ABSTRACT
The plasticity of metallic glasses depends largely on the atomic-scale structure. However, the details of the atomic-scale structure, which are responsible for their properties, remain to be clarified. In this study, in-situ high-energy synchrotron X-ray diffraction and strain-rate jump compression tests at different cryogenic temperatures were carried out. We show that the activation volume of flow units linearly depends on temperature in the non-serrated flow regime. A plausible atomic deformation mechanism is proposed, considering that the activated flow units mediating the plastic flow originate from the medium-range order and transit to the short-range order with decreasing temperature.
GRAPHICAL ABSTRACT
IMPACT STATEMENT
An atomic deformation mechanism in metallic glasses is proposed. Activated flow units mediating the plastic flow originate from the medium-range order and transit to the short-range order with decreasing temperature.
1. Introduction
Understanding the atomic structure in metallic glasses and its correlation with the mechanical deformation behavior is of crucial fundamental interest and a quite challenging topic [Citation1,Citation2]. It is generally accepted that the deformation of metallic glasses essentially involves all atoms that are spatially uniformly distributed, or originates from local atomic rearrangements associated with structural and chemical heterogeneity [Citation1–5]. Several models and concepts have been developed, and are now widely used to describe the deformation of the atomic structure, and cluster motifs in the glass in terms of free volume [Citation6], shear transformation zones (STZ) [Citation7,Citation8], flow units [Citation9,Citation10], geometrically unfavored motifs [Citation11], and the core–shell model [Citation12]. These cluster motifs are considered as the strain carriers in the glassy phase. However, the underlying mechanisms leading to plastic yielding have not been fully understood [Citation5,Citation13–16], mainly due to the absence of direct experimental characterization of the atomic structural evolution of metallic glasses. Especially, there is no consensus regarding the origin of the atomic structure that is responsible for the plastic deformation of metallic glasses. Although the short-range order (SRO) is believed to be closely related with the plasticity that may be dominated by distinct structural units in the glass [Citation17,Citation18], the characteristic-length scale for the formation of STZs on the medium-range order (MRO) length scale implies that the MRO mainly contributes to the plastic deformation of metallic glasses [Citation19–21]. Therefore, it is necessary to further characterize the SRO and MRO changes under different loading stresses.
The atomic structural response of metallic glasses can be influenced by external fields, including stress and temperature fields [Citation2,Citation14]. Although the influence of temperature on the structural evolution has been paid more attention in recent years [Citation22–25], these studies mostly focused on the effect of high temperature on the structural evolution in metallic glasses. In contrast, far less is known about the structural evolution at cryogenic temperatures. Decreasing the temperature to the cryogenic level usually improves the yield strength and enhances plastic deformability [Citation26–30], thus suggesting that metallic glasses are potentially useful materials for extremely low-temperature applications. Therefore, clarification of the structural evolution on the atomic scale at low temperatures to better understand the mechanical behavior is of crucial fundamental and practical importance.
For the present work, we choose a Zr64.13Cu15.75Ni10.12Al10 (at.%) metallic glass as a model material due to its excellent plasticity [Citation31]. Strain-rate jump compression tests at cryogenic temperatures were carried out to extract information on the flow behavior of the metallic glass. In-situ high-energy synchrotron X-ray diffraction measurements at cryogenic temperature were used to analyze the atomic structural evolutions of the SRO and MRO, which could shed light on the structural origin of the flow units in metallic glasses.
2. Experimental procedure
The alloy ingots were prepared by arc melting a mixture of pure metals (with a purity higher than 99.99%) at least four times in a titanium-gettered argon atmosphere, followed by suction casting into copper molds to form rod-shaped samples with a size of Φ 2 × 70 mm. Specimens with a length/diameter ratio of 2 were machined from the cast rods, and then carefully ground to ensure that the two ends are parallel. Strain-rate jump compression tests were carried out at strain rates from 1.24 × 10−5 to 8.40 × 10−4 s−1 at temperatures of 77, 108, 123, and 153 K. Before the compression tests, all specimens were cooled to the preset temperatures, and then held for one hour to stabilize the temperature throughout the samples and the contraction consistency of the load cell, by controlling the circulation rate of liquid nitrogen. Each temperature test was repeated at least five times to ensure the reliability of the experimental results.
