 2016;7:73–77.
- Guil-Guerrero J, Navarro-Juárez R, López-Martınez JC, et al. Functional properties of the biomass of three microalgal species. J Food Eng. 2004;65(4):511–517. DOI:10.1016/j.jfoodeng.2004.02.014.
- Kim -S-S, Rahimnejad S, Kim KW, Lee KJ, et al. Partial replacement of fish meal with Spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus). Turk J Fisher Aquat Sci. 2013;13:197–204.
- Wilson RP, Halver JE. Protein and amino acid requirements of fishes. Annu Rev Nutr. 1986;6(1):225–244.
- Sørensen M, Berge GM, Reitan KI, Ruyter B, et al. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar) —Effect on nutrient digestibility, growth and utilization of feed. Aquaculture. 2016;460:116–123.
- Annamalai SN, Das P, Thaher MIA, et al. Nutrients and energy digestibility of microalgal biomass for fish feed applications. Sustainability. 2021;13(23):13211. DOI:10.3390/su132313211.
- Tibbetts SM, Mann J, Dumas A. Apparent digestibility of nutrients, energy, essential amino acids and fatty acids of juvenile Atlantic salmon (Salmo salar L.) diets containing whole-cell or cell-ruptured Chlorella vulgaris meals at five dietary inclusion levels. Aquaculture. 2017;481:25–39.
- van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J Nutr. 2015;145(9):1981–1991.
- Barka A, Blecker C. Microalgae as a potential source of single-cell proteins. A review. Base. 2016;427–436. DOI:10.25518/1780-4507.13132
- YuCETEPE A, Saroglu O, Bildik F, et al. Optimisation of ultrasound-assisted extraction of protein from Spirulina platensis using RSM. Czech J Food Sci. 2018;36(1):98–108. DOI:10.17221/64/2017-CJFS.
- Buono S, Langellotti AL, Martello A, et al. Functional ingredients from microalgae. Food Funct. 2014;5(8):1669–1685. DOI:10.1039/C4FO00125G.
- Safi C, Charton M, Pignolet O, et al. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J Appl Phycol. 2013;25(2):523–529. DOI:10.1007/s10811-012-9886-1.
- Wu G. Functional Amino Acids in Growth, Reproduction, and Health. Adv Nutr. 2010;1(1):31–37.
- White RL, Ryan RA. Long-term cultivation of algae in open-raceway ponds: lessons from the field. Ind Biotechnol. 2015;11(4):213–220.
- Madhumathi M, Rengasamy R. Antioxidant status of Penaeus monodon fed with Dunaliella salina supplemented diet and resistance against WSSV. Int J Eng Sci Technol (IJEST). 2011;3(10):7249–7260.
- Walker AB, Berlinsky DL. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. North Am JAquacult. 2011;73(1):76–83.
- Cerezuela R, Guardiola FA, Meseguer J, et al. Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: effects on the immune system. Fish Physiol Biochem. 2012;38(6):1729–1739. DOI:10.1007/s10695-012-9670-9.
- Maliwat GC, Velasquez S, Robil JL, et al. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquacult Res. 2017;48(4):1666–1676. DOI:10.1111/are.13004.
- Hajiahmadian M, Vajargah MF, Farsani HG, Chorchi MM, et al. Effect of Spirulina platensis meal as feed additive on growth performance and survival rate in golden barb fish, punius gelius (Hamilton, 1822). J Fisher Int. 2012;7(3–6):61–64.
- Haas S, Bauer JL, Adakli A, et al. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J Appl Phycol. 2016;28(2):1011–1021. DOI:10.1007/s10811-015-0622-5.
- Kiron V, Phromkunthong W, Huntley M, et al. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquaculture Nutr. 2012;18(5):521–531. DOI:10.1111/j.1365-2095.2011.00923.x.
