Abstract
Oral, intestinal and genital mucosal epithelia have a barrier function to prevent paracellular penetration by viral, bacterial and other pathogens, including human immunodeficiency virus (HIV). HIV can overcome these barriers by disrupting the tight and adherens junctions of mucosal epithelia. HIV-associated disruption of epithelial junctions may also facilitate paracellular penetration and dissemination of other viral pathogens. This review focuses on possible molecular mechanisms of HIV-associated disruption of mucosal epithelial junctions and its role in HIV transmission and pathogenesis of HIV and acquired immune deficiency syndrome (AIDS).
Introduction
Oral, intestinal and genital mucosal epithelia have multiple barriers, including anatomical (multistratified and monostratified epithelial barrier with well-developed tight and adherens junctions) and biological (innate immune cells, antimicrobial factors and mucus), which resist penetration by multiple pathogens, including human immunodeficiency virus (HIV). Accumulating evidence indicates that HIV can overcome the mucosal barrier function by reducing or altering the anatomical barrier; i.e., the interaction of HIV envelope protein gp120 with mucosal epithelial cells disrupts epithelial junctions, leading to paracellular penetration by the virus. Penetration via paracellular gates substantially increases access of the virus to HIV-susceptible intraepithelial and subepithelial CD4+ T lymphocytes, Langerhans/dendritic cells (LC/DC) and macrophages, the first step in the establishment of systemic HIV infection. During systemic HIV/AIDS disease, viral proteins gp120 and tat and HIV-associated activation of inflammatory processes in the mucosal epithelia can also lead to the disruption of epithelial junctions, which may promote penetration and/or dissemination of other important viruses, including herpesviruses (HSV) and papillomaviruses (HPV).
This review describes the role of HIV in the disruption of mucosal epithelia and the possible molecular mechanisms of HIV-associated disruption of epithelial junctions. This review also discusses the potential implications of HIV-associated mucosal disruption in the penetration and spread of HIV and other viral pathogens and the progression of systemic HIV/AIDS disease.
HIV-Associated disruption of mucosal barriers and its role in HIV transmission
Mechanisms of HIV transmission through mucosal epithelia
Oropharyngeal, intestinal and genital mucosal epithelia play an important role in HIV transmission. Oral, intestinal and cervical epithelial cells may express one or more following proteins, which may facilitate HIV binding and entry: C-X-C chemokine receptor type 4 (CXCR4), C-C chemokine receptor type 5 (CCR5), galactosylceramide (GalCer), heparan sulfate proteoglycans (HSPGs), and mannose receptor.Citation1-7 However, the molecular mechanism of HIV transmission through mucosal epithelia is still not fully understood. It has been proposed that multiple pathways may facilitate initial HIV penetration into the body () by (i) infection of mucosal epithelial cells by HIV,Citation2,8-11 although HIV infection by the production of progeny virions in epithelial cells has not been comprehensively proved Citation12,13; (ii) transcytosis of virus across epithelia Citation1,3,Citation14-18; (iii) paracellular penetration by virus due to a lack of tight junctions or their disruption;Citation4,19-22 and (iv) capture of virus from the mucosal surface by intraepithelial and/or subepithelial LC/DC.Citation23 Recent studies showed that direct interaction of HIV through viral envelope protein gp120 in mucosal epithelial cells may disrupt the epithelial junctions, leading to paracellular penetration by the virus.Citation19,24-26
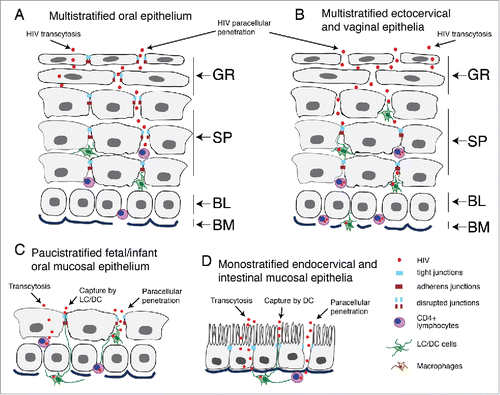