In-situ X-ray diffraction experiments were conducted at the PO2.1 beamline of the PETRA III electron storage ring (DESY Hamburg, Germany). Samples with a geometry of Φ 1 × 2 mm were cut from the metallic glass rods by a diamond saw. Then, the samples were placed inside a quartz capillary (diameter: 1.2 mm; wall thickness: 20 µm). The in-situ experiments were done under a protective argon atmosphere. The diffracted photons were collected using a 2D detector (Perkin Elmer) mounted orthogonal to the X-ray beam. An X-ray beam with a wavelength (λ) of 0.020727 nm was used to register intensity up to large values (∼200 nm−1) of the scattering vector, q (q = 4πsinθ/λ). The beam size was 0.6 × 0.6 mm2. The diffraction pattern from LaB6 was used to calibrate the sample-to-detector distance and tilting of the image plate detector with respect to the beam axis. The samples were cooled from 300 K down to approximately 79 K, followed by heating from 79 up to 300 K. The heating/cooling rates were 20 K/min.
3. Results
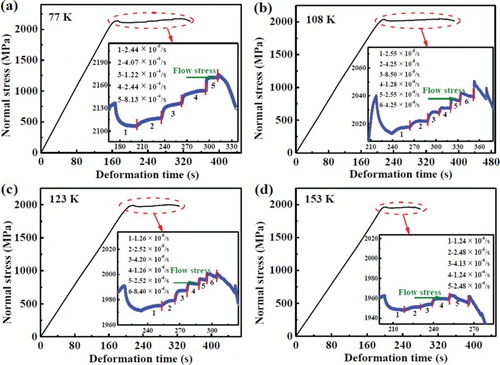
Compressive nominal stress–time curves of the metallic glass obtained upon strain-rate jump compression testing at temperatures of 77, 108, 123, and 153 K are displayed in . The curves demonstrate that both yield strength and deformation time (i.e. the plasticity) increase with decreasing test temperature [Citation26–30]. The plastic-deformation regimes of four stress–time curves were enlarged in the inset of (a)–(d). The flow stress at different strain rates was defined as the steady-state stress [Citation32], which is marked in the inset of (a)–(d). The flow stresses at different strain rates and four temperatures are listed in (a)–(d). It is noted that the flow stress exhibits an obvious increase with increasing strain rate, which is consistent with previous reports [Citation26,Citation29,Citation32]. According to the strain-rate jump compression tests, the deformation of the metallic glass at low temperature seems like a homogeneous flow behavior. However, it is spatially inhomogeneous, as found from scanning electron microscopic images clearly revealing the presence of shear bands on the surface of the tested specimens [Citation26,Citation28,Citation30].
Figure 1. Stress–time curves of the Zr64.13Cu15.75Ni10.12Al10 metallic glass measured at different temperatures by alternating the strain rates. The insets show the enlarged plastic region for the corresponding test temperature. (a) 77 K. (b) 108 K. (c) 123 K. (d) 153 K.
