Abstract
Co-existing paracellular and transcellular barrier defect in intestinal epithelium was documented in inflammatory bowel disease, celiac disease, and intestinal obstruction. Mechanisms regarding tight junction disruption have been extensively studied; however, limited progress has been made in research on bacterial transcytosis. Densely packed brush border (BB), with cholesterol-based lipid rafts in the intermicrovillous membrane invagination, serves as an ultrastructural barrier to prevent direct contact of luminal microbes with the cellular soma. Evidence in in vitro epithelial cell cultures and in vivo animal models of bowel obstruction and antibiotic-resistant bacterial infection had indicated that nonpathogenic, noninvasive enteric bacteria may hijack the lipid raft-mediated endocytic pathways. Our studies have shown that low dose interferon-gamma (IFNγ) causes long myosin light chain kinase (MLCK)-dependent terminal web (TW) contraction and BB fanning, allowing bacteria to pass through the consequently widened intermicrovillous cleft to be endocytosed via caveolin-associated lipid rafts. Activation of intracellular innate immune receptors by bacteria-containing endosomes may further induce inflammatory and oxidative stress, leading to secondary tight junction damage. The finding of bacterial internalization preceding tight junction damage suggests that abnormal bacterial uptake by epithelial cells may contribute to the initiation or relapse of chronic intestinal inflammation.
Introduction
The enteric microbiota inhabiting the gut lumen consists of up to 100 trillion (1014) bacteria, which is 10 times the number of cells in the human body. These commensal bacteria play critical roles in maintaining gut homeostasis, and form a peaceful symbiotic relationship with the healthy host.Citation1 Nevertheless, mutual benefit exists only if the bacteria are luminally confined by intestinal barriers. Disruption of the gut barrier, followed by penetration of microbial products into the gut mucosa and circulatory bloodstream, predisposes the host to enterocolitis, systemic inflammation, or septic complications.Citation2,3
Abnormal bacterial adherence and internalization by epithelial cells has been reported in patients and experimental models with colorectal cancers,Citation4-6 inflammatory bowel disease (IBD),Citation7,8 celiac disease,Citation9,10 chronic psychological stress, Citation11,12 surgical manipulation,Citation13 intestinal obstruction,Citation14,15 and antibiotic-resistant bacterial infection.Citation16 Recent evidence suggests that mucosa-associated bacteria may contribute to pathological phenomena since many exhibit adherent and invasive characteristics, and are therefore termed ‘pathobionts’ (a commensal with the potential to cause disease; see review papers elsewhere).Citation17,18 However, from the host's point of view, epithelial barrier dysfunction is an indispensable factor underlying the process of bacterial influx, irrespective of their commensal origins or pathogenic properties.
In many intestinal diseases exhibiting transcytotic bacterial influx, pathological signs of tight junction damage are also usually present in epithelial cells. Much effort has been placed on deciphering pathways responsible for paracellular permeability increases, while mechanisms of transcellular barrier dysfunction have been overlooked for years, with ensuing limited progress. This commentary review will focus on the transcellular routes of bacterial influx and will discuss the latest findings in ultrastructural modification and molecular mechanisms involved in bacterial transcytosis.
Intestinal Epithelial Barriers
Subcellular ultrastructures, including tight junctions (TJs) and brush borders (BBs), on polarized epithelial cells are crucial barriers against luminal bacteria.
Tight Junctions
Single-layered epithelial cells are linked by TJs, which are multi-protein complexes (e.g. claudins, occludin, and zonula occludens (ZO)) that seal the paracellular spaces between adjacent epithelial cells. Paracellular permeability is determined by the assembly of TJ proteins and regulated by contraction of the perijunctional actomyosin ring (PAMR). Phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK) causes contraction of the PAMR and the physical opening of the paracellular space.Citation19,20 On the other hand, phosphorylation of MLC by Rho-associated kinase (ROCK) leads to the endocytosis of TJ proteins into apical, vacuolar-associated compartments and the impairment of paracellular junctions.Citation21-23
Paracellular permeability is modulated by host-derived factors (e.g., mucosal immune cells, cytokines, enteric neurons) and gut environmental factors (e.g. dietary nutrients, commensals, and pathogens; for more information, see review articlesCitation24,25). Numerous studies using epithelial cell lines in vitro have shown that the proinflammatory cytokines, e.g., IFNγ, TNFα, and IL-1β, cause TJ disruption without affecting cell viability,Citation21,22,26 whereas free radicals induce cell death-dependent or –independent TJ disruption.Citation27-29
Brush Borders
Densely packed microvilli on the apical membrane of intestinal epithelial cells, collectively termed the BB, prevent physical contact between luminal bacteria and the cellular soma.Citation30,31 A long BB with high expression of membranous transporters and enzymes is a hallmark of fully differentiated epithelial cells. As a prominent site for digestive and absorptive functions, the length of the BB on epithelial cells of the small intestine is longer than that of the large intestine. The length of microvilli ranges from 1 to 2 μm, depending on the differentiation status of epithelial cells, and is around 0.1 μm in diameter.Citation32 Microvilli are packed in dense arrays forming minimal intermicrovillous spaces, Citation15 with each microvillus core composed of cross-linking filaments such as actin, villin, and fimbrin. The actin-core rootlets descend into a cytoskeletal meshwork, termed the terminal web (TW) region, which contains multiple proteins, e.g. actin, myosin, fodrin and spectrin, and extends down 0.5–1 μm into the cytoplasm.Citation33
Aside from lumen-projecting microvilli, the BB surface also consists of membrane invaginations between adjacent microvilli at the base of the intermicrovillous cleft. The membrane invaginations penetrate into the TW regions and form deep, apical tubules, termed caveolae.Citation34,35 This is the only part of the apical surface sterically accessible for membrane budding or endocytotic events.Citation34 The apical membrane of intestinal epithelial cells is enriched in sphingomyelin, glycospinogolipids and cholesterol, in addition to phospholipids.Citation36,37 From a functional point of view, the BB membrane is organized into cholesterol- or glycolipid-rich microdomains known as lipid rafts. The intermicrovillous membrane invaginations are rich in cholesterol-based lipid rafts, in comparison to the glycolipid-based rafts at the microvillar surface.Citation32,34 It remains unclear, however, how microbes of 0.5-1 μm in size gain access to the base of the intermicrovillous cleft for endocytosis.