Figure 1. Model of HIV penetration into mucosal epithelium through disrupted junctions. (A) Multistratified, nonkeratinized oral squamous mucosal epithelia have well-developed tight and adherens junctions within the strata granulosum, spinosum and parabasal layers. The intact tight junctions seal intercellular spaces, preventing paracellular penetration by HIV. (B) Nonkeratinized ectocervical and vaginal squamous mucosal epithelia also have multilayer organization, but their uppermost few layers lack the tight and adherens junctions, allowing paracellular penetration by HIV into the lower layers. These virions are more likely to reach interepithelial HIV-susceptible CD4+ T lymphocytes, LC/DC and macrophages and initiate systemic HIV infection. (C) Fetal and infant oral mucosal epithelia have paucistratified (2 to 5 layers) epithelia with tight and adherens junctions, which may play a role in preventing HIV mother-to-child transmission. (D) Monostratified columnar epithelia of rectal/intestinal and endocervical mucosa have tight and adherens junctions, which prevent paracellular penetration by HIV. (A–D) HIV transmission via stratified and nonstratified mucosal epithelia with intact tight junctions may occur by viral transcytosis; however, the efficiency of transcytosis is < 0.01% of the initial inoculum. Interaction of HIV with the surface of multistratified, paucistratified and monostratified mucosal epithelia may induce the disruption of epithelial tight junctions, promoting paracellular penetration by HIV and increasing its infectivity in CD4+ lymphocytes, LC/DC and monocyte/macrophages. (C and D) HIV systemic infection could also be initiated by the capture of virions across paucistratified fetal/infant oral and monostratified intestinal mucosa by submucosal LC/DC. GR, granulosum; SP, spinosum; BL, basal; BM, basement membrane.
Intercellular junctions of mucosal epithelia are critical for mucosal barrier function
The oropharyngeal, ectocervical, vaginal and foreskin epithelia consist of a multilayered, stratified squamous epithelium supported by an underlying layer of fibrous connective tissue, the lamina propria. The endocervical and intestinal mucosa is covered with monostratified simple epithelium. All mucosal epithelia form multiple intercellular junctions, including tight and adherens junctions,Citation3,4,Citation26-33 which are critical for maintaining the morphological and physiological features of mucosal epithelia. Tight junctions of mucosal epithelium form the physical tissue barrier between epithelial cells that protects the internal body from the external environment.Citation34 Tight junctions comprise the transmembrane proteins occludin and claudinsCitation1-24, which are associated with the cytoplasmic proteins zonula occludens-1 (ZO-1), ZO-2, and ZO-3 (). The zonula occludens themselves mediate linkage of occludin and claudins to the actin cytoskeleton.Citation35 The interaction of the intercellular transmembrane proteins facilitates the formation of tight junctions between adjacent epithelial cells near the apical surface, sealing the paracellular space between epithelial cells. Junctional adhesion molecule 1 (JAM-1) is specifically localized at the tight junctions of epithelial and endothelial cells and is involved in the regulation of junctional integrity and paracellular permeability.Citation36 Intercellular adherens junctions are formed by homotypic interaction of the transmembrane protein E-cadherin, which is connected to intracellular proteins p120 and β and α catenins and the actin cytoskeleton.Citation37 Both tight and adherens junctions are connected through the actin cytoskeleton, and the formation of adherens junctions is critical for the formation of tight junctions.Citation37 The tight and adherens junctions in epithelial cells play a critical role in the development of their distinct polarized apical and basolateral membranes, establishing a polarized organization of mucosal epithelial cells. Citation38
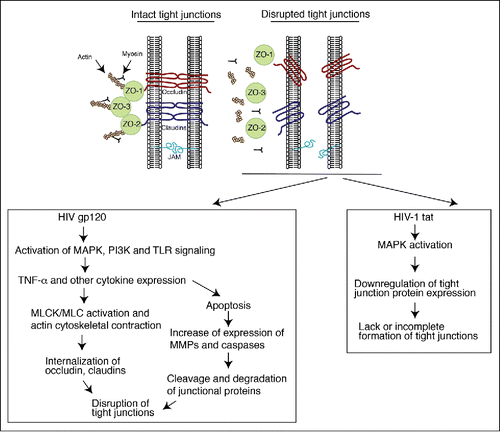