Usually, a low strain-rate sensitivity coupled with the lack of an intrinsic strain-hardening mechanism causes a fluid to diverge from Newtonian flow behavior, consequently leading to a tendency for developing an instability, that is, shear thinning behavior [Citation2,Citation16]. In the present study, according to the macroscopic compressive stress–strain curves at cryogenic temperatures, we can assume that the metallic glass essentially deforms exclusively through shear localization [Citation30]. However, the inhomogeneous deformation of the metallic glass is not easily analytically tractable although it has important influences on the strength and plasticity [Citation2]. Therefore, it is necessary to investigate the primary flow units mediated plastic flow of the metallic glass. In crystalline materials, the thermally triggered activation volume associated with the movement of dislocations or grain boundaries can be estimated [Citation33,Citation34]. Similarly, the flow units carrying the plastic deformation in metallic glasses are also supposed to be thermally activated. The plastic deformation is accommodated in localized shear bands. The plastic strain rate within the shear bands can be expressed as [Citation33–35]
(1)
where kB is the Boltzmann constant, T is the temperature,
is a temperature-independent plastic-deformation rate, ΔG is the Gibbs free energy of activation for the stress-assisted, thermally activated process, ΔG0 and ΔV* are the stress-independent activation energy and the activation volume, respectively. Since plastic deformation in metallic glass is dominated by shear stress, τ, a relation τ = σ/α is commonly used with the factor of 2 [Citation36]. Utilizing strain-rate jump compression tests, the activation volume, ΔV*, of stress-assisted thermally activated plastic flow can be obtained from the following equation [Citation7,Citation29,Citation33]:
(2)
where
and
are the two strain rates used in the strain-rate jump tests, respectively, and Δσflow (=σflow2 − σflow1) is the difference of flow stresses at the strain rates of
and
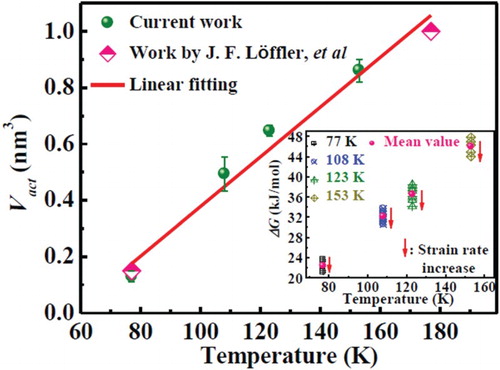
. According to Equation (2), the activation volume at different test temperatures can be evaluated as shown in , which increases linearly with increasing temperature, and can be expressed as: ΔV* = 0.00881T − 0.503. Although it is impossible to obtain an accurate activation volume from strain-rate jump tests at room temperature due to the presence of serrations [Citation29], the linear dependence of the activation volume on the temperatures well below 193 K (non-serrated flow regime) could well predict the changes in the activation volume. Based on Equation (2), the critical temperature (Tc = 57 K) can be obtained if assumed ΔV* = 0. This means that the above linear relationship is invalidate for temperature lower than 57 K due to the failure mode transition at lower temperatures [Citation37]. In the present study, the obtained activation volume of the metallic glass at 77 K (ΔV* ≈ 0.13 nm3) is very close to the values reported for other metallic glasses near or above the glass transition temperature [Citation29,Citation38], which clearly indicates that the defect concentration in the metallic glass deformed at 77 K is comparable with that in the supercooled liquid region [Citation29].
Figure 2. Activation volumes as a function of temperature, calculated from the strain-rate jump compression tests according to Equation (2). Inset shows the activation energy for the test temperatures.
In the plastic-deformation region, a β relaxation occurs to deliver the plastic strain [Citation9,Citation10]. Based on the theory of potential energy landscape, the β relaxation is treated as an atomic rearrangement process in which the atoms can jump the saddle points [Citation39]. With an increase in the shear stress, some atoms trapped in the basins can be thermally activated to traverse the saddle points, during which the shear stress could change the shape of the potential energy landscape, that is, change some stable configurations to be unstable by reducing the potential barrier to a neighboring configuration [Citation5]. Thus, a large shear stress and/or high strain rate can effectively lower the activation energy of the atoms jumping the basins. For the stress-assisted thermally activated plastic flow, the activation energy, ΔG, can be obtained by [Citation2]
(3)
where
is the compressive strain rate and
is the reference strain rate that is taken as half of
because of a geometrical consideration [Citation2]. Here,
, α0 (of order unity) is a parameter incorporating the volume fraction that is ready to deform in the glassy phase, ν0 is the attempt frequency, and γ0 ∼ 0.1 is the unit strain at which flow units transform on average [Citation2]. Accordingly, the activation energy at different strain rates and temperatures can be calculated in the inset of . It is clear that the mean value of ΔG increases from 22.35 to 45.99 kJ/mol with increasing temperature from 77 to 153 K. At one temperature, the value of ΔG decreases approximately 10% with increasing strain rate from 10−5 to 10−4 s−1.
