Abstract
Objectives: The edaravone development program established a study design in which a treatment effect slowing functional loss in amyotrophic lateral sclerosis (ALS) could be documented within a 24-week time frame. This report elucidates the strategic enrichment design utilized to create efficiency and precision in the development program. Methods: Post-hoc analyses describe learning, sequential iteration, and evolution in study design. Results: The first Phase 3 study of edaravone in ALS (Study MCI186-16) included a large proportion (35%) of placebo patients who were minimal progressors. These patients demonstrated high heterogeneity in change in ALSFRS-R score (−4 median with interquartile range [IQR] 7.5) and a modal distribution score of 0, suggesting evidence of minimal change in ALSFRS-R during the study. This level of variability and rate of progression may have made it difficult to detect a prospective treatment effect in the study. A strategic enrichment strategy provided the second Phase 3 study (Study MCI186-19) with the ability to detect a treatment effect. In Study MCI186-19, only 13% of the placebo patients were minimal progressors. Further, these placebo patients demonstrated less heterogeneity and greater functional progression of ALS, thereby providing greater likelihood of detecting a treatment effect. The enrichment strategy may have excluded some rapidly progressing patients, potentially supporting the detection of a treatment effect. As previously published, Study MCI186-19 prospectively documented a 33% reduction in rate of progression of ALS (p = 0.0013). Conclusions: Strategic choices in the design of Study MCI186-19 reduced the proportion of minimally progressing patients and supported detection of a treatment effect.
Keywords:
Introduction
Conducting randomized clinical trials in amyotrophic lateral sclerosis (ALS) has been particularly difficult. Numerous clinical trials have failed to identify a drug that could affect disease progression (Citation1–3). Diseases that show a high degree of heterogeneity in disease progression, such as ALS, can benefit from clinical study designs that enrich for patient populations that are more likely to show a response on the clinical study end points. Such enrichment strategies are recognized by the Food and Drug Administration (FDA) to be potentially valuable in designing efficient clinical trials (Citation4). The clinical development program for edaravone, approved by the FDA in 2017 for the treatment of ALS on the basis of evidence that it slows the decline in physical function (Citation5), has provided important insights relating to effective strategies for improving the sensitivity and efficiency of clinical trials in ALS (Citation5,Citation6).
The clinical development program for edaravone in ALS focused on testing the drug’s efficacy in a relatively short time frame (i.e. 24 weeks). An overview of this program is shown in (Citation7–13). The Phase 2 open-label investigation, Study MCI186-12, suggested that the drug delayed progression of functional decline as assessed by the Revised ALS Functional Rating Scale (ALSFRS-R) (Citation7). The results from Study MCI186-12 were used to guide development of Study MCI186-16, a randomized, double-blind, placebo-controlled Phase 3 study in ALS patients (Citation8).
Table 1 Overview of edaravone clinical development program.
Study MCI186-16 incorporated strategies to enhance detection of a treatment effect with edaravone in a 24-week period. This included a 12-week observation period designed to identify patients who would be expected to show disease progression during the double-blind period of the study. Patients were also required to have a diagnosis of “definite,” “probable,” or “probable laboratory-supported” ALS, as well as a forced vital capacity (FVC) of at least 70%, signs of the first symptoms of ALS within the 3 years prior to the study, and a Japanese ALS Severity Grade (Citation14) of 1 or 2. These inclusion criteria were intended to help enrich the study population with patients who were most likely to experience a significant, ALSFRS-R–measurable decline in function during 24 weeks, and to therefore document any change in disease progression attributable to edaravone. Although the results for the primary end point numerically favored edaravone, the effect was not statistically significant (Citation8).
Post-hoc analysis of Study MCI186-16 identified two subgroups demonstrating significant treatment effects (Citation13). The characteristics of these subgroups were defined in two steps.
Step 1: Identification of the “efficacy-expected subpopulation” (EESP), characterized by
○ A score of ≥2 points for all items in the ALSFRS-R at baseline.
○ FVC ≥80% at baseline.
Step 2: Identification of the “greater-efficacy-expected subpopulation” within the post-hoc EESP (designated as dpEESP2y), characterized by
○ A diagnosis of “definite” or “probable” ALS according to the El Escorial revised Airlie House diagnostic criteria.