Mechanisms of Intestinal Bacterial Endocytosis by Epithelial Cells
The phenomenum of co-existing transcellular and paracellular barrier damage in disease has rendered mechanistic studies of transcytotic pathways challenging, with data interpretation often confounded by secondary basolateral entry. A reductionist approach has been undertaken in recent years to establish models of increased transcytosis uncoupled from paracellular changes, in order to delineate mechanisms of intracellular bacterial flux. Herein, such cell culture and animal models with bacterial endocytosis in absence of TJ damage are described.Citation15,16,38
The actual mechanism of bacterial transcytosis was largely unclear until an elegant study, conducted by Clark et al. in 2005, described how low concentrations of IFNγ induced the transcellular influx of nonpathogenic, noninvasive bacteria into human Caco-2 cells.Citation38 In this study, low dose IFNγ did not alter cell viability nor affected the transepithelial electrical resistance (TER) and TJ protein expression in the monolayer.Citation38 This cell culture model revealed increased intracellular bacterial counts, determined using a gentamicin resistance assay, after low dose IFNγ stimulation.Citation38 A further study demonstrated that transcycotic bacterial passage in IFNγ-treated epithelial cells was dependent on extracellular signal-regulated kinase (ERK) 1/2 and ADP-ribosylation factor (ARF)-6 signaling.Citation39
Other reports indicated that metabolic and oxidative stress provokes transcellular bacterial passage in epithelial cells, albeit in the presence of TJ impairment. Variable stressors were tested, including uncoupling of mitochondrial oxidative phosphorylation,Citation40,41 hypoxia,Citation42 and low dose nitric oxide.Citation43 Mechanistic studies of bacterial transcytosis would however be difficult in these models since transcellular pathways are likely to be confounded by TJ disruption and cell death due to energy depletion.
Intestinal infection with the non-invasive parasite Giardia lamblia, or invasive pathogen Campylobacter jejuni, also induced transcellular translocation of commensal bacteria in epithelial cells.Citation44-46 The lumen-dwelling G. lamblia causes diffuse microvilli shortening, TJ disruption and epithelial cell apoptosis,Citation47,48 that may partly contribute to bacterial penetration. Moreover, it has been reported that intracellular C. jejuni-containing vacuoles deviate from the canonical endosomal pathways, avoiding delivery into lysosomes.Citation49 The perturbation of epithelial structures and intracellular trafficking pathways by pathogens may facilitate bystander survival and transcytosis of commensals.
Caveolin-associated, cholesterol-rich lipid raft-dependent endocytotic pathways
A number of studies have demonstrated that viable nonpathogenic bacteria, traditionally not known to possess invasive capability, can enter cells by exploiting endocytic pathways associated with cholesterol-rich lipid rafts and caveolin-1 (a cholesterol binding protein). Caveolin-1 is the main protein identified in the subcellular structure of caveolae, flask-shaped membrane invaginations situated at the base of intermicrovillous clefts on epithelial cells.Citation34,35 These studies have shown that depletion of membranous cholesterol by cholesterol-sequestering agents (methyl-β-cyclodextrin or filipin) and gene silencing of caveolin-1 abolished endocytosis and transcytosis of nonpathogenic bacteria by epithelial cells in in vitro models after IFNγ treatmentCitation15,38 and C. jejuni infection.Citation45,46 Moreover, caveolin-1 or cholesterol was found colocalized with bacteria-containing endosomes in epithelial cells.Citation15,45
Invasive pathogens (e.g., Salmonella spp., Shigella spp, and enterohemorrhagic or enteroinvasive Escherichia coli) use their needle-like type III secretion systems to inject effector proteins into epithelial cells, and subsequently manipulate the host cytoskeletal actins for anchorage and entry.Citation50 In contrast to pathogens with specialized machinery to invade cells, the lipid raft/caveolae-mediated pathway was utilized by noninvasive mucosal bacteria to penetrate cells by hijacking the host endocytic machinery. These studies pinpointed the location of the portal as lipid rafts on apical membrane invaginations, but have left open the question of how bacteria pass through the BB and gain access to the base of the intermicrovillous cleft.
