ABSTRACT
Multiferroic phase-transforming alloys demonstrate intriguing multicaloric effects, but they are intrinsically brittle and their elastocaloric effect shows poor cyclability, which remains a major challenge for exploiting more efficient multicaloric refrigeration. Here, by employing a novel strategy of strengthening grain boundary, the cyclability of elastocaloric effect in the prototype Ni-Mn-based multiferroic phase-transforming alloys is strikingly enhanced by two orders of magnitude. Ultrahigh cyclability of a large elastocaloric effect is achieved. This not only paves the way for exploiting multicaloric effects for more efficient cooling, but also provides a strategy for overcoming the cyclability issues in the ubiquitous brittle intermetallic phase-transforming materials.
GRAPHICAL ABSTRACT

IMPACT STATEMENT
The cyclability of elastocaloric effect in the prototype Ni-Mn-based multiferroic phase-transforming alloys has been strikingly enhanced by two orders of magnitude through employing the strategy of strengthening grain boundary.
1. Introduction
Refrigeration is becoming increasingly important to industry, transportation and households and it accounts for a considerable fraction of the global energy consumption. Nowadays the most commonly used cooling technology is based on vapor compression, which produces ozone-depleting or greenhouse gases that are detrimental to the global environment [Citation1]. It is imperative to develop alternative cooling technologies that are environment-friendly and highly efficient to address the environmental concerns [Citation2]. Elastocaloric refrigeration, which is a novel solid-state cooling technology, is considered to be the most promising alternative to the traditional vapour-compression-based technology [Citation1]. Such refrigeration relies on the elastocaloric effect of the refrigerant material, which refers to the temperature change of the material under adiabatic conditions or the entropy change under isothermal conditions upon the application of a uniaxial stress [Citation3]. Large elastocaloric effects usually occur in ferroelastic phase-transforming materials, such as Ti-Ni-based [Citation4], Cu-based [Citation5] and Ni-Mn-based shape memory alloys [Citation6,Citation7]. For elastocaloric refrigeration, the cyclability of elastocaloric effect, which determines the service life of refrigerants, is of utmost importance [Citation1,Citation8]. Multiferroic phase-transforming alloys, which combine ferroelastic order and ferromagnetic order, exhibit intriguing multicaloric (elastocaloric, magnetocaloric and barocaloric) effects [Citation1]. This offers the unique opportunity for multicaloric refrigeration which yields much stronger cooling effect than single-caloric refrigeration [Citation9]. However, these materials are highly brittle and exhibit poor cyclability of elastocaloric effect, which remains a major challenge for refrigeration applications. In this work we demonstrate that, with a novel yet simple strategy of strengthening grain boundary, we have strikingly enhanced the cyclability of elastocaloric effect in the prototype Ni-Mn-based multiferroic phase-transforming alloys, by two orders of magnitude. This represents a significant advance in developing high-performance fatigue-tolerant elastocaloric materials.
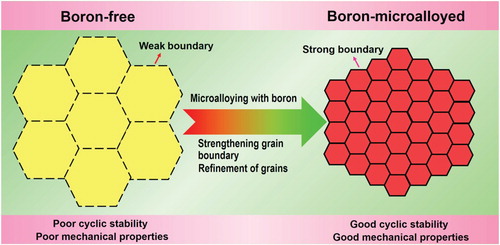
The basic concept of our strategy is illustrated in Figure . The great brittleness of the multiferroic phase-transforming alloys, which are ordered intermetallics in nature, is directly related to the grain boundary weakness. The elastocaloric effect in these alloys originates from the stress-induced reversible transformation between austenite and martensite that releases or absorbs heat upon forward or reverse transformation [Citation3]. During transformation, the stress-induced martensitic variants that are formed asynchronously and oriented differently in neighboring grains result in stress concentrations and crack initiation at grain boundaries [Citation10], leading to poor cyclic stability of elastocaloric effect. Therefore, we expect that strengthening grain boundary and refining grains (to increase yield strength) should be effective in enhancing mechanical properties and cyclability of elastocaloric effect. Furthermore, the enhanced mechanical properties enable the alloys to withstand a stress that is high enough to induce the complete transformation, which facilitates the achievement of a large elastocaloric effect. With inspirations from the work on structural intermetallics such as Ni3Al [Citation11], Ni3Fe [Citation12], and FeAl [Citation13] alloys, we envisage that microalloying with boron could effectively strengthen grain boundary and suppress intergranular fracture in the present functional intermetallic alloys. Meanwhile, similar to the case for TiAl structural intermetallics [Citation14], microalloying with boron could also lead to refinement of grains. Hence, in this work we microalloyed the Ni-Mn-based multiferroic phase-transforming alloys with boron to simultaneously strengthen grain boundary and refine grains. With this strategy, we have achieved ultrahigh cyclability of a very large elastocaloric effect in a boron-microalloyed Ni-Mn-In-based alloy. A high adiabatic temperature change (ΔTad) of ∼5.6 K remains stable for 2700 cycles of loading and unloading. Our findings may serve as the foundation for exploiting multicaloric effects in multiferroic phase-transforming alloys for environment-friendly and high-efficiency solid-state cooling applications.