- Ibrahem MD, Mohamed MF, Ibrahim MA, The role of Spirulina platensis (Arthrospira platensis) in growth and immunity of Nile tilapia (Oreochromis niloticus) and its resistance to bacterial infection. 2013.
- Begum H, Yusoff FM, Banerjee S, et al. Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr. 2016;56(13):2209–2222. DOI:10.1080/10408398.2013.764841.
- Wichuk K, Brynjólfsson S, Fu W. Biotechnological production of value-added carotenoids from microalgae. Bioengineered. 2014;5(3):204–208.
- Christaki E, Bonos E, Florou-Paneri P. Innovative microalgae pigments as functional ingredients in nutrition. In: Handbook of marine microalgae. Elsevier; 2015. p. 233–243.
- Parmar RS, Singh C. A comprehensive study of eco-friendly natural pigment and its applications. Biochem Biophys Rep. 2018;13:22–26.
- Raja R, Hemaiswarya S, Kumar NA, et al. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34(2):77–88. DOI:10.1080/10408410802086783.
- Rammuni M, Ariyadasa, TU, Nimarshana, PHV, Attalage, RA, et al. Comparative assessment on the extraction of carotenoids from microalgal sources: astaxanthin from H. pluvialis and β-carotene from D. salina. Food chemistry. 2019;277:128–134.
- Ambati RR, Gogisetty, D, Aswathanarayana, RG, Ravi, S, Bikkina, PN, Bo, L, Yuepeng, S, et al. Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Critical reviews in food science and nutrition. 2019;59(12):1880–1902. DOI:10.1080/10408398.2018.1432561.
- Chen B, Wan C, Mehmood MA, et al. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products–a review. Bioresour Technol. 2017;244:1198–1206.
- Sun X, Chang Y, Ye Y, et al. The effect of dietary pigments on the coloration of Japanese ornamental carp (koi, Cyprinus carpio L.). Aquaculture. 2012;342:62–68.
- Ribeiro AR, Gonçalves A, Barbeiro M, et al. Phaeodactylum tricornutum in finishing diets for gilthead seabream: effects on skin pigmentation, sensory properties and nutritional value. J Appl Phycol. 2017;29(4):1945–1956. DOI:10.1007/s10811-017-1125-3.
- Paniagua-Michel J. Chapter 16 - microalgal nutraceuticals. In: Kim S-K, editor. Handbook of marine microalgae. Boston: Academic Press; 2015. p. 255–267.
- Jin E-S, Polle JE, Lee HK, Hyun SM, Chang M, et al. Xanthophylls in microalgae: from biosynthesis to biotechnological mass production and application. J Microbiol Biotechnol. 2003;13(2):165–174.
- Herrero M, Jaime L, Martín-Álvarez PJ, et al. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J Agric Food Chem. 2006;54(15):5597–5603. DOI:10.1021/jf060546q.
- Pauline S, Joannis-Cassan C, Duran E, et al. Commercial applications of microalgae. J Biosci Bioeng. 2006;101(2):87–96. DOI:10.1263/jbb.101.87.
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14(1):217–232.
- Hu -C-C, Lin J-T, Lu F-J, et al. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008;109(2):439–446. DOI:10.1016/j.foodchem.2007.12.043.
- Moheimani NR, Borowitzka MA. Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnol Bioeng. 2007;96(1):27–36.
- Borowitzka MA. Dunaliella: biology, production, and markets. In: Richmond A, Hu Q, editors. Handbook of microalgal culture; 2013. p. 359–368.
- Medina-Félix D, López-Elías JA, Martínez-Córdova LR, et al. Evaluation of the productive and physiological responses of Litopenaeus vannamei infected with WSSV and fed diets enriched with Dunaliella sp. J Invertebr Pathol. 2014;117:9–12.
- Ruangsomboon S, Choochote S, Taveekijakarn P. Growth performance and nutritional composition of red tilapia (Oreochromis niloticus x O. mossambicus) fed diets containing raw Spirulina platensis. in The International Conference on Sustainable Community Development. 2010. Khon Kaen University, Nongkhai Campus Nong Kom Ko, Thailand.