Figure 2. Model of HIV-associated disruption of tight junctions in initial HIV entry and systemic HIV/AIDS disease. (Upper panel) Tight junctions are formed between epithelial cells of oral, genital and intestinal mucosa by lateral interaction of integral membrane proteins occludin and claudins, which are associated with the cytoplasmic proteins ZO-1, ZO-2, and ZO-3. These proteins themselves link occludin and claudins to the actin cytoskeleton. Junctional adhesion molecule 1 (JAM-1) is also localized at the tight junctions of mucosal epithelia. (Lower left panel) HIV-associated disruption of mucosal epithelium may occur upon initial infection of HIV and during systemic HIV/AIDS disease. Initial HIV gp120 interaction with mucosal epithelia is facilitated by binding of viral gp120 to one or more HIV coreceptors (GalCer, CCR5 and/or CXCR4), as well as to HSPG, TLR2 and TLR4. Such interaction activates MAPK, PI3K and/or TLR signaling, leading to upregulation of proinflammatory cytokines, including TNF-α, which induce the activation of MLCK and MLC phosphorylation and actin cytoskeletal contraction. These events induce mislocalization of ZO-1 from junctional areas and internalization of occludin and claudins 1, 3 and 4 from assembled junctions. An increase in the expression of proinflammatory cytokines may activate the apoptotic pathway in epithelial cells, leading to MMP- and/or caspase-mediated degradation of junctional proteins. Disruption of epithelial tight junctions facilitates opening of the paracellular space between epithelial cells and paracellular penetration by HIV. During HIV/AIDS disease, HIV-infected subepithelial CD4+ lymphocytes, LC/DC and macrophages migrate into the mucosal epithelium and release virions and envelope protein gp120, which disrupt epithelial junctions. In addition, HIV-infected cells secrete the viral transactivator protein tat, which also contributes to the disruption of tight junctions. (Lower right panel) HIV tat binds to integrins and induces MAPK activation, which downregulates expression of tight junction proteins ZO-1, occludin, and claudins 1, 3 and 4. The lack of expression of tight junction proteins leads to a lack or incomplete formation of tight junctions.
The ability of HIV to transmigrate through mucosal epithelia varies considerably at different epithelial sites. While HIV transmission through adult oral epithelium is rare,Citation39,40 mother-to-child transmission through fetal/neonatal/infant oral epithelium is more common.Citation41 This is mainly due to stratification of epithelial layers with well-developed tight junctions. The more highly stratified adult oral epithelium limits viral penetration more efficiently than does the less stratified fetal/neonatal/infant oral epithelium.Citation4 Formation of functional tight junctions in adult oral epithelium in all layers, including the upper stratum granulosum, is critical for prevention of HIV paracellular penetration and low-level HIV oral transmission. In contrast, in the stratified ectocervical and vaginal epithelium, tight junctions are present within the parabasal and spinosum layers. The surface granulosum layers lack tight junctions,Citation32 allowing HIV paracellular penetration toward intramucosal virus-susceptible immune cells.Citation20 This may explain one of the possible mechanisms of the higher level of HIV genital transmission compared to oral transmission in adult populations.Citation42,43 Epithelial tight junctions are critical for the prevention of HIV transmission through mucosal epithelia.Citation4
HIV-induced upregulation of proinflammatory cytokines leads to disruption of epithelial barriers
In vitro studies using polarized endocervical, ectocervical, intestinal and oral epithelia showed that direct interaction of HIV with the apical (mucosal) surface of epithelial cells disrupts tight junction proteins (claudins 1 and 4, occludin and ZO-1) and results in increased paracellular permeability Citation19,24 (). Treatment of polarized epithelial cells with purified HIV envelope protein gp120 disrupts tight junctions,Citation19,24,Citation26 indicating that the interaction of viral envelope with the mucosal surface is critical for the disruption of epithelial junctions and paracellular penetration by HIV.