2. Materials and methods
Polycrystalline button ingots were prepared by arc-melting high-purity constituent elements. Rod ingots of Φ3 mm were prepared by suction casting using parts of the arc-melted ingots. These rod ingots were subsequently sealed into evacuated quartz tubes and annealed at 1173 K for 2 h followed by water quenching.
The phase transformation temperatures were determined by differential scanning calorimetry (DSC) measurements. Microstructure examination was conducted by using scanning electron microscopy (SEM) operated at 15 kV. Chemical distribution at the atomic scale was investigated by 3D atom probe tomography (APT) on a LEAP 5000 XR. The APT tip specimen was prepared using a focused ion beam.
The mechanical properties and elastocaloric effect were studied using a mechanical testing machine (Instron-5966) under a compression mode. Samples of Φ3 × 6 mm3, cut from the rod ingots, were used. The strain was recorded with a dynamic strain gauge extensometer. The adiabatic temperature change during loading and unloading was measured by a K-type thermocouple, which was first soldered on the sample surface and then wrapped together with sample using an aluminium wire (diameter: 0.1 mm). The temperature data were recorded using an OM-DAQ-USB-2401 data acquisition module.
3. Results and discussion
For solid-state refrigeration, the phase transformation temperatures of the refrigerant materials should be around room temperature. Since microalloying the Ni-Mn-In alloys with B decreases the martensitic transformation temperature [Citation6], we substituted Fe for In and Ni simultaneously to slightly increase the electron concentration e/a and transformation temperature. For this kind of alloys, the phase transformation temperature generally increases with e/a [Citation15], and therefore the Fe substitution compensates the effect of B microalloying on transformation temperature. In this way, we designed and prepared a series of Ni-Mn-In-based bulk polycrystalline alloys whose detailed information is shown in Table . Calorimetric investigation shows that, with B microalloying and Fe substitution we have obtained a (Ni51Mn33In14Fe2)99.4B0.6 alloy with an austenitic transformation finish temperature (Af) slightly below room temperature (Figure S1). Stress–strain tests demonstrate that the mechanical properties of the alloys are indeed remarkably enhanced by B microalloying; both the yield strength and fracture strength increase with B microalloying (Figure S2). The (Ni51Mn33In14Fe2)99.4B0.6 alloy exhibits very good mechanical properties.
Table 1. Composition, electronic concentration e/a and phase transformation temperatures of the Ni-Mn-In-based alloys.
The cyclic stability of elastocaloric effect was investigated by monitoring the sample temperature variation during cyclic loading and unloading. The results for the Ni51.5Mn33In15.5, Ni51Mn33In14Fe2 and (Ni51Mn33In14Fe2)99.4B0.6 alloys are shown as a function of time in Figure . The compressive stress with a maximum of 350 MPa, was applied at a temperature slightly higher than Af for each alloy, at which reversible stress-induced transformation occurs. Since the samples were exposed to air during testing, a relatively high strain rate of 2.8 × 10−2 s−1 was employed for both loading and unloading to approximate adiabaticity. For each cycle, the sample was firstly loaded to the target stress, followed by holding for 10 s, and then unloaded followed by further holding for 10 s. As can be seen (refer to the inset of Figure (b) for more details), in each cycle the sample temperature rapidly increases upon loading, tends to recover to the testing temperature during holding and rapidly decreases upon unloading. The adiabatic temperature change ΔTad during loading and unloading, which is an important parameter that characterizes the elastocaloric effect, was determined from the temperature variation, as demonstrated in Figure (b).