- Becker W. Microalgae in human and animal nutrition. In: Richmond A, editors. Handbook of microalgal culture. Blackwell, Oxford: Wiley Online Library; 2004.
- Depeint F, Bruce WR, Shangari N, et al. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163(1–2):94–112. DOI:10.1016/j.cbi.2006.04.014.
- Barbosa MJ, Zijffers JW, Nisworo A, et al. Optimization of biomass, vitamins, and carotenoid yield on light energy in a flat‐panel reactor using the A‐stat technique. Biotechnol Bioeng. 2005;89(2):233–242. DOI:10.1002/bit.20346.
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci IJBS. 2008;4(2):89.
- Hernández-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JI, Muñoz-Ochoa M, Castillo-Domínguez RM, et al. Monthly variation in the chemical composition of Eisenia arborea JE areschoug. Journal of applied phycology. 2009;21(5):607–616. DOI:10.1007/s10811-009-9454-5.
- Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211.
- Markou G, Angelidaki I, Georgakakis D. Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol. 2012;96(3):631–645.
- Raven JA, Beardall J. Carbohydrate metabolism and respiration in algae. In: Larkum AWD, Douglas SE, Raven JA, editors. Photosynthesis in Algae. Advances in Photosynthesis and Respiration, Vol. 14. Springer, Dordrecht; 2003. p. 205–224.
- Milledge JJ. Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Bio/Technol. 2011;10(1):31–41.
- Van Krimpen MM, Bikker P, Van der Meer IM, Van der Peet-Schwering CMC, Vereijken JM, et al. Cultivation, processing and nutritional aspects for pigs and poultry of European protein sources as alternatives for imported soybean products. Wageningen UR Livestock Research; 2013.
- Rismani-Yazdi H, Haznedaroglu, BZ, Bibby, K, Peccia, J, et al. Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: pathway description and gene discovery for production of next-generation biofuels. BMC Genomics. 2011;12(1):1–17. DOI:10.1186/1471-2164-12-148.
- Chacón‐Lee T, González‐Mariño G. Microalgae for “healthy” foods—possibilities and challenges. Compr Rev Food Sci Food Saf. 2010;9(6):655–675.
- Iwamoto H. Industrial production of microalgal cell-mass and secondary products - major industrial species: Chlorella. In: Richmond A, editors. Handbook of microalgal culture: biotechnology and applied phycology. 2003. p. 253–263.
- Wee KL Aquaculture nutrition research in Australia. in Proceedings of Aquaculture Nutrition Workshop, Salamander Bay. 1991.
- Niccolai A, Chini Zittelli G, Rodolfi L, et al. Microalgae of interest as food source: biochemical composition and digestibility. Algal Res. 2019;42:101617.
- Sarker PK, Kapuscinski AR, Lanois AJ, et al. Towards sustainable aquafeeds: complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PloS one. 2016;11(6):e0156684. DOI:10.1371/journal.pone.0156684.
- Skrede A, Mydland L, Ahlstrøm Ø, et al. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. J Anim Feed Sci. 2011;20(1):131–142. DOI:10.22358/jafs/66164/2011.
- Sahoo PK, Mukherjee SC. Effect of dietary β-1,3 glucan on immune responses and disease resistance of healthy and aflatoxin B1-induced immunocompromised rohu (Labeo rohita Hamilton). Fish Shellfish Immunol. 2001;11(8):683–695.
- Angelis SD, Novak AC, Sydney EB, et al. Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: process preliminary optimization and partial characterization. Appl Biochem Biotechnol. 2012;167(5):1092–1106. DOI:10.1007/s12010-012-9642-7.