○ Being within 2 years of initial ALS symptom onset at the time of giving informed consent.
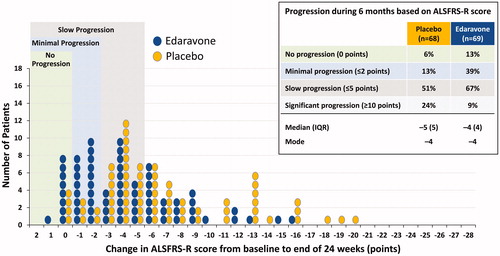
Step 1 and Step 2 subgroup patients experienced significantly less decline in ALSFRS-R with edaravone vs placebo () (Citation13). Therefore, Step 2 post-hoc criteria were utilized as the inclusion criteria for the subsequent Phase 3 trial, Study MCI186-19 () (Citation11). Study MCI186-19 demonstrated a significant effect of edaravone, with least-squares mean ± standard error (LS mean ± SE) values for the change from baseline in ALSFRS-R during 24 weeks of −7.50 ± 0.66 for placebo vs −5.01 ± 0.64 for edaravone, a difference between groups of 2.49 ± 0.76 (p = 0.0013) (Citation11).
Figure 1 Changes from baseline in ALSFRS-R score during treatment in the Study MCI186-16 FAS, Step 1 (EESP), and Step 2 (dpEESP2y) groups (LOCF for patients who completed at least the third cycle). Least-squares mean ± standard error of the inter-group differences in the ALSFRS-R score during treatment was 0.65 ± 0.78 (p = 0.4108) in the FAS group, 2.20 ± 1.03 (p = 0.0360) in the Step 1 group, and 3.01 ± 1.33 (p = 0.0270) in the Step 2 group.
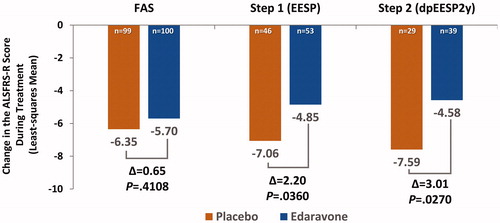
Table 2 Inclusion criteria for groups included in the post-hoc subgroup analysis of study MCI186-16 and study MCI186-19 (Citation9,Citation12,Citation13).
This study describes post-hoc analyses of the Study MCI186-16 and Study MCI186-19 populations to elucidate more fully the enrichment strategy used in the edaravone clinical development program and to highlight the key learnings.
Methods
The designs and outcomes for Study MCI186-16 and Study MCI186-19 have been previously published (Citation8, Citation11). Post-hoc analyses of these studies were performed to analyze the distributions of the changes from baseline ALSFRS-R scores at week 24 for the full analysis set (FAS) for each study and for the Step 1 and Step 2 subgroups of Study MCI186-16. Based on an analysis of the PRO-ACT database, which indicated that, on average, patients experience a change in ALSFRS-R score of −1.02 points per month (Citation15), the following classifications were defined: no progression: 0-point decline during 6 months; minimal progression: ≤2-point decline during 6 months; slow progression: ≤5-point decline during 6 months; and significant progression: ≥10-point decline during 6 months.
Results
Baseline characteristics
shows the baseline characteristics for the study populations. Overall, baseline characteristics were well balanced between treatment arms within each study and subgroup.
Table 3 Baseline characteristics of the post-hoc analysis subgroups.
Study MCI186-16 ALSFRS-R distribution analysis
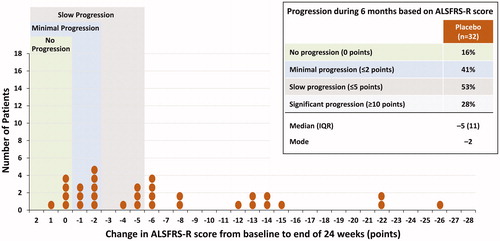
Over the 24-week placebo-controlled period in Study MCI186-16, the FAS had nearly equal distributions of patients across the two study arms who had no progression, minimal progression, slow progression, and significant progression (). At the end of 24 weeks, the FAS placebo group had a median change in ALSFRS-R score of −4 (interquartile range [IQR], 7.5) and a modal change of 0, with 17% of patients experiencing no progression and 35% experiencing minimal progression (). In the placebo minimal progression group, the mean change in ALSFRS-R score was only −0.13 points per month. Similarly, in the placebo arm of the Step 1 subgroup, the proportion of patients with slow progression was comparable to that of the placebo patients in the FAS (; compare with ). In contrast, the proportion of slow progressors in the placebo arm of the Step 2 subgroup was 53% (), vs 63% and 62% in the FAS and Step 1 groups, respectively.