As shown in , the activation volume of the flow units, Vact, varies from 1.2 to 0.13 nm3 as the temperature decreases from 193 to 77 K. This activation volume is nearly close to the volume of the atoms participating in the shear transformation. The average atomic radius, R, statistically estimated by , where Ai and ri are the atomic fraction and the atomic radius of each element, respectively. According to the dense-packing hard-sphere model of metallic glasses, the number of the atoms, N, involved in the flow unit can be readily obtained by
, and the calculation results are listed in .
Table 1. Activation volume size at different temperatures, the corresponding diameter, and the equivalent number of atoms.
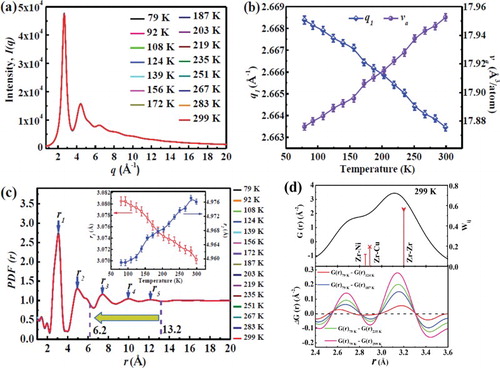
Previous studies [Citation26–30] and the above-described compression tests have revealed that the strength and the plasticity of metallic glasses are significantly enhanced with decreasing temperature, which is believed to be related to the atomic structural differences at different temperatures. The high-energy X-ray in synchrotron investigations provides diffraction patterns of the metallic glass at different temperatures. The integrated diffraction curves, I(q), at different temperatures are plotted ((a)). The first maximum in I(q) reflects the information of the MRO structure. The positions of the first maximum in I(q) calculated by Gaussian fitting as a function of temperature is plotted in (b). According to q1·va0.433 = 9.3 [Citation40], the atomic volume as a function of temperature is calculated to be shown in (b). It is evident that the atomic volume of the metallic glass decreases from approximately 17.95 to 17.88 Å3/atom with decreasing temperature, indicative of contraction of the MRO. The pair correlation functions, PDF(r), at different temperatures are shown in (c). Through Gaussian fitting of the first and second maxima of PDF(r), it can be revealed that the position of the first maximum shifts from 3.070 to 3.080 Å when the temperature is decreased from 299 to 79 K, while the position of the second maximum decreases from 4.976 to 4.960 Å [the inset in (c)]. Hence, it is obvious that the first atomic shell abnormally expands when decreasing the temperature to the cryogenic level, whereas shrinkage occurs on the other atomic shells [Citation41]. (d) shows the first maximum in reduced PDF, G(r), at 299 K, suggesting the atomic nearest-neighborhoods on a length scale of ∼2.4–3.6 Å. Each partial PDF comprises its X-ray scattering weight wij [Citation42]. Based on the type and concentration of the constituent elements in the Zr64.13Cu15.75Ni10.12Al10 metallic glass, it is assumed that Zr–Zr, Zr–Cu, and Zr–Ni are the dominant atomic pairs constituting the first maximum of G(r). The differences between G(r) at T (=299, 235, 187, and 124 K) and 79 K, that is, ΔG(r) = G(r)79K − G(r)T are also shown in (d), indicating a highly spatially localized process of atomic rearrangement around the large solvent Zr atoms due to the decrease in temperature. In other words, the decrease in temperature causes the formation of more Zr–Zr pairs and fewer Zr–Cu or Zr–Ni pairs in the first nearest-neighbor shell. This change in the chemical SRO may result in the abnormal expansion of the first atomic shell [Citation3].
Figure 3. High-energy X-ray diffraction results of the Zr64.13Cu15.75Ni10.12Al10 metallic glass. (a) Integrated diffraction curves, I(q), at different temperatures. (b) Position of the first maximum in I(q) and averaged atomic volume as a function of temperature, indicating shrinkage of MRO with decreasing temperature. (c) Pair correlation function, PDF(r), at different temperatures. The corresponding atomic shells with the diameter of the activation volume are labeled. The inset shows the positions of the first and the second maxima as functions of temperature. (d) The first G(r) maximum and the X-ray scattering weights wij (Top panel). ΔG(r) changes in the first maximum from ∼2.4 to 3.6 Å (Bottom panel).