Terminal web contraction and brush border fanning
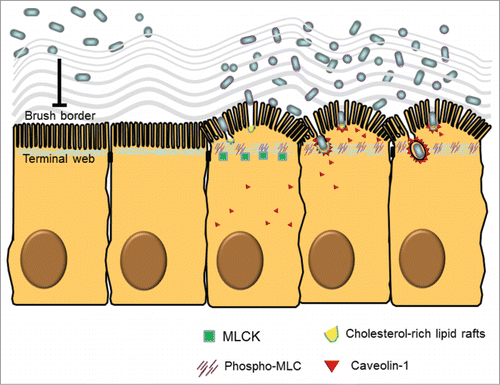
Microvilli in dense array are considered a physical barrier to luminal bacteria in steady-state conditions. It remains unclear how microbes penetrate the BB to enter cells. Although, in early literature, morphological evidence of ultrastructural motility was found in the BB after induction of TW contraction by the addition of Ca2+ and ATP,Citation51,52 the pathophysiological significance of the phenomenon was unknown. Previous reports indicated that the Ca2+/calmodulin-dependent phosphorylation of MLC induced TW contraction associated with BB fanning on isolated brush border sheets interconnected by TJs.Citation51,52 We speculated that ultrastructural changes of the BB were required to allow entry of intestinal bacteria into cells. By using human C2BBe cell cultures (a clone of Caco-2 cells with long BB), we demonstrated that IFNγ stimulation caused internalization of nonpathogenic bacteria by epithelial cells through a cholesterol-rich lipid raft and caveolin-1-dependent endocytotic pathways.Citation15 Our data showed that IFNγ increased MLCK-dependent MLC phosphorylation in the TW region associated with arc formation and fanning of the BB, thereby increasing the intermicrovillous space and exposing the cholesterol-rich lipid rafts on BB membrane for bacterial entry ().Citation15
Figure 1. Ultrastructural and molecular mechanisms of bacterial endocytosis by intestinal epithelial cells. A brush border (BB), composed of densely packed actin-cored microvilli rooted in the terminal web (TW), serves as a barrier to prevent physical contact between luminal microbes and cellular soma. After low dose interferon gamma (IFNγ) stimulation, myosin light chain kinase (MLCK)-dependent phosphorylation of myosin light chain (MLC) in the TW region causes contraction and arc formation, leading to BB fanning. The consequently enlarged intermicrovillous cleft exposes the bacteria to cholesterol-rich lipid microdomains situated at the BB membrane invagination, and allows bacteria to be internalized via lipid raft- and caveolin-1-dependent endocytic pathways.
The phenomenon of bacterial endocytosis was observed in the absence of TJ damage in our cell culture model.Citation15 No change in TER or apical-to-basolateral dextran flux was observed following IFNγ stimulation or after exposure to nonpathogenic bacteria, and thus, ruling out the possibility of paracellular entry. The dose of IFNγ (100 IU/ml) chosen was far lower than the concentration (1000–3000 IU/ml) reported for the induction of TJ damage in Caco-2 cells.Citation38,53,54 A similar observation was made in T84 cells, in which IFNγ higher than 10 IU/ml disrupted TJs but a concentration of 1 IU/ml induced bacterial internalization without TJ damage.Citation55,56 These findings suggest that minute fluctuations of cytokine levels below the threshold of full-blown inflammation may instigate transcellular leakiness but not paracellular permeability changes.
Epithelial cell culture studies have yielded mechanistic details of bacterial endocytosis; however, in vivo models are needed to support these findings. As many previously established models, such as chemically induced colitis and pathogen infection, are confounded by co-existing transcellular and paracellular bacterial influx, we attempted to overcome this limitation by developing novel animal models that displayed transcellular passage of bacteria without TJ disruption. Two models with enteric bacterial dysbiosis (changes in numbers or composition of gut microbiota) were established, including bowel obstructionCitation15,29,57 and infection with antibiotic-resistant enterobacteria.Citation16
The bowel obstruction model induced commensal bacterial overgrowth after loop ligation in the distal small intestine. Bacterial endocytosis by epithelial cells was observed after 6 h of obstruction, whereas TJ damage was seen only after 24 h.Citation15,29,57 The TJ damage in gut tissues after 24 h of obstruction was evidenced by occludin cleavage, ZO-1 destruction, and increased tissue conductance and macromolecular permeability.Citation15,29,57 Interestingly, continuous epithelial lining on villus surface with no sign of cell death was found at both time points, ruling out loss of cell viability as a cause of bacterial transcellular passage. Normal crypt/villus ratio was observed after 6 h in comparison to villus blunting without crypt hyperplasia after 24 h, suggesting cell sloughing on villus tip may partly contribute to TJ damage at the later time point. Additional advantages of this model are the presence of resident bacterial overgrowth, which is confined in a particular gut segment without being flushed out by peristaltic force, as well as the ligation of the intestinal loop for easy intraluminal administration of inhibitors.