Figure 2. (a–c) Temperature variation during cyclic loading, holding, and unloading, shown as a function of time for (a) Ni51.5Mn33In15.5, (b) Ni51Mn33In14Fe2 and (c) (Ni51Mn33In14Fe2)99.4B0.6, respectively. The inset of (b) shows the temperature variation during one of the cycles for Ni51Mn33In14Fe2. The maximum applied stress is 350 MPa and the strain rate is 2.8 × 10−2 s−1. For the results shown in (c), the test was interrupted after each 300 cycles to save the data while the sample temperature was still being monitored during that time period. The testing temperature for (c) is 304 K. The determination of adiabatic temperature change ΔTad is illustrated in (b). (d) Temperature variation recorded during the 300th, 900th, 1500th, 2100th and 2700th cycles of loading, holding, and unloading for (Ni51Mn33In14Fe2)99.4B0.6. (e) Adiabatic stress-strain curve recorded during the 300th cycle of loading, holding, and unloading (with a strain rate of 2.8 × 10−2 s−1) [the corresponding temperature variation is shown in (d)] and isothermal stress-strain curves with different maximum applied stresses (the strain rate is 2.8 × 10−4 s−1), for (Ni51Mn33In14Fe2)99.4B0.6.
![Figure 2. (a–c) Temperature variation during cyclic loading, holding, and unloading, shown as a function of time for (a) Ni51.5Mn33In15.5, (b) Ni51Mn33In14Fe2 and (c) (Ni51Mn33In14Fe2)99.4B0.6, respectively. The inset of (b) shows the temperature variation during one of the cycles for Ni51Mn33In14Fe2. The maximum applied stress is 350 MPa and the strain rate is 2.8 × 10−2 s−1. For the results shown in (c), the test was interrupted after each 300 cycles to save the data while the sample temperature was still being monitored during that time period. The testing temperature for (c) is 304 K. The determination of adiabatic temperature change ΔTad is illustrated in (b). (d) Temperature variation recorded during the 300th, 900th, 1500th, 2100th and 2700th cycles of loading, holding, and unloading for (Ni51Mn33In14Fe2)99.4B0.6. (e) Adiabatic stress-strain curve recorded during the 300th cycle of loading, holding, and unloading (with a strain rate of 2.8 × 10−2 s−1) [the corresponding temperature variation is shown in (d)] and isothermal stress-strain curves with different maximum applied stresses (the strain rate is 2.8 × 10−4 s−1), for (Ni51Mn33In14Fe2)99.4B0.6.](/cms/asset/5e7c5d74-541d-4b6b-9fdf-5ba6a7c38248/tmrl_a_1566182_f0002_oc.jpg)
As shown in Figure (a), the boron-free Ni51.5Mn33In15.5 alloy shows poor cyclability of elastocaloric effect; the sample fractured after only 27 loading-unloading cycles, owing to the great brittleness of the polycrystalline intermetallic multiferroic phase-transforming alloys. The Fe substitution leads to some improvement of the cyclability of elastocaloric effect. The elastocaloric effect of the Ni51Mn33In14Fe2 alloy remained almost stable for 109 loading-unloading cycles and then the sample fractured, as shown in Figure (b). In contrast, the cyclic stability of elastocaloric effect is strikingly enhanced by boron microalloying. The boron-microalloyed (Ni51Mn33In14Fe2)99.4B0.6 alloy exhibits ultrahigh cyclability of elastocaloric effect. As demonstrated in Figure (c,d), the elastocaloric effect of (Ni51Mn33In14Fe2)99.4B0.6 showed almost no degradation during the 2700 loading-unloading cycles we performed, and the structural integrity of the sample was well maintained after the test. This represents the highest cyclability of elastocaloric effect reported heretofore in multiferroic phase-transforming alloys. Actually, such cyclability, with at least 2700 cycles, far exceeds that reported in other multiferroic phase-transforming alloys [Citation6,Citation16]. Furthermore, the elastocaloric effect of the boron-microalloyed (Ni51Mn33In14Fe2)99.4B0.6 alloy is very large, with a high ΔTad of ∼5.6 K during loading (Figure (c,d)). It should be mentioned that the ΔTad we achieved during loading is higher than that during unloading (Figure (d)); this is mainly because that, with the program control the sample could only be rapidly unloaded to ∼50 MPa using the relatively high strain rate of 2.8 × 10−2 s−1 (see Figure (e)), and heat exchange between the sample and environment happened during further slow manual unloading to 0 MPa. In principle, the ΔTad values during loading and unloading are roughly the same if the adiabaticity is satisfied. As indicated from the isothermal stress-strain curves in Figure (e), under the isothermal condition applying a stress of 350 MPa (with a strain rate of 2.8 × 10−4 s−1) can only induce a part of the transformation. Considering the fact that the stress required for inducing martensitic transformation under the adiabatic condition (the strain rate is 2.8 × 10−2 s−1) is higher than that under the isothermal condition (Figure (e)), the application of 350 MPa in the cyclic elastocaloric effect measurement (Figure (c)) could only induce partial transformation. The ultrahigh cyclability of the large elastocaloric effect confers the boron-microalloyed Ni-Mn-In alloys great potential for elastocaloric and multicaloric refrigeration.