4. Discussion
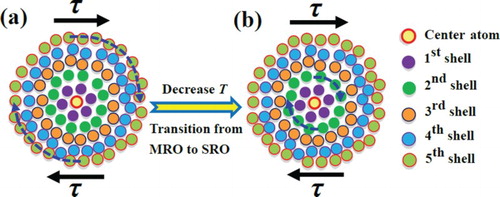
Recent experimental and simulation evidence has shown that the mechanical properties of metallic glasses are determined by liquid-like regions or flow units originating from the intrinsically heterogeneous structure [Citation9–12,Citation27,Citation43,Citation44]. The size of the flow units is believed to be ranged from tens of atoms to several hundred atoms, implying that the flow units may nucleate in both SRO and MRO [Citation21]. Based on the pair correlation function, previous studies have measured the strain distribution in different atomic shells of metallic glasses at room temperature [Citation19,Citation21], which revealed that in the r values from 13 to 14 Å, corresponding to the fifth or sixth atomic shell, the strain is larger than those in other values of r. In this case, it was believed that the atoms in the length scale of r = 13–14 Å have the largest contribution to the shear transformation [Citation21]. In the present study, the equivalent diameters of the activation volumes are calculated in , which clearly shows that the activation volume size of 13.2 Å at 193 K can be compared to the size of the fifth atomic shell ((c)). Therefore, our results are in good agreement with the reported characteristic MRO length scales. On the other hand, it has been demonstrated that the atomic pairs in the SRO have the least contribution to the flow units [Citation21]. When the temperature decreases to 77 K, the activation volume will annihilate due to the gradual cooling contraction. However, the abnormal expansion in the first atomic shell (SRO) will leave some vacancy-like or loosely packed regions, which can be potential sites for the formation of flow units when compared with the case at high temperatures. In this case, the observation that the numbers of flow units increase with decreasing temperature indicates that the density of the potential sites for the nucleation of shear banding is improved. Thus, more shear bands are activated at lower temperatures, which causes that the serrated flow behavior becomes insignificantly with decreasing temperature [Citation28].
depicts a schematic illustration of the microscopic deformation mechanism associated with the evolution of flow units. Due to the intrinsic structural heterogeneity, there are no equivalent atoms in metallic glass. Experimental observations show that the local shear events are located in ∼1 nm soft spots, which are related to the MRO [Citation6,Citation12,Citation13,Citation19–21]. When stress is applied to the sample, the isolated flow units hidden in the MRO will be activated in spite of the occurrence of structure contraction at 193 K ((a)). However, the flow units are enveloped by elastic surroundings. The operation of such flow units is so localized that the flow unit can be treated as a soft region surrounded by hard atomic clusters behaving elastically [Citation12]. The operation of these isolated flow units must lead to a local increase in the inelastic strain and energy. In this case, the atoms surrounding the flow units must shift concordantly with the activated flow units to counterbalance the energy changes [Citation40,Citation44–47]. With increasing stress, more potential sites are activated, which then causes the flow units to be aggregated. This process must involve the surrounding atoms to participate in the shear deformation, which then leads the flow units to be proliferated [Citation44,Citation45]. When the volume fraction of flow units reaches a critical value upon increasing the stress to the yield stress, the flow units will percolate through the surrounding elastic shells, that is, cage-breaking takes place, and coalesce to form larger flow units [Citation44,Citation48,Citation49]. These activated flow units and elastic shells interact with each other cooperatively, resulting in localized plastic flow events [Citation44]. Thus, the pairing of flow units and elastic shells constitutes the elementary cluster in the accommodation of plastic flow in metallic glasses [Citation12]. With a decrease in the temperature, the cooling shrinkage results in the annihilation of the flow units in the MRO, and makes packing of the clusters in the MRO to be more denser ((b)). However, the abnormal expansion of the SRO can provide potential sites for the formation of flow units (). The dissipation of strain energy is possibly accommodated by the flow units in the SRO due to the reduction in the numbers of the flow units in the MRO. Furthermore, the decrease in the temperature causes that the activation volume decreases (). Considering the coordination number of the first atomic shell (SRO) to be approximately 12 (which is already very close to the atoms in the activation volume at 77 K), we can rationalize that the size of the activation volume can be compared to the size of the first atomic shell ((b)). When the temperature is increased to 193 K, the number of the atoms in the activation volume is very close to the numbers covered in the range from the first atomic shell to the fifth atomic shell (MRO) ((a)). In this case, we can rationally assume the activation volume to be a spherical shape. With decreasing the size of the activation volume to be compared to the size of SRO, since the atomic shell in SRO is more stiffer than those in MRO [Citation21], the activation of the flow units at low temperature, such as 77 K, becomes more difficult. Hence, it is easy to understand that the yield strength increases notably as temperature decreases (). Moreover, increasing the strain rate requires more flow units to be activated to compensate the strain evolution, which directly results in a higher flow stress (insets in ).