At early time points of obstruction, disarray of the microvilli and a 3-fold increase in intermicrovillous space were accompanied by the trapping of bacteria on the BB and the presence of intracellular microbes in endosomes. Colocalization of intracellular bacteria with caveolin-1 was observed in mouse epithelial cells by electron microscopy, supporting lipid raft/caveolin-mediated endocytic pathways for bacterial internalization in vivo.Citation15 Intraluminal administration of ML-7 (a specific MLCK inhibitor) prevented TW myosin phosphorylation and BB fanning, as well as reduced bacterial internalization by enterocytes.Citation15 Moreover, TW and BB changes and bacterial endocytosis were not seen in mice genetically deficient in IFNγ or MLCK-210 (a non-muscle form of MLCK, also called epithelial-specific MLCK).Citation15 Our findings support roles for IFNγ and MLCK in bacterial endocytosis and provide in vivo evidence linking TW contraction and BB fanning with bacterial internalization ().
The antibiotic-resistant enterobacterial infection model was developed by depleting commensals with oral antibiotics before orogastric inoculation of antibiotic-resistant E. coli. A high incidence of bloodstream infection with antibiotic-resistant superbugs has been reported in critical care units, of which the clinical isolates are mostly members of the Enterobacteriaceae family (e.g., E. coli and Klebsiella pneumoniae).Citation58 Clinical findings suggested that, in some cases, superbugs colonized the intestines rather than were acquired from skin wounds. In our model, antibiotic treatment enabled intestinal colonization and transient dominance of orally acquired, antibiotic-resistant E. coli in mice.Citation16 Moreover, colonization by resistant E. coli was mainly detected in the large intestine and paralleled abnormal bacterial endocytosis (by both resistant bacteria and commensals) by colonocytes.Citation16 Colonization by antibiotic-resistant E. coli was associated with increased proinflammatory cytokine production in the gut mucosa and bacterial translocation to liver and spleen, but no sign of TJ damage was seen in any intestinal segments.Citation16 The tissue conductance, macromolecular permeability, and occludin and ZO-1 expression in jejunum, cecum, and colon after colonization of resistant E.coli were comparable to those without infection.Citation16 Mice deficient in MLCK-210 were also investigated using this model; however, the level of microbial internalization by colonocytes was only slightly decreased compared to wild type mice and without statistical significance.Citation16 In comparison to the findings of the small intestine obstruction model, the lack of dependency of bacterial endocytosis on MLCK may be partly explained by the short microvilli on colonocytes, or by the emergence of invasive resident bacteria in the colon after antibiotic manipulation and superbug exposure.
Other clinical diseases showing evidence of bacterial endocytosis and BB abnormality, such as IBD and celiac disease, may also make use of an MLCK-dependent mechanism and further investigations are warranted.Citation59-62 Moreover, regulatory mechanisms pinpointing MLCK activation in various subcellular regions, i.e. TW and PAMR, for differential control of transcellular and paracellular barriers remain poorly understood. Questions concerning whether these effects are orchestrated by multiple isoforms of MLCK, by different kinase-dependent signals, or by calcium mobilization, need to be answered.
Faulty Host or Bug, or Both?
Our data demonstrated that low dose IFNγ-induced BB fanning allowed passage of nonpathogenic bacteria through enlarged intermicrovillous space and facilitated bacterial internalization via lipid rafts, stressing an important role of host factors in promoting noninvasive bacterial endocytosis. It is noteworthy that physical trapping of bacteria on intermicrovillous cleft is different from pathogenic adherence that requires specific machinery such as pili, fimbriae, adhesin, intimin etc.Citation63 We cannot rule out changes in virulent properties (e.g., adherent ability) of enteric bacteria which may also contribute to and are not mutually exclusive from our findings in regards to abnormal host-microbe interaction.
Accumulating evidence suggests that commensals may become ‘pathobionts’ upon antibiotic pressure and colitis injury. A recent paper demonstrated that antibiotics induced the expansion of resistant E. coli that harbor virulence factors associated with invasion and motility, and caused septic syndromes following chemically induced colitis.Citation64 Other studies have documented that mucosa-associated E.coli expressing pathogenicity islands of afimbrial adhesin (afa), long polar fimbriae (lpf) and polyketide synthase (pks) are frequently seen in IBD and colon cancer patients.Citation5,65 The afa and lpf operons are known to confer adhesive characteristics on E.coli for epithelial cells.Citation5,65 Recent studies in mouse models of colitis-associated colorectal cancer have demonstrated that pks-encoding colibactin produced by mucosa-associated, adherent E.coli causes DNA damage and cell senescence, and drives tumor progression.Citation66,67 The newly acquired adherent and cytotoxic characteristics of enteric bacteria are likely to stimulate host immune response through pathogen-associated or damage-associated molecular patterns, triggering the onset of proinflammatory signaling. Production of low levels of cytokines such as IFNγ may then activate BB ultrastructural changes for exposure of membranous lipid rafts and unknown receptors for binding and entry of bacteria. Overall, the finding of BB fanning in promoting bacterial endocytosis is consistent with the notion of invasive pathobionts, further emphasizing that bi-directional crosstalk between host and bug underlies the dysbiotic relationship.