Figure 2 Distribution of changes in ALSFRS-R at 24 weeks in the Study MCI186-16 FAS population (LOCF). Each circle represents one patient in the study. The mode for the distribution of each arm is shown in the figure. The table lists the percentage of patients in each arm who experienced no progression, minimal progression, slow progression, or significant progression, as described in the text. Descriptive statistics (median, interquartile range [IQR], and mode) are also listed in the table for each arm.
![Figure 2 Distribution of changes in ALSFRS-R at 24 weeks in the Study MCI186-16 FAS population (LOCF). Each circle represents one patient in the study. The mode for the distribution of each arm is shown in the figure. The table lists the percentage of patients in each arm who experienced no progression, minimal progression, slow progression, or significant progression, as described in the text. Descriptive statistics (median, interquartile range [IQR], and mode) are also listed in the table for each arm.](/cms/asset/40a59e73-b4da-4189-87fe-08d31819fb71/iafd_a_1599955_f0002_c.jpg)
Figure 3 Distribution of changes in ALSFRS-R at 24 weeks in the Study MCI186-16 placebo patients, Step 1 subgroup (LOCF).
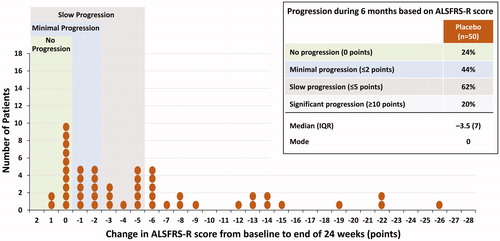
Figure 4 Distribution of changes in ALSFRS-R at 24 weeks in the Study MCI186-16 placebo patients, Step 2 subgroup (LOCF).
displays the distribution of changes in ALSFRS-R score by inclusion criteria. Study MCI186-16 FAS placebo patients are color-coded for the individual criteria applied to Step 1 and Step 2 post-hoc subgroup analyses. shows the inclusion criteria and treatment effect for each group. The circles in each represent one patient. Within each circle, wedge symbols represent the inclusion criteria of Study MCI186-19 that would have excluded that patient from enrollment in Study MCI186-19.
Figure 5 Distribution of changes in ALSFRS-R at 24 weeks in the Study MCI186-16 FAS population, with symbols indicating the Step 2 criteria that applied to each patient. Brown circles represent patients who met all Step 2 criteria. White circles with colored wedges represent patients who did not meet one or more Step 2 criteria, as indicated in the figure legend.
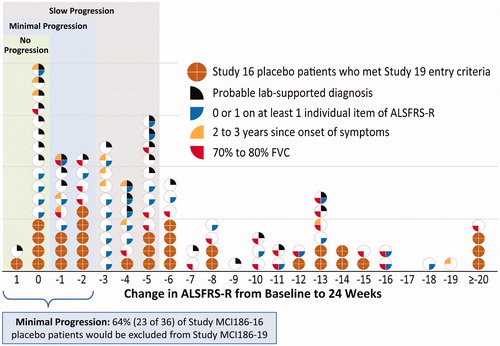
Table 4 Changes in ALSFRS-R score during treatment in the post-hoc analysis subgroups.Table Footnotea
With the application of the Study MCI186-19 inclusion criteria to the Study MCI186-16 FAS patients, 74% (48/65) of placebo slow progressors would not have been eligible for Study MCI186-19. Similarly, 64% (23/36) of placebo minimal progressors would not have been eligible for Study MCI186-19. This enrichment was mainly due to 3 of the inclusion criteria: score of ≥2 points for all items in the ALSFRS-R at baseline, diagnosis of “definite” or “probable” ALS, and <2 years since ALS symptom onset (blue, black, and yellow wedge symbols). On the other hand, the FVC ≥80% at baseline inclusion criterion (red wedge symbol) would have excluded patients across the entire distribution, including 62% (24/39) of faster-progressing patients (i.e. those showing a reduction of ≥6 points).