5. Conclusions
In summary, the flow behavior of Zr64.13Cu15.75Ni10.12Al10 at different cryogenic temperatures has been investigated through strain-rate jump compression tests. Based on the atomic structural evolutions observed by in-situ high-energy synchrotron X-ray radiation, we successfully elucidated the atomic structural origin of flow units, and the mechanism of yielding and plastic deformation. It is found that the structural transformation of the metallic glass undergoes a transition from MRO-dominated to SRO-dominated flow units with decreasing temperature. Aspects of these results are key to the understanding of the deformation mechanisms in metallic glasses. They may be adapted to structurally disordered systems in general, and thus contribute to the tailoring of their mechanical properties.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen MW. Mechanical behavior of metallic glasses: microscopic understanding of strength and ductility. Annu Rev Mater Res. 2008;38:445–469. doi: 10.1146/annurev.matsci.38.060407.130226
- Schuh CA, Hufnagel TC, Ramamurty U. Mechanical behavior of amorphous alloys. Acta Mater. 2007;55:4067–4109. doi: 10.1016/j.actamat.2007.01.052
- Liu YH, Fujita T, Aji DPB, et al. Structural origins of Johari-Goldstein relaxation in a metallic glass. Nat Commun. 2014;5:3238.
- Chen HS, Coleman E. Structure relaxation spectrum of metallic glasses. Appl Phys Lett. 1976;28:245–247. doi: 10.1063/1.88725
- Hufnagel TC, Schuh CA, Falk ML. Deformation of metallic glasses: recent developments in theory, simulations, and experiments. Acta Mater. 2016;109:375–393. doi: 10.1016/j.actamat.2016.01.049
- Spaepen F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses. Acta Metall. 1977;25:407–415. doi: 10.1016/0001-6160(77)90232-2
- Argon AS. Plastic deformation in metallic glasses. Acta Metall. 1979:27:47–58. doi: 10.1016/0001-6160(79)90055-5
- Miracle DB. A structural model for metallic glasses. Nat Mater. 2004;3:697–702. doi: 10.1038/nmat1219
- Wang Z, Sun BA, Bai HY, et al. Evolution of hidden localized flow during glass-to-liquid transition in metallic glass. Nat Commun. 2014;5:5823. doi: 10.1038/ncomms6823
- Lu Z, Jiao W, Wang WH, et al. Flow unit perspective on room temperature homogeneous plastic deformation in metallic glasses. Phys Rev Lett. 2014;113:045501. doi: 10.1103/PhysRevLett.113.045501
- Ding J, Patinet S, Falk ML, et al. Soft spots and their structural signature in a metallic glass. Proc Nat Acad Sci USA. 2014;111:14052–14056. doi: 10.1073/pnas.1412095111
- Ye JC, Lu J, Liu CT, et al. Atomistic free-volume zones and inelastic deformation of metallic glasses. Nat Mater. 2010;9:619–623. doi: 10.1038/nmat2802
- Johnson WL, Samwer K. A universal criterion for plastic yielding of metallic glasses with a (T/Tg)2/3 temperature dependence. Phys Rev Lett. 2005;95:195501. doi: 10.1103/PhysRevLett.95.195501
- Cao XF, Gao M, Zhao LZ, et al. Microstructural heterogeneity perspective on the yield strength of metallic glasses. J Appl Phys. 2016;119:084906. doi: 10.1063/1.4942625
- Mondal K, Ohkubo T, Toyama T, et al. The effect of nanocrystallization and free volume on the room temperature plasticity of Zr-based bulk metallic glasses. Acta Mater. 2008;56:5329–5339. doi: 10.1016/j.actamat.2008.07.012
- Liu ZY, Chen MW, Liu CT, et al. Origin of yielding in metallic glass: stress-induced flow. Appl Phys Lett. 2014;104:251901. doi: 10.1063/1.4884066
- Zhang Y, Greer AL. Thickness of shear bands in metallic glasses. Appl Phys Lett. 2006;89:1907.