- Ismail MM, Ismail GA, El-Sheekh MM. Potential assessment of some micro-and macroalgal species for bioethanol and biodiesel production. Energy Sources Part A. 2020;1–17. DOI:10.1080/15567036.2020.1758853
- Mišurcová L, Škrovánková S, Samek D, Ambrožová J, Machů L, et al. Health benefits of algal polysaccharides in human nutrition. Advances in food and nutrition research. 2012;66:75–145.
- Lopatina N, Klochkova N, Usov A. Polysaccharides of algae 69. monosaccharide composition of polysaccharides of several Pacific red algae studied by reductive hydrolysis of biomass. Russ Chem Bull. 2017;66(5):915–921.
- Wang Y, Guo W, Yen H-W, et al. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol. 2015;198:619–625.
- Scholz MJ, Weiss TL, Jinkerson RE, et al. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell. 2014;13(11):1450–1464. DOI:10.1128/EC.00183-14.
- Palinska KA, Krumbein WE. Perforation patterns in the peptidoglycan wall of filamentous cyanobacteria. J Phycol. 2000;36(1):139–145.
- Teuling E, Wierenga PA, Agboola JO, et al. Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture. 2019;499:269–282.
- Valente LMP, Custódio M, Batista S, et al. Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol Biochem. 2019;45(3):1067–1081. DOI:10.1007/s10695-019-00621-w.
- Bitou N, Ninomiya M, Tsujita T, et al. Screening of lipase inhibitors from marine algae. Lipids. 1999;34(5):441–445. DOI:10.1007/s11745-999-0383-7.
- Sinha AK, Kumar V, Makkar HPS, et al. Non-starch polysaccharides and their role in fish nutrition – a review. Food Chem. 2011;127(4):1409–1426. DOI:10.1016/j.foodchem.2011.02.042.
- Sarker PK, Kapuscinski AR, Bae AY, et al. Towards sustainable aquafeeds: evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS One. 2018;13(7):e0201315. DOI:10.1371/journal.pone.0201315.
- Karapanagiotidis IT, Bell MV, Little DC, et al. Replacement of dietary fish oils by alpha‐linolenic acid‐rich oils lowers omega 3 content in tilapia flesh. Lipids. 2007;42(6):547–559. DOI:10.1007/s11745-007-3057-1.
- Wee K Aquaculture nutrition research in Australia. in Proceedings of Aquaculture Nutrition Workshop, Salamander Bay. 1991.
- Falge R, Schpanof L, Jurss K. Amylase, esterase and protease activity in the intestine content of rainbow salmo gairdneri rich., after feeding with feed containing different amounts of starch and protein. J Ichthyol. 1978;18:283–287.
- Rodehutscord M, Borchert F, Gregus Z, et al. Availability and utilisation of free lysine in rainbow trout (Oncorhynchus mykiss): 2. Comparison of l-lysine· HCl and l-lysine sulphate. Aquaculture. 2000;187(1–2):177–183. DOI:10.1016/S0044-8486(99)00389-0.
- Encarnação P, de Lange C, Rodehutscord M, et al. Diet digestible energy content affects lysine utilization, but not dietary lysine requirements of rainbow trout (Oncorhynchus mykiss) for maximum growth. Aquaculture. 2004;235(1–4):569–586. DOI:10.1016/j.aquaculture.2004.01.001.
- Tibbetts SM, Milley JE, Lall SP. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol. 2015;27(3):1109–1119.
- Goiris K, Muylaert K, Fraeye I, et al. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol. 2012;24(6):1477–1486. DOI:10.1007/s10811-012-9804-6.
- Li H-B, Cheng K, Wong C, et al. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007;102(3):771–776. DOI:10.1016/j.foodchem.2006.06.022.
- Evans DH, Claiborne JB, Currie S, The physiology of fishes. 2013.
- Yang L, Li H, Lu Q, et al. Emerging trends of culturing microalgae for fish‐rearing environment protection. J Chem Technol Biot. 2021;96(1):31–37. DOI:10.1002/jctb.6563.