Intracellular Immune Signals Triggered by Internalized Bacteria may Cause Secondary TJ Damage in Epithelial Cells
Host-driven trapping/endocytosis and pathogen-driven adherence/invasion of bacteria represent 2 distinct mechanisms for microbial entry, yet the intracellular presence of bacterial structural components resulting from either pathway may trigger activation of innate immune responses. Previous studies had shown that epithelial infection with invasive pathogens (i.e., Salmonella enterica and enteroinvasive E. coli) activated intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs).Citation68-70 Bacterial structural components of peptidoglycan, i.e. tripeptide l-Ala-γ-D-Glu-meso-diaminopimelic acid (Tri-DAP) and muramyl dipeptide (MDP), are ligands for NOD-1 and -2, respectively. Presence of NOD-1 and -2 have been reported in human intestinal epithelial cells, and downstream inflammasome complex and proinflammatory signaling was observed following infection with invasive pathogens or exposure to Tri-DAP and MDP.Citation68-70
Direct evidence linking endocytic pathways and NOD activation was recently reported; 2 independent studies showed that co-localization of cytosolic NOD-1 and NOD-2 with early endosomes containing bacterial membrane products, invasive Salmonella, or MDP-coated 1 μm latex beads in epithelial and myeloid cells.Citation71,72 The sensing of bacterial products and induction of proinflammatory cytokines required the recruitment of NOD-2 to endosomal membranes forming a complex with peptide transporters that mediate cytosolic transfer of endosomally-derived MDP.Citation71 Other studies have demonstrated that NOD-2 activation by MDP or invasive pathogens stimulated the production of reactive oxygen species (ROS) and nitric oxide (NO) in epithelial cells and macrophages to engage in bactericidal and bacteriostatic activity.Citation73-75 Although the innate immune response as an early warning system is aiming for bacterial elimination, excessive inflammatory and oxidative/nitrosative stress may act as double-edged swords and result in bystander damage to intestinal barrier integrity. Death-dependent TJ disruption or cell death-independent signaling for TJ impairment have been documented in intestinal epithelial cells following exposure to ROS and NO,Citation27-29 as well as IFNγ, TNFα, and IL-1β.Citation21,22,26
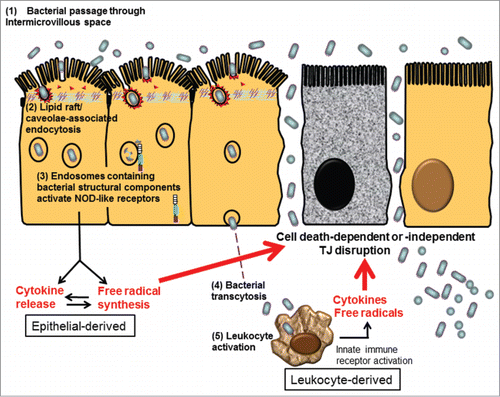
We and others demonstrated that bacterial endocytosis by epithelial cells preceded TJ damage under inflammatory stress in a dose- and time-dependent manner.Citation15,29,38,57 Therefore, we suspect that bacterial entry through intermicrovillous lipid rafts may trigger activation of intracellular NLRs by the sensing of endosomally derived bacterial structural components (unrelated to virulence factors). The NLR-mediated immune responses may adversely cause aggravated inflammatory and oxidative stress, leading to secondary TJ damage in epithelial cells ().
Figure 2. Proposed schema of bacterial endocytosis preceding tight junction damage in epithelial cells. (1) Bacteria passed through the widened intermicrovillous cleft and were exposed to lipid rafts on apical membrane invagination. (2) Bacterial endocytosis by epithelial cells was mediated by lipid raft/caveolae-dependent pathways. (3) Cytosolic innate immune receptors, such as nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), were recruited to the membrane of endosomes containing microbes and bacterial structural components. Activation of NLR-dependent signaling leads to epithelial synthesis of proinflammatory cytokines and free radicals, aiming for bacterial elimination. However, excessive inflammatory or oxidative stress may act as a double-edged sword causing cell death-dependent or -independent TJ disruption in epithelial cells. (4) Internalized bacteria, if managed to evade intracellular killing, may transcytose to the lamina propria. (5) Transcytosed bacteria activate leukocytes, resulting in even higher levels of proinflammatory cytokines and free radicals to aggravate TJ damage, leading to uncontrollable inflammation.
Concluding Remarks
The finding that bacterial endocytosis precedes breaches of TJs suggests that abnormal bacterial transcytosis may contribute to the initiation or relapse of chronic intestinal inflammation. Since low dose proinflammatory cytokines are sufficient to induce bacterial endocytosis by epithelial cells, sub-clinical or low grade changes below the threshold may tip the balance of tolerance toward full blown inflammation owing to subsequent intracellular microbial sensing and paracellular permeability damage. With the recent knowledge of adherent mucosa-associated bacteria playing a critical role in IBD and colitis-associated colorectal cancers, increased understanding of the abnormal interaction between epithelium and bacteria could aid in the development of novel strategies for managing chronic inflammatory diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We thank the funding support from Ministry of Science and Technology, Taiwan (MOST 102-2628-B-002-009-MY3) and National Taiwan University (NTU-CDP-104R7798).