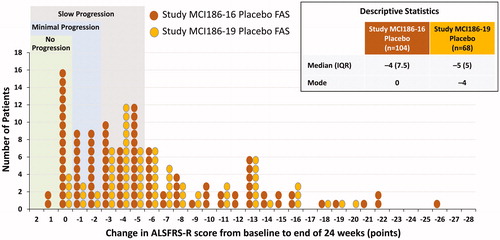
shows the change from baseline in ALSFRS-R score for the FAS placebo groups in Study MCI186-16 and Study MCI186-19. The inclusion criteria for Study MCI186-19 resulted in a group of patients who had a more bell-shaped distribution in the change in ALSFRS-R score than was seen for Study MCI186-16. For example, the median change in ALSFRS-R score was −4 (IQR, 7.5) for Study MCI186-16 and −5 (IQR, 5) for Study MCI186-19, demonstrating more progression and a narrower IQR for Study MCI186-19 (). Moreover, the mode of the populations was 0 for Study MCI186-16 and −4 for Study MCI186-19. In addition, there was a reduction in the proportion of placebo patients who had minimal progression in Study MCI186-19 (13%), as compared with Study MCI186-16 (35%).
Figure 6 Comparison of Study MCI186-16 and Study MCI186-19 distributions of changes in ALSFRS-R score at 24 weeks for placebo patients (FAS, LOCF). The median (IQR) and mode for each distribution are indicated in the figure. Hodges–Lehmann estimate of median shift (MCI186-19 to MCI186-16): –1.0, Wilcoxon 2-sample test, 2-sided p = 0.0607.
Based on the learnings from the post-hoc analysis performed on Study MCI186-16 patient data, Study MCI186-19 employed the inclusion criteria defined in Step 2. Results showed a statistically significant, 33% reduced functional loss favoring edaravone in the ALSFRS-R score at 24 weeks (primary end point) (). shows the proportion of slow progressors: 66.7% (46/69) of edaravone patients at the end of 24 weeks, as compared with 51.4% of placebo patients (35/68). Similar to the findings in the Study MCI186-16 Step 2 group, significant progression in Study MCI186-19 occurred in 24% of placebo patients but in only 9% of edaravone patients.
Discussion
Conducting clinical trials in rare diseases such as ALS can be particularly challenging due to significant disease heterogeneity, low numbers of patients, and the lack of reliable biomarkers, among other factors (Citation1). One approach to addressing these challenges is the use of patient data from rare disease registries as historical controls; however, the applicability of these approaches is currently limited and under debate (Citation16). To improve trial efficiency, clinical study enrichment strategies have been utilized to enhance signal detection (Citation4,Citation17,Citation18). Enrichment strategies, however, may slow the rate of study enrollment, unintentionally exclude potentially responsive patients, or complicate the interpretation of study results to patients within the non-enriched population (Citation4,Citation17,Citation18). The selectivity required for enrichment may require clinician-researchers to turn away patients who are interested in research participation, and the acceptance of difficult compromises.
Testing the efficacy of edaravone in ALS within a 24-week time window required a novel study design that accounted for disease heterogeneity and the relative insensitivity of outcomes measures while keeping the total patient number within reason. Thus, the study population would need to demonstrate a strong tendency for disease progression during the 24-week study time frame. Post-hoc analyses of Study MCI186-16 suggested that the trial design had not been fully successful in its optimization for disease progression. Study MCI186-19 prospectively refined its inclusion criteria, learning from the experience of Study MCI186-16, further optimizing the potential for disease progression while simultaneously minimizing heterogeneity. The current analyses described in this publication provide evidence that the strategic design of Study MCI186-19 appears to have been successful in its intent. Less heterogeneity and faster progression, overall, are documented. It is possible, however, that some faster-progressing patients, who may have further supported detection of a treatment effect, were potentially excluded by the study criteria.
Post-hoc analyses of Study MCI186-16 identified subgroups of patients (the Step 1 and Step 2 subgroups) that demonstrated statistically significant effects of edaravone on disease progression (Citation13). The investigators explained the rationale for each of the subgroup criteria. In this discussion, we further explore the rationale for each of the criteria and how they evolved into the inclusion criteria for Study MCI186-19.