HIV-induced disruption of intestinal and endocervical junctions is associated with upregulation of proinflammatory cytokines.Citation19 HIV exposure to intestinal epithelial cells induces expression of tumor necrosis factor α (TNF-α) and interleukins (IL)-6 and IL-8. HIV interaction with endometrial cells also increases expression of TNF-α, IL-1β and IL-6. HIV gp120 interacts with Toll-like receptors (TLR)2 and TLR4 on endometrial and endocervical epithelial cells, leading to NF-κB activation, which upregulates expression of cytokines including TNF-α.Citation44 gp120 binding to HSPGs is important in the interaction between gp120 and TLRs and in the activation of NF-κB. Interestingly, activated TNF-α-stimulated NF-κB signaling inhibits expression of ZO-1.Citation45 Thus, it is possible that HIV-associated induction of expression of TNF-α and activation of NF-κB may maintain each other's active status, leading to substantial disruption of epithelial junctions.
HIV-associated elevation of proinflammatory cytokines, including TNF-α, causes disruption of intestinal and endothelial epithelia by actin cytoskeletal contraction and internalization of tight junction proteins.Citation45-47 TNF-α induces activation of myosin light chain kinase (MLCK), which activates the phosphorylation of myosin light chain (MLC), leading to reorganization of the membrane-associated actin/myosin cytoskeleton and tight junction remodeling. Activation of MLC induces internalization of junctional proteins, including JAM-1, occludin and claudins 1 and 4, leading to the disappearance of junctional proteins from cell border and junctional areasCitation46-48 (). In vivo studies showed that TNF-α-induced MLCK activation triggers caveolin-1-dependent internalization of occludin and disassembly of ZO-1 from junctions of mouse intestinal cells.Citation49
HIV-induced expression of proinflammatory cytokines, particularly TNF-α, in the oral, intestinal, endothelial and renal epithelia may also cause disruption of epithelial junctions by an apoptotic mechanism, i.e., activation of matrix metalloproteinase (MMP)-9 and caspase-3, which may digest junctional proteins and disrupt cell junctionsCitation50-54 (). Activation of MMP-2 and -9 is also induced by HIV gp120 binding to mannose receptor on vaginal epithelial cells.Citation55
Interleukins IL-1, IL-1β, IL2, IL-4 and IL-13 also play a role in the disruption of tight junctions in epithelial cells. In mouse intestinal epithelial cells, IL-1β induces paracellular permeability through an increase in MLCK mRNA expression and induction of NF-κB activation.Citation56 IL-2 induces intestinal epithelial permeability by induction of claudin-2 expression and activation of the Jun amino-terminal kinases (JAK) signaling cascade.Citation57 In epithelial cells, claudin-2 expression induces the formation of paracellular pores within the tight junctions, leading to an increase in the paracellular permeability of mucosal epithelium.Citation58 IL-4 and IL-13 reduce the barrier function of lung epithelial cells through activation of phosphatidylinositol 3-kinase (PI3K) and JAK signaling pathways.Citation59 HIV-associated induction of expression of these cytokines in mucosal epithelium may lead to disruption of epithelial junctions and subsequent paracellular virus entry.
HIV-induced disruption of epithelial junctions by MAPK activation
HIV gp120-induced disruption of oral and corneal epithelial junctions is associated with activation of MAPK.Citation24,60 HIV gp120 interacts with GalCer, CCR5 and/or CXCR4 on the oral, genital and intestinal epithelial surface.Citation1,3,Citation6,7,Citation14-18,Citation61 HIV gp120 binding to GalCer, CCR5 and CXCR4 induces intracellular calcium elevation and activation of MAPK signaling.Citation62-65 This causes disruption of epithelial and endothelial junctions by reducing the expression of ZO-1, occludin and claudins 1, 3 and 4 Citation65-70 (). In corneal and kidney epithelial cells, activation of MAPK also reduces tyrosine phosphorylation of occludin and ZO-1, which is critical for the formation of functional tight junctions.Citation60,69 Activation of MAPK p38 reduces the expression of occludin and ZO-1 in gastric epithelial cells.Citation71 In intestinal epithelial cells, a TNF-α-induced increase in tight junction permeability is required for activation of the extracellular signal-regulated kinase (ERK)1/2, indicating that HIV-induced MAPK activation may also contribute to TNF-α-induced disruption of epithelial junctions.Citation72
HIV-associated disruption of tight junctions via proteasomal degradation of ZO-1 and ZO-2
HIV gp120 induces disruption of tight junctions of endothelial cells through activation of proteasome-mediated degradation of ZO-1 and ZO-2.Citation73 HIV-1 gp120-induced degradation of ZO1/2 proteins is neutralized by treatment of cells with the proteasome inhibitor lactacystin, confirming the specific role of proteasomes in degradation of tight junction proteins. The molecular mechanisms of proteasome- mediated degradation of ZO1/2 proteins are unknown.