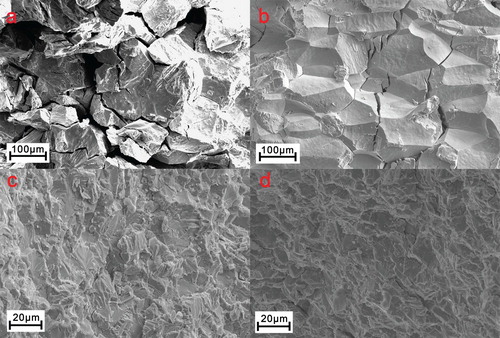
As demonstrated above, the cyclability of elastocaloric effect of the Ni-Mn-In alloys is strikingly enhanced by two orders of magnitude by boron microalloying. This is attributed to the strengthening of grain boundary and grain refinement as a result of boron microalloying. The increase in fracture strength in the boron-microalloyed sample (Figure S2) is one indication for the strengthening of grain boundary resulting from boron microalloying. To provide further evidence, we examined the fracture modes (see Figure ). The boron-free Ni51.5Mn33In15.5 and Ni51Mn33In14Fe2 alloys both exhibit intergranular fracture (Figure (a,b)), owing to the grain boundary weakness. In contrast, the fracture mode in the boron-microalloyed (Ni51.5Mn33In15.5)99.4B0.6 and (Ni51Mn33In14Fe2)99.4B0.6 alloys is predominantly transgranular (Figure (c,d)). This provides unambiguous evidence that the grain boundary in the boron-microalloyed samples is indeed strengthened.
Figure 3. Scanning electron fractographs for the (a) Ni51.5Mn33In15.5, (b) Ni51Mn33In14Fe2, (c) (Ni51.5Mn33In15.5)99.4B0.6 and (d) (Ni51Mn33In14Fe2)99.4B0.6 alloys, respectively.
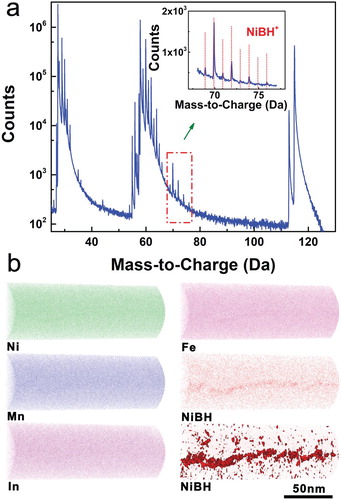
To elucidate how the boron microalloying strengthens grain boundary, we studied the chemical distribution at the atomic scale by 3D atom probe tomography (APT). A (Ni51Mn33In14Fe2)99.4B0.6 tip specimen was taken from a region around the grain boundary, as illustrated in Figure S3d. The 3D APT results are shown in Figure . The peak assignment of the mass spectrum confirms that the complex ion NiBH+ forms (Figure (a)). It is clear that, while the single atoms of Ni, Mn, In and Fe show homogenous distribution, the complex ion NiBH+ exhibits strong segregation to grain boundary (Figure (b)). Due to the segregation and clustering of NiBH+ at grain boundary, the hydrogen is bonded to B and Ni and trapped into the NiBH clusters. This trapping effectively reduces the hydrogen diffusion along grain boundary and thus suppresses the hydrogen embrittlement which is a well-known reason for intergranular fracture in ordered intermetallics [Citation17]. In this way, the grain boundary strength is enhanced due to boron microalloying. The strengthening of grain boundary suppresses the fatigue crack formation and propagation along gain boundaries during stress-induced transformation, and thus enhances the cyclability of elastocaloric effect.