Score of 0 or 1 on at least one individual item of ALSFRS-R
Study MCI186-19 required a score of ≥2 points for each item of the ALSFRS-R. The rationale was that including patients with a baseline ALSFRS-R score of 0 or 1 on any items might truncate the range of potential change within the study period. We concur that this is an appropriate method for preserving the potential for dynamic change in ALSFRS-R scores. However, this rationale fails to suggest that the advantages of edaravone are unique to the included patients alone. Although measuring the magnitude of change is facilitated by this criterion in the context of the study design, it does not necessarily follow that individual item scores of 0 or 1 should uniquely predict whether or not an individual patient will benefit from edaravone.
FVC <80%
Patients with respiratory dysfunction may show rapid progression, potentially introducing variability in the study design (Citation13). Therefore, the criterion requiring FVC ≥80% was added, which helped enroll a patient population with “normal” respiratory function. Although one-quarter of placebo patients in the Study MCI186-16 FAS had an FVC <80%, the majority of these patients did not contribute to the “slow progressor” group (). Therefore, this cutoff for respiratory function in Study MCI186-19 did not substantially contribute to the magnitude of change in ALSFRS-R, but it may have helped to decrease overall variability.
"Probable laboratory-supported" diagnosis
Study MCI186-19 excluded patients with a “probable laboratory-supported” diagnosis in order to focus on patients with a diagnosis of “definite” or “probable” ALS. In previous studies, changes in ALSFRS-R scores in patients with a “probable laboratory-supported” diagnosis were smaller in magnitude (Citation8), and could therefore limit the potential of the study design to detect change. In post-hoc analyses of Study MCI186-16 placebo patients, “probable laboratory-supported” was the most prevalent contributor to the “minimal decline” group. It is possible that this criterion (i.e. the presence of lower and upper motor neuron involvement in multiple regions) in the context of the other criteria (e.g. requiring a score of ≥2 points for each item of the ALSFRS-R, reflecting good functioning in all regions) may have created patient recruitment challenges, potentially prolonging enrollment or excluding some patients whose progression might have otherwise supported detection of a treatment effect. Overall, we believe this modification from Study MCI186-16 to Study MCI186-19 represented a key strategic contribution, increasing the likelihood that the population would demonstrate measurable progression within the study time frame.
>2 years since onset of symptoms
Restricting the time since symptom onset from within 3 years in Study MCI186-16 to within 2 years in Study MCI186-19 was based on the rationale that patients who retain broad functionality and normal respiratory function more than 2 years since initial symptomatic onset might exhibit less pronounced ALSFRS-R changes. This hypothesis appeared to have been confirmed, as 11 of the 13 placebo patients from Study MCI186-16 who were >2 years from first symptom onset were shown in post-hoc analysis to have been “slow progressors.” Therefore, from the perspective of signal detection in a clinical trial, we agree with the investigators’ rationale for shortening the required time since symptom onset. Meeting the criteria for “definite” or “probable” ALS within 2 years since symptom onset helped to ensure adequate progression to measure a treatment effect in the study time frame.
Observation period
To select for an adequately progressing patient population, Study MCI186-19 required patients to show a decrease in ALSFRS-R score of between 1 and 4 points during a 12-week observation period. In retrospect, this may not necessarily have been a critical component of the study design in the enrichment for ALS progression. Current values for ALSFRS-R decline are estimated at ∼1 point per month (Citation15). Given that the observation period was 12 weeks, a portion of the patients meeting the study protocol requirements were, by definition, slow progressors. Also, of the 192 patients enrolled in Study MCI186-19, 55 discontinued during the observation period, with one reason being a decline in FVC to <80% in 21 patients. However, FVC was not a main contributor to the slow progressing group, and therefore the observation period may have eliminated patients who would have enhanced the study robustness. A possible contribution of the requirements of the observation period toward reduced heterogeneity is noted.
Our analyses also appear to reveal that mean change values in ALSFRS-R are best considered in the context of population distribution. The Study MCI186-16 FAS placebo group had a mean change in ALSFRS-R score (−6.35 points during 24 weeks) consistent with the PRO-ACT mean monthly change of −1.02 points per month (Citation15). However, the distribution analysis revealed that the mean was highly influenced by patients on the outer limits of the spectrum (i.e. slow progressors and fast progressors).