- Jiang MQ, Dai LH. Short-range-order effects on intrinsic plasticity of metallic glasses. Phil Mag Lett. 2010;90:269–277. doi: 10.1080/09500831003630781
- Hufnagel TC, Ott RT, Almer J. Structural aspects of elastic deformation of a metallic glass. Phys Rev B. 2006;73:064204. doi: 10.1103/PhysRevB.73.064204
- Schuh CA, Lund AC, Nieh TG. New regime of homogeneous flow in the deformation map of metallic glasses: elevated temperature nanoindentation experiments and mechanistic modeling. Acta Mater. 2004;52:5879–5891. doi: 10.1016/j.actamat.2004.09.005
- Shahabi HS, Scudino S, Kaban I, et al. Structural aspects of elasto-plastic deformation of a Zr-based bulk metallic glass under uniaxial compression. Acta Mater. 2015;95:30–36. doi: 10.1016/j.actamat.2015.05.011
- Jakse N, Hennet L, Price DL, et al. Structural changes on supercooling liquid silicon. Appl Phys Lett. 2003;83:4734–4736. doi: 10.1063/1.1631388
- Kelton KF, Lee GW, Gangopadhyay AK, et al. First X-ray scattering studies on electrostatically levitated metallic liquids: demonstrated influence of local icosahedral order on the nucleation barrier. Phys Rev Lett. 2003;90:195504. doi: 10.1103/PhysRevLett.90.195504
- Lou HB, Wang XD, Cao QP, et al. Negative expansions of interatomic distances in metallic melts. Proc Nat Acad Sci USA. 2013;110:10068–10072. doi: 10.1073/pnas.1307967110
- Mattern N, Stoica M, Vaughan G, et al. Thermal behaviour of Pd40Cu30Ni10P20 bulk metallic glass. Acta Mater. 2012;60:517–524. doi: 10.1016/j.actamat.2011.10.032
- Li HQ, Fan C, Tao KX, et al. Compressive behavior of a Zr-based metallic glass at cryogenic temperatures. Adv Mater. 2006;18:752–754. doi: 10.1002/adma.200501990
- Ketov SV, Sun YH, Nachum S, et al. Rejuvenation of metallic glasses by non-affine thermal strain. Nature 2015;524:200–203. doi: 10.1038/nature14674
- Liu ZY, Wang G, Chan KC, et al. Temperature dependent dynamics transition of intermittent plastic flow in a metallic glass. I. Experimental investigations. J Appl Phys. 2013;114:033520. doi: 10.1063/1.4815943
- Dubach A, Dalla Torre FH, Löffler JF. Constitutive model for inhomogeneous flow in bulk metallic glasses. Acta Mater. 2009;57:881–892. doi: 10.1016/j.actamat.2008.10.027
- Maaß R, Klaumünzer D, Preiß EI, et al. Single shear-band plasticity in a bulk metallic glass at cryogenic temperatures. Scr Mater. 2012;66:231–234. doi: 10.1016/j.scriptamat.2011.10.044
- Liu YH, Wang G, Wang RJ, et al. Super plastic bulk metallic glasses at room temperature. Science 2007;315:1385–1388. doi: 10.1126/science.1136726
- Kato H, Kawamura Y, Inoue A, et al. Newtonian to non-Newtonian master flow curves of a bulk glass alloy Pd40Ni10Cu30P20. Appl Phys Lett. 1998;73:3665. doi: 10.1063/1.122856
- Caillard D, Martin J-L. Thermally activated mechanisms in crystal plasticity. London: Elsevier; 2003.