References
- Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J Gastrointest Pathophysiol 2012; 3:27-43; PMID:22368784; http://dx.doi.org/10.4291/wjgp.v3.i1.27
- Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007; 22:464-71; PMID:17376034; http://dx.doi.org/10.1111/j.1440-1746.2007.04933.x
- Leaphart CL, Tepas JJ, III. The gut is a motor of organ system dysfunction. Surgery 2007; 141:563-9; PMID:17462455; http://dx.doi.org/10.1016/j.surg.2007.01.021
- Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Dechelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res 2014; 20:859-67; PMID:24334760; http://dx.doi.org/10.1158/1078-0432.CCR-13-1343
- Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, Knight P, Codling C, Marchesi JR, Winstanley C, et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 2014; 63:761-70; PMID:23846483; http://dx.doi.org/10.1136/gutjnl-2013-304739
- Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 2004; 127:80-93; PMID:15236175; http://dx.doi.org/10.1053/j.gastro.2004.03.054
- Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002; 122:44-54; PMID:11781279; http://dx.doi.org/10.1053/gast.2002.30294
- Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol 2002; 37:10034-41; PMID:12374228; http://dx.doi.org/10.1080/003655202320378220
- Forsberg G, Fahlgren A, Hörstedt P, Hammarström S, Hernell O, Hammarström ML. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol 2004; 99:894-904; PMID:15128357; http://dx.doi.org/10.1111/j.1572-0241.2004.04157.x
- Sjöberg V, Sandström O, Hedberg M, Hammarström S, Hernell O, Hammarström ML. Intestinal T-cell responses in celiac disease - impact of celiac disease associated bacteria. PLoS One 2013; 8:e53414; PMID:23326425; http://dx.doi.org/10.1371/journal.pone.0053414
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007; 56:1522-8; PMID:17339238; http://dx.doi.org/10.1136/gut.2006.117176
- Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 2002; 123:1099-108; PMID:12360472; http://dx.doi.org/10.1053/gast.2002.36019
- Engel DR, Koscielny A, Wehner S, Maurer J, Schiwon M, Franken L, Schumak B, Limmer A, Sparwasser T, Hirner A, et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat Med 2010 16:1407-13; PMID:21113155; http://dx.doi.org/10.1038/nm.2255
- Samel S, Keese M, Kleczka M, Lanig S, Gretz N, Hafner M, Sturm J, Post S. Microscopy of bacterial translocation during small bowel obstruction and ischemia in vivo - a new animal model. BMC Surgery 2002; 2:6; PMID:12174194; http://dx.doi.org/10.1186/1471-2482-2-6
- Wu LL, Peng WH, Kuo WT, Huang CY, Ni YH, Lu KS, Turner JR, Yu LC. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-gamma. Am J Pathol 2014; 184:2260-74; PMID:24911373; http://dx.doi.org/10.1016/j.ajpath.2014.05.003
- Yu LC, Shih YA, Wu LL, Lin YD, Kuo WT, Peng WH, Lu KS, Wei SC, Turner JR, Ni YH. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol 2014; 307:G824-35; PMID:25059827; http://dx.doi.org/10.1152/ajpgi.00070.2014
- Chassaing B, Gewirtz AT. Pathobiont hypnotises enterocytes to promote tumour development. Gut 2014; 63:1837-8; PMID:24780742
- Kahrstrom CT. Bacterial pathogenesis: E. coli claims the driving seat for cancer. Nat Rev Microbiol 2012; 10:670; PMID:22926206
- Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol 2006; 169:1901-9; PMID:17148655; http://dx.doi.org/10.2353/ajpath.2006.060681
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 2006; 119:2095-106; PMID:16638813; http://dx.doi.org/10.1242/jcs.02915
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 2005; 19:923-33; PMID:15923402; http://dx.doi.org/10.1096/fj.04-3260com
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 2005; 16:5040-52; PMID:16055505; http://dx.doi.org/10.1091/mbc.E05-03-0193
- Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol 2009; 10:36; PMID:19422706; http://dx.doi.org/10.1186/1471-2121-10-36
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70:631-59; PMID:22782113
- Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010; 177:512-24; PMID:20581053; http://dx.doi.org/10.2353/ajpath.2010.100168
- Al-Sadi R, Guo S, Ye D, Dokladny K, Alhmoud T, Ereifej L, Said HM, Ma TY. Mechanism of IL-1beta modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol 2013; 190:6596-606; PMID:23656735; http://dx.doi.org/10.4049/jimmunol.1201876
- Salzman AL, Menconi MJ, Unno N, Ezzell RM, Casey DM, Gonzalez PK, Fink MP. Nitric oxide dilates tight junctions and depletes ATP in cultured Caco-2BBe intestinal epithelial monolayers. Am J Physiol Gastrointestinal Liver Physiol 1995; 268:G361-G73; PMID:7864133
- Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2008; 294:G1060-G9; PMID:18292183; http://dx.doi.org/10.1152/ajpgi.00202.2007
- Wu LL, Chiu HD, Peng WH, Lin BR, Lu KS, Lu YZ, Yu LCH. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med 2011; 39:2087-98; PMID:21552122; http://dx.doi.org/10.1097/CCM.0b013e31821cb40e
- Shifrin D, McConnell R, Nambiar R, Higginbotham J, Coffey R, Tyska M. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol 2012; 22:627-31; PMID:22386311; http://dx.doi.org/10.1016/j.cub.2012.02.022
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 2005; 16:2443-57; PMID:15758024; http://dx.doi.org/10.1091/mbc.E04-12-1116
- Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta 2003; 1617:1-9; PMID:14637014; http://dx.doi.org/10.1016/j.bbamem.2003.09.005
- Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 1992; 102 ( Pt 3):581-600; PMID:1506435
- Hansen GH, Pedersen J, Niels-Christiansen LL, Immerdal L, Danielsen EM. Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J 2003; 373:125-32; PMID:12689332; http://dx.doi.org/10.1042/BJ20030235
- Field FJ, Born E, Murthy S, Mathur SN. Caveolin is present in intestinal cells: role in cholesterol trafficking? J Lipid Res 1998; 39:1938-50; PMID:9788240
- Christiansen K, Carlsen J. Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim Biophys Acta 1981; 647:188-95; PMID:6170331; http://dx.doi.org/10.1016/0005-2736(81)90245-5
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992; 68:533-44; PMID:1531449; http://dx.doi.org/10.1016/0092-8674(92)90189-J
- Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 2005; 128:1258-67; PMID:15887109; http://dx.doi.org/10.1053/j.gastro.2005.01.046
- Smyth D, McKay CM, Gulbransen BD, Phan VC, Wang A, McKay DM. Interferon-gamma signals via an ERK1/2-ARF6 pathway to promote bacterial internalization by gut epithelia. Cell Microbiol 2012; 14:1257-70; PMID:22463716; http://dx.doi.org/10.1111/j.1462-5822.2012.01796.x
- Lewis K, Lutgendorff F, Phan V, Soderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 2010; 16:1138-48; PMID:20024905; http://dx.doi.org/10.1002/ibd.21177
- Wang A, Keita AV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 2014; 184:2516-27; PMID:25034594; http://dx.doi.org/10.1016/j.ajpath.2014.05.019
- Wells CL, VandeWesterlo EM, Jechorek RP, Erlandsen SL. Effect of hypoxia on enterocyte endocytosis of enteric bacteria. Crit Care Med 1996; 24:985-91; PMID:8681603; http://dx.doi.org/10.1097/00003246-199606000-00019
- Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci USA 2006; 103:1295-300; PMID:16432212
- Chen TL, Chen S, Wu HW, Lee TC, Lu YZ, Wu LL, Ni YH, Sun CH, Yu WH, Buret AG, et al. Persistent gut barrier damage and commensal bacterial influx following eradication of Giardia infection in mice. Gut Pathog 2013; 5:26; PMID:23991642; http://dx.doi.org/10.1186/1757-4749-5-26
- Kalischuk L, Inglis GD, Buret A. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog 2009; 1:2; PMID:19338680
- Kalischuk LD, Leggett F, Inglis GD. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog 2010; 2:14; PMID:21040540; http://dx.doi.org/10.1186/1757-4749-2-14
- Scott KG, Yu LCH, Buret AG. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immun 2004; 72:3536-42; PMID:15155662; http://dx.doi.org/10.1128/IAI.72.6.3536-3542.2004
- Yu LCH, Huang CY, Kuo WT, Sayer H, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects human intestinal epithelial cells against Giardia duodenalis-induced apoptosis. Int J Parasitol 2008; 38:923-34; PMID:18281046; http://dx.doi.org/10.1016/j.ijpara.2007.12.004
- Watson RO, Galan JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 2008; 4:e14; PMID:18225954
- Tosi T, Pflug A, Discola KF, Neves D, Dessen A. Structural basis of eukaryotic cell targeting by type III secretion system (T3SS) effectors. Res Microbiol 2013; 164:605-19; PMID:23541478; http://dx.doi.org/10.1016/j.resmic.2013.03.019
- Keller TC, III, Conzelman KA, Chasan R, Mooseker MS. Role of myosin in terminal web contraction in isolated intestinal epithelial brush borders. J Cell Biol 1985; 100:1647-55; PMID:3988804; http://dx.doi.org/10.1083/jcb.100.5.1647
- Keller TC, III, Mooseker MS. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J Cell Biol 1982; 95:943-59; PMID:6897550; http://dx.doi.org/10.1083/jcb.95.3.943
- Di Paolo MC, Merrett MN, Crotty B, Jewell DP. 5-Aminosalicylic acid inhibits the impaired epithelial barrier function induced by gamma interferon. Gut 1996; 38:115-9; PMID:8566837; http://dx.doi.org/10.1136/gut.38.1.115