Role of HIV/AIDS-Associated disruption of mucosal epithelia in disease progression and spread of other viral pathogens
Interaction of HIV and viral proteins gp120 and tat with mucosal epithelial cells induces disruption of epithelial junctions
In HIV-infected individuals with AIDS manifestation, tight junctions in oral, intestinal and genital mucosal epithelia are disrupted, leading to barrier impairment.Citation26,74-76 HIV infection is associated with severe impairment of the barrier function of intestinal mucosal epithelium, which leads to diarrhea and malabsorption.Citation77-79 Analysis of the epithelial barrier function of duodenal biopsy samples from HIV-infected patients with diarrhea shows barrier defects of mucosal epithelium, including reduction of transepithelial resistance (TER) and increase of paracellular leakage.Citation80 As discussed above, the interaction of viral envelope gp120 with mucosal epithelial cells may induce disruption of epithelial tight junctions by various mechanisms. The HIV transactivator protein tat, which is expressed in HIV-infected patients, also induces disruption of tight junctions. HIV-1 tat contains the tripeptide arginine–glycine–aspartic acid, and its interaction with α5β1, α5β3 and αVβ3 integrins activates ras-dependent MAPK signaling,Citation81-85 leading to disruption of tight junctions of endothelial and epithelial cells.Citation24,26,Citation66-68,Citation86,87
Prolonged interaction of HIV-1 tat with oral epithelial cells for 3 to 5 days leads to constitutive activation of ERK1/2 MAPK and substantial disruption of ZO-1, occludin and claudin-1 Citation26 (). In retinal pigment epithelial cells, HIV tat induces activation of ERK1/2 and NF-κB signaling and reduces expression of claudins 1, 3 and 4.Citation66 In endothelial cells, HIV-1 tat-induced activation of MAPK is responsible for the reduction of occludin, ZO-1 and ZO-2 expression.Citation67,68 The active status of MAPK signaling prevents the association of ZO-1, occludin and claudin-1 with the cell-to-cell contact areas and thus inhibits the formation of tight junctions in canine kidney epithelial cells.Citation69 In endothelial cells, oxidative stress-associated MAPK activation downregulates occludin expression.Citation70 In astrocytes, HIV-1 tat-activated MAPK and NF-κB signaling induces MMP-9 expression,Citation88 suggesting that tat may also activate MMP-9 in epithelial cells, leading to the degradation of epithelial junctions. In monocytes/macrophages, HIV-1 tat interacts with TLR4 and induces expression of TNF-α, IL-6 and IL-8 via NF-κB activation,Citation89,90 suggesting that tat-induced disruption of epithelial junctions may occur by a similar mechanism. HIV-1 tat increases the accumulation of ZO-1 in the nucleus via Rho signaling and reduces the development of tight junctions in endothelial cells.Citation91
If HIV infection is indeed a biologically relevant contributor to the disruption of mucosal epithelial junctions, then HIV virions and proteins should be present in the mucosal environment. Accumulating evidence indicates that HIV-infected lymphocytes, macrophages and LC/DC, and cell-free HIV-1 virions can be detected in the oral and genital mucosal epithelium, as well as in the saliva and cervicovaginal secretions of HIV-positive individuals.Citation26,92-99 Furthermore, HIV-1 tat and gp120 are detected in blood and saliva,Citation26,100-103 and HIV tat- and gp120-expressing CD4+ lymphocytes, macrophages and LC are detected in the oral and anogenital epithelia of HIV-infected individuals.Citation26 HIV gp120 is detected in submucosal lymphoid tissues and in plasma,Citation102-104 and HIV-infected CD4+ lymphocytes in the mucosa-associated lymphoid tissue of the gut are localized beneath intestinal mucosa.Citation105,106
HIV-infected lymphocytes, macrophages and LC/DC that penetrate the mucosal epithelium may secrete virions and shed and secrete viral proteins gp120 and tat, respectively, in the mucosal environment. Interaction of virions and viral proteins with one or more of their coreceptors—GalCer, CCR5, CXCR4, integrins and/or mannose receptors of mucosal epithelial cells—may lead to disruption of epithelial tight junctions through activation of MAPK, PI3K and/or TLR signaling. Indeed, the presence of HIV tat- and gp120-infected cells in the oral and anal mucosal epithelium is correlated with the disruption of epithelial tight junction proteins ZO-1, occludin and claudin-1.Citation26 HIV infection of CD4+ lymphocytes in gut mucosa is also associated with disruption of intestinal epithelial tight junctions.Citation105-108 TER and expression of claudin-1 in the duodenal epithelium of HIV-infected individuals is reduced.Citation74,75,Citation80 In contrast, expression of claudin-2 is increased in HIV-infected human intestine and in the gut of humanized mice infected with HIV.Citation19,109,Citation110