- Chen Z, Qiu S, Amadu AA, Shen Y, Wang L, Wu Z, Ge S, et al. Simultaneous improvements on nutrient and Mg recoveries of microalgal bioremediation for municipal wastewater and nickel laterite ore wastewater. Bioresource Technology. 2020;297:122517.
- Chen Z, Qiu S, Yu Z, Li M, Ge S, et al. Enhanced secretions of algal cell-adhesion molecules and metal ion-binding exoproteins promote self-flocculation of Chlorella sp. Cultivated Munic Wastewater Environ Sci Technol. 2021;55(17):11916–11924.
- Bulgariu D, Bulgariu L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour Technol. 2012;103(1):489–493.
- Leong YK, Chang J-S. Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour Technol. 2020;303:122886.
- Ahmad A, Bhat AH, Buang A. Enhanced biosorption of transition metals by living Chlorella vulgaris immobilized in Ca-alginate beads. Environ Technol. 2019;40(14):1793–1809.
- Kumar KS, Dahms H-U, Won E-J, et al. Microalgae–a promising tool for heavy metal remediation. Ecotoxicol Environ Saf. 2015;113:329–352.
- Tran HT, Vu ND, Matsukawa M, et al. Heavy metal biosorption from aqueous solutions by algae inhabiting rice paddies in Vietnam. J Environ Chem Eng. 2016;4(2):2529–2535. DOI:10.1016/j.jece.2016.04.038.
- Deng L, Su Y, Su H, et al. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater. 2007;143(1–2):220–225. DOI:10.1016/j.jhazmat.2006.09.009.
- Ahmad A, Bhat AH, Buang A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: kinetic and equilibrium modeling. J Clean Prod. 2018;171:1361–1375.
- Ashfaq A, Bhat A, Azizul B. Immobilized Chlorella vulgaris for efficient palm oil mill effluent treatment and heavy metals removal. Desalin Water Treat. 2017;81:105–117.
- Tian LX, Liu YJ, Yang HJ, et al. Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp (Ctenopharyngodon idella). Aquacult Int. 2012;20(2):283–293. DOI:10.1007/s10499-011-9456-6.
- Lin S-M, Shi C-M, Mu -M-M, et al. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2018;78:121–126.
- Polakof S, Panserat, S, Soengas, JL, Moon, TW, et al. Glucose metabolism in fish: a review. Journal of Comparative Physiology B. 2012;182(8):1015–1045.
- Ekpo I, Bender J. Digestibility of a commercial fish feed, wet algae, and dried algae by Tilapia nilotica and silver carp. The Progressive Fish-Culturist. 1989;51(2):83–86.
- Kiron V, Sørensen M, Huntley M, et al. Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front Mar Sci. 2016;3:67.
- Sørensen M, Gong Y, Bjarnason F, et al. Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PloS one. 2017;12(7):e0179907. DOI:10.1371/journal.pone.0179907.
- Ahmad MT, Shariff M, Md. Yusoff F, et al. Applications of microalga Chlorella vulgaris in aquaculture. Revi Aquacult. 2020;12(1):328–346. DOI:10.1111/raq.12320.
- Abro R, Digestion and metabolism of carbohydrates in fish. Vol. 2014. 2014.
- Krogdahl Å, Hemre GI, Mommsen T. Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquaculture Nutr. 2005;11(2):103–122.
- Córdova O, Passos F, Chamy R. Enzymatic pretreatment of microalgae: cell wall disruption, biomass solubilisation and methane yield increase. Appl Biochem Biotechnol. 2019;189(3):787–797.
- Ometto F, Quiroga G, Pšenička P, et al. Impacts of microalgae pre-treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014;65:350–361.
- Gong Y, Guterres HADS, Huntley M, et al. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquaculture Nutr. 2018;24(1):56–64. DOI:10.1111/anu.12533.