Figure 4. (a) Mass spectrum for the (Ni51Mn33In14Fe2)99.4B0.6 tip specimen taken from a region around the grain boundary (as illustrated in Figure S3d). The inset shows the assignment of the peaks enclosed in the rectangular frame in the main panel; the red vertical lines denote the peak positions of NiBH+. (b) 3D APT reconstructions of atomic distributions of Ni, Mn, In and Fe and distribution of NiBH+ around the grain boundary, together with the iso-concentration surface for NiBH+ at 0.8% (lower right panel).
In order to gain information on grain size of the alloys, we performed microstructure examination (see Figure S3). Evidently, boron microalloying is indeed very effective in grain refinement. While the boron-free Ni51.5Mn33In15.5 alloy exhibits a microstructure with coarse grains of ∼100 µm (Figure S3a), the boron-microalloyed (Ni51Mn33In14Fe2)99.4B0.6 alloy shows significantly refined grains of ∼15 µm (Figure S3d). The grain refinement increases the yield strength and thus impedes local plastic deformation during stress-induced transformation [Citation18], which may become the crack initiation sites during cycling. Moreover, since the grains are refined, the misorientation between neighboring grains becomes smaller, and this leads to more synchronous stress-induced transformation in neighboring grains and thus diminishes stress concentrations at grain boundaries. These factors contribute to the enhanced cyclability of elastocaloric effect and are also beneficial for attaining a larger elastocaloric effect in the boron-microalloyed samples. It should be noted that boron microalloying results in the formation of precipitates, especially at grain boundaries (Figure S3c and S3d). Our energy dispersive spectroscopy (EDS) analysis shows that the precipitates are rich in Ni and lean in In (Table S1). High-resolution transmission electron microscopy (TEM) experiment reveals that the interface between the precipitate and the matrix is incoherent with a large lattice misfit (Figure S4). Hence, the precipitates have little contribution to the strengthening of the alloys. After all, it is the strengthening of grain boundary and grain refinement that contribute to the good mechanical properties and high cyclability of elastocaloric effect in the boron-microalloyed samples.
To demonstrate that our present strategy (Figure ) can readily be applied to other multiferroic phase-transforming alloys, we also microalloyed the Ni-Mn-Sn alloys with boron to simultaneously strengthen grain boundary and refine grains. A high-performance (Ni51.5Mn35Sn12.5Fe1)99.4B0.6 alloy with transformation temperatures around room temperature was achieved. Figure S5 shows the temperature variation during cyclic loading and unloading for this alloy. Clearly, this alloy also exhibits ultrahigh cyclability of elastocaloric effect. The elastocaloric effect remained almost stable for the 3000 loading-unloading cycles we performed. The sample remained intact after the test. This clearly evidences the applicability of our strategy to other multiferroic phase-transforming alloy systems. It should be noted that, in addition to the bulk polycrystalline samples, the present strategy is expected to yield significant enhancement of the cyclability of elastocaloric effect in thin films that have fine and uniform grains. Since our strategy is based on grain boundary engineering, it is not expected to be applicable to single crystals that are free of grain boundaries.
For a caloric material, the cyclic stability which is directly related to operation life is of crucial importance for refrigeration applications. Meanwhile, the field-induced adiabatic temperature change ΔTad is an important parameter quantifying the caloric effect. Figure demonstrates the comparison of the cyclability of elastocaloric effect and ΔTad of the alloys designed here with other elastocaloric materials in the polycrystalline form [Citation4–6,Citation16,Citation19–23]. As compared to other materials except Ti-Ni-based alloys, the present (Ni51Mn33In14Fe2)99.4B0.6 and (Ni51.5Mn35Sn12.5Fe1)99.4B0.6 alloys exhibit the highest cyclability of elastocaloric effect reported hitherto. It should be noted that the elastocaloric effect of Ti-Ni-based alloys degrades considerably during cycling [Citation4,Citation21] while that of the present (Ni51Mn33In14Fe2)99.4B0.6 and (Ni51.5Mn35Sn12.5Fe1)99.4B0.6 alloys remains very stable for more than 2700 cycles. More importantly, the Ti-Ni-based ferroelastic phase-transforming alloys display only elastocaloric effect, while the present multiferroic phase-transforming alloys show attractive multicaloric effects [Citation1]. Besides stress-induced transformation, the present alloys also exhibit magnetic-field-induced transformation (as shown in Figure S6). This opens opportunities for exploiting multicaloric effects for more efficient refrigeration. Synergic harnessing of the multicaloric effects under optimized coupling of stress, magnetic field and temperature could yield a significantly larger caloric effect in these alloys as compared to the materials showing only a single-caloric effect.