Role of HIV-associated inflammation in disruption of the mucosal barrier
In HIV/AIDS disease, the upregulation of proinflammatory cytokines may play a critical role in inducing the disruption of mucosal epithelial junctions. It is well known that expression of proinflammatory cytokines, including TNF-α, interferon gamma (IFN-γ), IL-6 and IL-8, is elevated in the blood and various tissues in HIV-infected individuals.Citation111-115 This could be due to the pathogenesis of systemic HIV/AIDS disease, including viral, bacterial, fungal and parasitic opportunistic infections. In addition, HIV tat and gp120 directly or indirectly induce expression of TNF-α and IFN-γ as well as other cytokines in immune and epithelial cells.Citation19,116-120 Secretion of the proinflammatory cytokines TNF-α, IFN-γ, IFN-α, and IL-1β by HIV-infected immune cells induces apoptosis in intestinal epithelial cells, leading to the activation of MMP-2 and -9 and caspase-3 and -6, which digest junctional proteins.Citation51,121-125 Substantial disruption of gut epithelium in HIV-infected cells was also confirmed by detection of zonulin-1 in plasma,Citation108 a marker for disruption of intestinal junctions.Citation126 An increase in claudin-2 expression in inflamed intestinal mucosa forms paracellular pores and therefore is associated with a reduction in the gut barrier.Citation127 HIV infection of intestinal mucosa reduces the expression of small noncoding RNAs, specifically, microRNA. These mRNAs are involved in regulating subsets of gene expression critical for cell differentiation and death, which are important for maintenance of the functional epithelial barrier.Citation128 HIV-associated disruption of intestinal junctions is a complex process that includes direct and indirect roles of HIV infection in the activation of apoptotic and inflammatory signaling.Citation107 Depletion of HIV-infected CD4+ lymphocytes by massive apoptosis and release of MMPs and caspases in the mucosal environment may play a critical role in the disruption of intestinal mucosa.Citation105-108 The disruption of intestinal epithelial junctions allows penetration of commensal gut microbiota and their products into lamina propria and systemic circulation, leading to chronic inflammation and further disruption of gut epithelium, accelerating HIV/AIDS disease progression.Citation129-132
In HIV-infected individuals, the elevation of TNF-α in serum is correlated with the activation of recurrent oral mucosal aphthous ulcers with severe disruption of epithelial junctions. Treatment of these lesions with thalidomide, an inhibitor of TNF-α or anti-TNF-α antibody, leads to their resolution.Citation133,134 These findings suggest that in systemic HIV/AIDS disease, oral epithelia and activated immune cells may express high levels of proinflammatory cytokines such as TNF-α and thereby induce the disruption of epithelial junctions of oral mucosa.
To confirm the role of HIV-associated elevation of proinflammatory cytokines in the disruption of oral epithelial junctions, we examined the expression of 2 critical proinflammatory cytokines, TNF-α and IFN-γ, in oral (buccal) mucosal biopsy samples obtained from HIV-infected and -uninfected individuals. Expression of the tight junction protein occludin and cytokines TNF-α and IFN-γ was evaluated by immunofluorescence assay in oral biopsy samples from 12 HIV-positive and 4 HIV-negative donors. Analysis of occludin expression in samples from all HIV-uninfected individuals showed that approximately 80 to 90% of cells had intact tight junctions with ring-shaped occludin localization (, left panel). However, the oral epithelium of 67% (8/12) of HIV-infected individuals showed that approximately 50 to 90% of epithelial cells lost occludin expression or its ring-shaped localization (, right panels), indicating the disruption of tight junctions. In most cells, the pattern of occudin expression was cytoplasmic and vesicular, suggesting the aberrant internalization of tight junction proteins.