- Becker W. Microalgae for aquaculture. The nutritional value of microalgae for aquaculture. In: Richmond A, editor. Handbook of microalgal cultureBlackwell, Oxford; 2004. p. 380.
- Guedes AC, Sousa-Pinto I, Malcata FX. Application of microalgae protein to aquafeed, in handbook of marine microalgae. Elsevier, Academic Press; 2015. p. 93–125.
- Halim R, Danquah MK, Webley PA. Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv. 2012;30(3):709–732.
- Gong Y, Bandara T, Huntley M, et al. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture. 2019;501:455–464.
- Savoie A, Le François NR, Lamarre SG, et al. Dietary protein hydrolysate and trypsin inhibitor effects on digestive capacities and performances during early-stages of spotted wolffish: suggested mechanisms. Comp Biochem Physiol Part A. 2011;158(4):525–530. DOI:10.1016/j.cbpa.2010.12.017.
- de Carvalho Carneiro D, Oliveira MM, da Cunha Lima ST. Estimating protein quantities from microalgae: protein per biomass percentage, spectroscopic concentration, and lectin content. Chem Papers. 2019;73(10):2535–2540.
- Silva A, Cavalcanti VLR, Porto ALF, et al. The green microalgae Tetradesmus obliquus (Scenedesmus acutus) as lectin source in the recognition of ABO blood type: purification and characterization. J Appl Phycol. 2020;32(1):103–110. DOI:10.1007/s10811-019-01923-5.
- Wu L-C, Ho J-AA, Shieh M-C, et al. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem. 2005;53(10):4207–4212. DOI:10.1021/jf0479517.
- Li Y, Ai Q, Mai K, et al. Comparison of high-protein soybean meal and commercial soybean meal partly replacing fish meal on the activities of digestive enzymes and aminotransferases in juvenile Japanese seabass, Lateolabrax japonicus (Cuvier, 1828). Aquacult Res. 2014;45(6):1051–1060. DOI:10.1111/are.12042.
- Seo S-H, Cho S-J. Changes in allergenic and antinutritional protein profiles of soybean meal during solid-state fermentation with Bacillus subtilis. Lwt. 2016;70:208–212.
- Ströher R, Stenzel M, Pereira NC, et al. Enzymatic extraction of protein from toasted and not toasted soybean meal. Proc Food Sci. 2011;1:463–469.
- Olukomaiya OO, Adiamo OQ, Fernando WC, et al. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020;315:126238.
- Srivastava A, Kar R. Application of immobilized tannase from Aspergillus Niger for the removal of tannin from myrobalan juice. Indian J Microbiol. 2010;50(1):46–51.
- Alonso R, Orue E, Marzo F. Effects of extrusion and conventional processing methods on protein and antinutritional factor contents in pea seeds. Food Chem. 1998;63(4):505–512.
- Ayalew D, Keber T, Ayenew M. Evaluation of anti-nutritional factor reduction techniques for triticale improved utilization system in Amhara region. J Food Process Technol. 2017;8(7):681.
- Mukhopadhyay N, Sarkar S, Bandyopadhyay S. Effect of extrusion cooking on anti-nutritional factor tannin in linseed (Linum usitatissimum) meal. Int J Food Sci Nutr. 2007;58(8):588–594.
- Van Haver L, Nayar S. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017;24:167–180.
- Kim B, Im H, Lee JW. In situ transesterification of highly wet microalgae using hydrochloric acid. Bioresour Technol. 2015;185:421–425.
- Zhang J, Hu B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour Technol. 2012;114:529–535.
- Hameed A, Hussain SA, Yang J, et al. Antioxidants potential of the filamentous fungi (Mucor circinelloides). Nutrients. 2017;9(10):1101. DOI:10.3390/nu9101101.
- Hong K-J, Lee C-H, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004;7(4):430–435.
- Hu Y, Xiao Y, Liao K, et al. Development of microalgal biofilm for wastewater remediation: from mechanism to practical application. J Chem Technol Biot. 2021;96(11):2993–3008. DOI:10.1002/jctb.6850.