Figure 5. Comparison of the cyclability of elastocaloric effect and adiabatic temperature change ΔTad of the alloys designed in this work with other elastocaloric materials in the polycrystalline form. The ‘Cycles’ denote the number of cycles of elastocaloric effect reported in literature; since most of the studies did not mention whether failure occurred after such cycles, this number does not necessarily corresponds to fatigue life. Data in this figure were taken from the present study and literature: Ti-Ni film [Citation4], Ti-Ni-Cu film [Citation4], Ti-Ni-V [Citation21], Ti-Ni [Citation23], Ni-Mn-In [Citation6], Cu-Zn-Al [Citation5], Ni-Co-Mn-In [Citation16,Citation19], Ni-Mn-Ga [Citation20], Ni-Fe-Ga [Citation22].
![Figure 5. Comparison of the cyclability of elastocaloric effect and adiabatic temperature change ΔTad of the alloys designed in this work with other elastocaloric materials in the polycrystalline form. The ‘Cycles’ denote the number of cycles of elastocaloric effect reported in literature; since most of the studies did not mention whether failure occurred after such cycles, this number does not necessarily corresponds to fatigue life. Data in this figure were taken from the present study and literature: Ti-Ni film [Citation4], Ti-Ni-Cu film [Citation4], Ti-Ni-V [Citation21], Ti-Ni [Citation23], Ni-Mn-In [Citation6], Cu-Zn-Al [Citation5], Ni-Co-Mn-In [Citation16,Citation19], Ni-Mn-Ga [Citation20], Ni-Fe-Ga [Citation22].](/cms/asset/9e303e96-a786-44ea-9de0-ea39f55b79fc/tmrl_a_1566182_f0005_oc.jpg)
4. Conclusions
In conclusion, by employing the strategy of simultaneously strengthening grain boundary and refining grains, we have strikingly enhanced the cyclability of elastocaloric effect by two orders of magnitude in the prototype Ni-Mn-based multiferroic phase-transforming alloys. Ultrahigh cyclability of a very large elastocaloric effect has been achieved in the (Ni51Mn33In14Fe2)99.4B0.6 bulk polycrystalline alloy: the high adiabatic temperature change ΔTad of ∼5.6 K remains stable for 2700 cycles of loading and unloading. This (Ni51Mn33In14Fe2)99.4B0.6 alloy contains neither expensive nor toxic elements and is easy to fabricate. It shows great potential for environment-friendly solid-state refrigeration. Our achievement of the ultrahigh cyclability of a large elastocaloric effect in multiferroic phase-transforming alloys paves the way for exploiting multicaloric effects for more efficient cooling applications. Furthermore, the present strategy is not believed to be limited to the multiferroic phase-transforming alloys, but could readily be applied to the ubiquitous brittle intermetallic phase-transforming materials to enhance their cyclability for advanced sensing, actuating and energy harvesting applications.