- Eun CS, Kim YS, Han DS, Choi JH, Lee AR, Park YK. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS 2011; 119:49-56; PMID:21143526; http://dx.doi.org/10.1111/j.1600-0463.2010.02691.x
- Donato RP, El-Merhibi A, Gundsambuu B, Mak KY, Formosa ER, Wang X, Abbott CA, Powell BC. Studying permeability in a commonly used epithelial cell line: T84 intestinal epithelial cells. Methods Mol Biol 2011; 763:115-37; PMID:21874448; http://dx.doi.org/10.1007/978-1-61779-191-8_8
- Smyth D, Phan V, Wang A, McKay DM. Interferon-gamma-induced increases in intestinal epithelial macromolecular permeability requires the Src kinase Fyn. Lab Invest 2011; 91:764-77; PMID:21321534; http://dx.doi.org/10.1038/labinvest.2010.208
- Wu CC, Lu YZ, Wu LL, Yu LCH. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol 2010; 10:39-50; PMID:20403206; http://dx.doi.org/10.1186/1471-230X-10-39
- Nasa P, Juneja D, Singh O, Dang R, Singh A. An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Eur J Intern Med 2012; 23:192-5; PMID:22284253; http://dx.doi.org/10.1016/j.ejim.2011.06.016
- Fratila OC, Craciun C. Ultrastructural evidence of mucosal healing after infliximab in patients with ulcerative colitis. J Gastrointestinal Liver Dis 2010; 19:147-53; PMID:20593047
- Shields HM, Bates ML, Goldman H, Zuckerman GR, Mills BA, Best CJ, Bair FA, Goran DA, DeSchryver-Kecskemeti K. Scanning electron microscopic appearance of chronic ulcerative colitis with and without dysplasia. Gastroenterology 1985; 89:62-72; PMID:4007414
- Iancu TC, Manov I. Ultrastructural aspects of enterocyte defects in infancy and childhood. Ultrastruct Pathol 2010; 34:117-25; PMID:20455660; http://dx.doi.org/10.3109/01913121003648410
- Mones RL, Yankah A, Duelfer D, Bustami R, Mercer G. Disaccharidase deficiency in pediatric patients with celiac disease and intact villi. Scand J Gastroenterol 2011; 46:1429-34; PMID:21936724; http://dx.doi.org/10.3109/00365521.2011.619276
- Le Bouguenec C. Adhesins and invasins of pathogenic Escherichia coli. Int J Med Microbiol 2005; 295:471-8; PMID:16238021; http://dx.doi.org/10.1016/j.ijmm.2005.07.001
- Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012; 18:799-806; PMID:22522562; http://dx.doi.org/10.1038/nm.2729
- Raisch J, Buc E, Bonnet M, Sauvanet P, Vazeille E, de Vallee A, Dechelotte P, Darcha C, Pezet D, Bonnet R, et al. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J Gastroenterol 2014; 20:6560-72; PMID:24914378; http://dx.doi.org/10.3748/wjg.v20.i21.6560
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012; 338:120-3; PMID:22903521; http://dx.doi.org/10.1126/science.1224820
- Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Dechelotte P, Bonnet M, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 2014; 63:1932-42; PMID:24658599; http://dx.doi.org/10.1136/gutjnl-2013-305257
- Richmond AL, Kabi A, Homer CR, Marina-Garcia N, Nickerson KP, Nesvizhskii AI, Sreekumar A, Chinnaiyan AM, Nunez G, McDonald C. The nucleotide synthesis enzyme CAD inhibits NOD2 antibacterial function in human intestinal epithelial cells. Gastroenterology 2012; 142:1483-92 e6; PMID:22387394; http://dx.doi.org/10.1053/j.gastro.2012.02.040
- Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun 2004; 72:1487-95; PMID:14977954; http://dx.doi.org/10.1128/IAI.72.3.1487-1495.2004
- Laroui H, Yan Y, Narui Y, Ingersoll SA, Ayyadurai S, Charania MA, Zhou F, Wang B, Salaita K, Sitaraman SV, et al. L-Ala-gamma-D-Glu-meso-diaminopimelic acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J Biol Chem 2011; 286:31003-13; PMID:21757725; http://dx.doi.org/10.1074/jbc.M111.257501
- Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Maziere A, Klumperman J, Schlatter M, Delamarre L, Mellman I. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 2014; 509:240-4; PMID:24695226; http://dx.doi.org/10.1038/nature13133
- Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner LJ, Thomas BJ, Malosse C, Gantier MP, Casillas LN, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 2014; 15:623-35; PMID:24746552; http://dx.doi.org/10.1016/j.chom.2014.04.001
- Corcionivoschi N, Alvarez LA, Sharp TH, Strengert M, Alemka A, Mantell J, Verkade P, Knaus UG, Bourke B. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe 2012; 12:47-59; PMID:22817987; http://dx.doi.org/10.1016/j.chom.2012.05.018
- Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology 2002; 122:1070-87; PMID:11910358; http://dx.doi.org/10.1053/gast.2002.32372
- Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 2009; 122:3522-30; PMID:19759286; http://dx.doi.org/10.1242/jcs.050690