- Lu Q, Han P, Chen F, et al. A novel approach of using zeolite for ammonium toxicity mitigation and value-added Spirulina cultivation in wastewater. Bioresour Technol. 2019;280:127–135.
- Quinn JC, Davis R. The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour Technol. 2015;184:444–452.
- Ruiz J, Olivieri G, de Vree J, et al. Towards industrial products from microalgae. Energy Environ Sci. 2016;9(10):3036–3043. DOI:10.1039/C6EE01493C.
- Palmegiano GB, Gai F, Daprà F, et al. Effects of Spirulina and plant oil on the growth and lipid traits of white sturgeon (Acipenser transmontanus) fingerlings. Aquacult Res. 2008;39(6):587–595. DOI:10.1111/j.1365-2109.2008.01914.x.
- Pizzera A, Scaglione D, Bellucci M, et al. Digestate treatment with algae-bacteria consortia: a field pilot-scale experimentation in a sub-optimal climate area. Bioresour Technol. 2019;274:232–243.
- Bongiorno T, Foglio L, Proietti L, et al. Microalgae from biorefinery as potential protein source for siberian Sturgeon (A. baerii) aquafeed. Sustainability. 2020;12(21):8779. DOI:10.3390/su12218779.
- Ahmad A, Buang A, Bhat AH. Renewable and sustainable bioenergy production from microalgal co-cultivation with palm oil mill effluent (POME): a review. Renew Sust Energ Rev. 2016;65:214–234.
- da Silva TL, Reis A Scale-up Problems for the Large Scale Production of Algae. In: Das D, editor. Algal Biorefinery: An Integrated Approach. Springer, Cham; 2015. p. 125–149.
- Smetana S, Sandmann M, Rohn S, et al. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: life cycle assessment. Bioresour Technol. 2017;245:162–170.
- Becker EW. Micro-algae as a source of protein. Biotechnol Adv. 2007;25(2):207–210.
- Chen S, Chi Z, O’Fallon JV, et al. System integration for producing microalgae as biofuel feedstock. Biofuels. 2010;1(6):889–910. DOI:10.4155/bfs.10.52.
- Hussein EES, Dabrowski K, El-Saidy DMSD, et al. Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquacult Res. 2013;44(6):937–949. DOI:10.1111/j.1365-2109.2012.03100.x.
- Ahmad A, Shah SMU, Othman MF, et al. Aerobic and anaerobic co-cultivation of Nannochloropsis oculata with oil palm empty fruit bunch for enhanced biomethane production and palm oil mill effluent treatment. Desalin Water Treat. 2015;56(8):2055–2065. DOI:10.1080/19443994.2014.960458.
- Li J, Liu Y, Cheng JJ, et al. Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. N Biotechnol. 2015;32(6):588–596. DOI:10.1016/j.nbt.2015.02.001.
- Ahmad A, Shah, SMU, Othman, MF, Abdullah, MA, et al. Biomethane production and palm oil mill effluent treatment by co-cultivation of Nannochloropsis oculata. In: Applied mechanics and materials. Trans Tech Publications Ltd; 2014. p. 818–821.
- Hannon M, Gimpel J, Tran M, et al. Biofuels from algae: challenges and potential. Biofuels. 2010;1(5):763–784. DOI:10.4155/bfs.10.44.
- Nagappan S, Devendran S, Tsai P-C, et al. Metabolomics integrated with transcriptomics and proteomics: evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020;91:1–14.
- Berge G, Hatlen B, Odom JM, et al. Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquaculture Nutr. 2013;19:110–121.
- Sørensen I, Rose JKC, Doyle JJ, et al. The Charophycean green algae as model systems to study plant cell walls and other evolutionary adaptations that gave rise to land plants. Plant Signal Behav. 2012;7(1):1–3. DOI:10.4161/psb.7.1.18574.