The strategies described may provide a model for successful and efficient study conduct in ALS. Nonetheless, as ALS clinical trials consider the adoption of enrichment strategies, the relevance of core and progressive features of the disease in the enriched population will determine the perceived credibility of the generalization. Study MCI186-19 included individuals remarkable for the clarity of diagnosis due to active loss of function in multiple anatomic domains, thus establishing core features and progression. However, real-world applicability will be further determined by analysis, study, discussion, and experience. Practical application of the rationale and methodology of the clinical trial design, and for generalization to the larger population of individuals diagnosed with ALS, gains support from acceptance by a number of global health authorities, including the United States FDA and the Japanese Pharmaceuticals and Medical Devices Agency.
Conclusions
The results of these post-hoc analyses support the concept that the selection of strategic inclusion criteria, enriching for (a) anatomically widespread progression of ALS within 24 months of initial symptom onset, and (b) reduced variability in the study population, facilitated the successful demonstration of a treatment effect of edaravone. Optimization of dynamic change and management of heterogeneity in an enriched study population, defined with diagnostic certainty, enabled detection of clinically and significantly important efficacy intended to be generalizable to a larger population. These advantages in signal detection are noted within the context of challenges inherent to clinical enrichment. It is hoped that key learnings from this development program may contribute to fruitful discussion and to further potential refinement in the successful design of efficient future clinical trials in ALS.
Declaration of interest
J.P. and S.L. are employees of Mitsubishi Tanabe Pharma Development America, Jersey City, NJ, USA; J.P., K. Takei, and K. Tsuda are employees of Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan; S.A., and W.A. are employees of Mitsubishi Tanabe Pharma America, Jersey City, NJ, USA (MTPA). J.H. was an employee of MTPA during the time that the analyses described in this publication were conducted. J.Z. is under contract with MTPA.
Acknowledgments
Medical writing assistance was provided by Alex Morla, Ph.D., of p-value communications.
Additional information
Funding
References
- Brooks BR, Jorgenson JA, Newhouse BJ, Shefner JM, Agnese W. Edaravone in the treatment of amyotrophic lateral sclerosis: efficacy and access to therapy – a roundtable discussion. Am J Manag Care. 2018;24:S175–86.
- Katyal N, Govindarajan R. Shortcomings in the current amyotrophic lateral sclerosis trials and potential solutions for improvement. Front Neurol. 2017;8:521.
- Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 2014;13:1127–38.
- Center for Drug Evaluation and Research (CDER). Guidance for industry: enrichment strategies for clinical trials to support approval of human drugs and biological products. 2012. Available at: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm332181.pdf. Accessed January 7, 2019.
- Beydoun SR, Rosenfeld J. Edaravone in amyotrophic lateral sclerosis—lessons from the clinical development program and the importance of a strategic clinical trial design. US Neurol. 2018;14:47–53.
- Maragakis NJ. What can we learn from the edaravone development program for ALS? Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:98–103.
- Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Scler. 2006;7:241–5.
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–7.
- Writing Group on Behalf of the Edaravone ALS 17 Study Group. Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:20–31.
- Writing Group on Behalf of the Edaravone ALS 18 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:40–8.
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–12.
- Writing Group on Behalf of the Edaravone ALS 19 Study Group. Open-label 24-week extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:55–63.
- Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:11–9.
- Japan Intractable Diseases Information Center. Amyotrophic lateral sclerosis (ALS) [Japanese]. Available at: http://www.nanbyou.or.jp/entry/214. Accessed July 19, 2018.
- Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83:1719–25.
- Jansen-van der Weide MC, Gaasterland CMW, Roes KCB, Pontes C, Vives R, Sancho A, et al. Rare disease registries: potential applications towards impact on development of new drug treatments. Orphanet J Rare Dis. 2018;13:154.
- Karam C, Berry JD. Heterogeneity, urgency, generalizability, and enrollment: the HUGE balance in ALS trials. Neurology. 2019;92:215–6.
- van Eijk RPA, Westeneng HJ, Nikolakopoulos S, Verhagen IE, van Es MA, Eijkemans MJC, et al. Refining eligibility criteria for amyotrophic lateral sclerosis clinical trials. Neurology. 2019;92:e451–e460.