- Wang YM, Hamza AV, Ma E. Temperature-dependent strain rate sensitivity and activation volume of nanocrystalline Ni. Acta Mater. 2006;54:2715–2726. doi: 10.1016/j.actamat.2006.02.013
- Yang XS, Wang YJ, Wang GY, et al. Time, stress, and temperature-dependent deformation in nanostructured copper: stress relaxation tests and simulations. Acta Mater. 2016;108:252–263. doi: 10.1016/j.actamat.2016.02.021
- Zhang ZF, Eckert J, Schultz L. Difference in compressive and tensile fracture mechanisms of Zr59Cu20Al10Ni8Ti3 bulk metallic glass. Acta Mater. 2003;51:1167–1179. doi: 10.1016/S1359-6454(02)00521-9
- Jiang MQ, Wilde G, Chen JH, et al. Cryogenic-temperature-induced transition from shear to dilatational failure in metallic glasses. Acta Mater. 2014;77:248–257. doi: 10.1016/j.actamat.2014.05.052
- Bletry M, Guyot P, Brechet Y, et al. Homogeneous deformation of bulk metallic glasses in the super-cooled liquid state. Mater Sci Eng A. 2004;387–389:1005–1011. doi: 10.1016/j.msea.2004.02.085
- Debenedetti PG, Stillinger FH. Supercooled liquids and the glass transition. Nature. 2001;410:259–267. doi: 10.1038/35065704
- Ma D, Stoica AD, Wang XL. Power-law scaling and fractal nature of medium-range order in metallic glasses. Nat Mater. 2009;8:30–34. doi: 10.1038/nmat2340
- Tan J, Wang G, Liu ZY, et al. Correlation between atomic structure evolution and strength in a bulk metallic glass at cryogenic temperature. Sci Rep. 2013;4:3897.
- Stoica M, Das J, Bednarcik J, et al. Strain distribution in Zr64.13Cu15.75Ni10.12Al10 bulk metallic glass investigated by in situ tensile tests under synchrotron radiation. J Appl Phys. 2008;104:013522. doi: 10.1063/1.2952034
- Bian XL, Wang G, Chen HC, et al. Manipulation of free volumes in a metallic glass through Xe-ion irradiation. Acta Mater. 2016;106:66–77. doi: 10.1016/j.actamat.2016.01.002
- Zhao LZ, Xue RJ, Li YZ, et al. Revealing localized plastic flow in apparent elastic region before yielding in metallic glasses. J Appl Phys. 2015;118:244901. doi: 10.1063/1.4938567
- Wang G, Mattern N, Bednarčík J. Correlation between elastic structural behavior and yield strength of metallic glasses. Acta Mater. 2012;60:3074–3083. doi: 10.1016/j.actamat.2012.02.012
- Bian XL, Wang G, Chan KC, et al. Shear avalanches in metallic glasses under nanoindentation: deformation units and rate dependent strain burst cut-off. Appl Phys Lett. 2013;103:101907.
- Huang R, Suo Z, Prevost JH, et al. Inhomogeneous deformation in metallic glasses. J Mech Phys Solids. 2002;50:1011–1027. doi: 10.1016/S0022-5096(01)00115-6
- Sun BA, Liu ZY, Yang Y, et al. Delayed shear banding and evolution of local plastic flow in a metallic glass. Appl Phys Lett. 2014;105:091904. doi: 10.1063/1.4894860
- Wang Q, Zhang ST, Yang Y, et al. Unusual fast secondary relaxation in metallic glass. Nat Commun. 2015;6:7876. doi: 10.1038/ncomms8876