Supplemental Material
Download PDF (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mañosa L, Planes A. Materials with giant mechanocaloric effects: cooling by strength. Adv Mater. 2017;29(11):1603607. doi: 10.1002/adma.201603607
- Lloveras P, Stern-Taulats E, Barrio M, et al. Giant barocaloric effects at low pressure in ferrielectric ammonium sulphate. Nat Commun. 2015;6:8801. doi: 10.1038/ncomms9801
- Bonnot E, Romero R, Mañosa L, et al. Elastocaloric effect associated with the martensitic transition in shape-memory alloys. Phys Rev Lett. 2008;100(12):3436–3440. doi: 10.1103/PhysRevLett.100.125901
- Bechtold C, Chluba C, de Miranda RL, et al. High cyclic stability of the elastocaloric effect in sputtered TiNiCu shape memory films. Appl Phys Lett. 2012;101(9):091903. doi: 10.1063/1.4748307
- Mañosa L, Jarque-Farnos S, Vives E, et al. Large temperature span and giant refrigerant capacity in elastocaloric Cu-Zn-Al shape memory alloys. Appl Phys Lett. 2013;103(21):211904. doi: 10.1063/1.4832339
- Yang Z, Cong DY, Sun XM, et al. Enhanced cyclability of elastocaloric effect in boron-microalloyed Ni-Mn-In magnetic shape memory alloys. Acta Mater. 2017;127:33–42. doi: 10.1016/j.actamat.2017.01.025
- Yang Z, Cong DY, Huang L, et al. Large elastocaloric effect in a Ni–Co–Mn–Sn magnetic shape memory alloy. Mater Des. 2016;92:932–936. doi: 10.1016/j.matdes.2015.12.118
- Tušek J, Engelbrecht K, Eriksen D, et al. A regenerative elastocaloric heat pump. Nat Energy. 2016;1:16134. doi: 10.1038/nenergy.2016.134
- Vopson MM. The multicaloric effect in multiferroic materials. Solid State Commun. 2012;152(23):2067–2070. doi: 10.1016/j.ssc.2012.08.016
- Wilkes KE, Liaw PK. The fatigue behavior of shape-memory alloys. JOM. 2000;52(10):45–51. doi: 10.1007/s11837-000-0083-3
- Liu CT, Stiegler JO. Ductile ordered intermetallic alloys. Science. 1984;226:636–642. doi: 10.1126/science.226.4675.636
- Liu Y, Liu CT, Heatherly L, et al. Effect of boron on the fracture behavior and grain boundary chemistry of Ni3Fe. Scr Mater. 2011;64(3):303–306. doi: 10.1016/j.scriptamat.2010.08.027
- Liu CT, George EP. Environmental embrittlement in boron-free and boron-doped FeAl (40 at.% Al) alloys. Scr Mater. 1990;24(7):1285–1290. doi: 10.1016/0956-716X(90)90343-F
- Larsen DE, Christodoulou L, Kampe SL, et al. Investment-cast processing of XD TM near-γ titanium aluminides. Mater Sci Eng A. 1991;144(1-2):45–49. doi: 10.1016/0921-5093(91)90208-5
- Moya X, Mañosa L, Planes A, et al. Martensitic transition and magnetic properties in Ni–Mn–X alloys. Mater Sci Eng A. 2006;438-440:911–915. doi: 10.1016/j.msea.2006.02.053
- Lu B, Liu J. Elastocaloric effect and superelastic stability in Ni–Mn–In–Co polycrystalline Heusler alloys: hysteresis and strain-rate effects. Sci Rep. 2017;7:2084. doi: 10.1038/s41598-017-02300-3
- Cohron JW, George EP, Heatherly L, et al. Hydrogen-boron interaction and its effect on the ductility and fracture of Ni3Al. Acta Mater. 1997;45(7):2801–2811. doi: 10.1016/S1359-6454(96)00383-7
- Norfleet DM, Sarosi PM, Manchiraju S, et al. Transformation-induced plasticity during pseudoelastic deformation in Ni–Ti microcrystals. Acta Mater. 2009;57 (12):3549–3561. doi: 10.1016/j.actamat.2009.04.009
- Lu B, Zhang P, Xu Y, et al. Elastocaloric effect in Ni45Mn36.4In13.6Co5 metamagnetic shape memory alloys under mechanical cycling. Mater Lett. 2015;148:110–113. doi: 10.1016/j.matlet.2015.02.076
- Hu Y, Li Z, Yang B, et al. Combined caloric effects in a multiferroic Ni–Mn–Ga alloy with broad refrigeration temperature region. APL Mater. 2017;5(4):046103. doi: 10.1063/1.4980161
- Kim Y, Jo M-G, Park J-W, et al. Elastocaloric effect in polycrystalline Ni50Ti45.3V4.7 shape memory alloy. Scr Mater. 2018;144:48–51. doi: 10.1016/j.scriptamat.2017.09.048
- Xu Y, Lu B, Sun W, et al. Large and reversible elastocaloric effect in dual-phase Ni54Fe19Ga27 superelastic alloys. Appl Phys Lett. 2015;106(20):201903. doi: 10.1063/1.4921531
- Engelbrecht K, Tušek J, Sanna S, et al. Effects of surface finish and mechanical training on Ni-Ti sheets for elastocaloric cooling. APL Mater. 2016;4(6):064110. doi: 10.1063/1.4955131