Figure 3. Disruption of tight junctions of HIV-infected oral mucosal epithelia is associated with infiltration of activated lymphocytes, macrophages and natural killer cells expressing proinflammatory cytokines TNF-α and IFN-γ. (A) Buccal biopsy samples from HIV-infected and HIV-uninfected individuals were immunostained for occludin (red). EP, epithelium. (B) Buccal tissues from HIV-infected and HIV-uninfected individuals were coimmunostained for TNF-α or IFN-γ (green) and the markers for T lymphocytes (CD3) and natural killer cells (CD56) (red), respectively. EP, epithelium; LP, lamina propria. (A and B) Cell nuclei were counterstained with TO-PRO-3 iodide (blue). Cells were analyzed using a krypton-argon laser coupled with a Bio-Rad MRC2400 confocal head. The data were analyzed using Laser Sharp software. In panel B, yellow indicates colocalization of TNF-α or IFN-γ immune cell markers. (C) For evaluation of tight junction integrity of oral mucosal epithelium, buccal tissue sections from HIV-infected and HIV-uninfected individuals were immunostained for occludin, and epithelial cells in ring-shaped patterns were counted. For quantitative evaluation of TNF-α-expressing cells, CD3-, CD68-, and CD1a-positive cells were costained for TNF-α. In parallel experiments, CD3- and CD56-positive cells were costained for IFN-γ. Total numbers of TNF-α- and IFN-γ-expressing cells were counted in the mucosal epithelium. Cells were counted in a minimum of 10 separate, randomly chosen fields, and average numbers were presented in mm2. The total number of intraepithelial immune cells expressing TNF-α or IFN-γ was compared with the percentage of epithelial cells expressing a normal pattern of tight junction protein occludin. Statistical analysis was performed using Spearman rank correlation test. Collection of all biopsy tissue samples was approved by the Committee on Human Research of the University of California, San Francisco (IRB approval # H8597-30664-03).
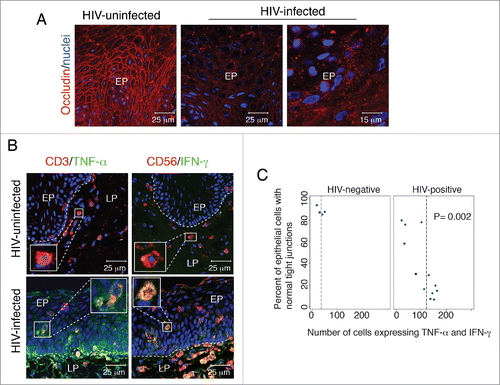
TNF-α expression was detected in T lymphocytes (), macrophages and LC (data not shown), and IFN-γ was detected in natural killer cells () and T lymphocytes (data not shown) in the epithelia and lamina propria of oral biopsy samples. To examine the infiltration of TNF-α- and IFN-γ-positive immune cells into mucosal epithelium, we performed quantitative analysis. To determine the relationship between the infiltration of TNF-α- and IFN-γ-positive immune cells into epithelium and epithelial tight junction disruption, we compared the total number of intraepithelial immune cells expressing TNF-α and IFN-γ with the percentage of epithelial cells exhibiting normal localization of occludin (). Statistical analysis using the Spearman rank correlation test showed that HIV-negative tissues had a lower number of TNF-α- and IFN-γ-expressing cells (8-25 cells/mmCitation2) than did tissues from HIV-positive donors. This result correlated with a high number of oral epithelial cells with intact tight junctions (80-90%), indicating that the lower level of TNF-α and IFN-γ expression in HIV-negative epithelium is not sufficient for tight junction disruption. In contrast, in HIV-positive tissues, the total number of cells expressing TNF-α and IFN-γ was substantially higher (100-245 cells/mmCitation2), and the percentage of intact tight junctions was substantially lower (80-90%), indicating a contribution of TNF-α and IFN-γ to disruption of tight junctions.
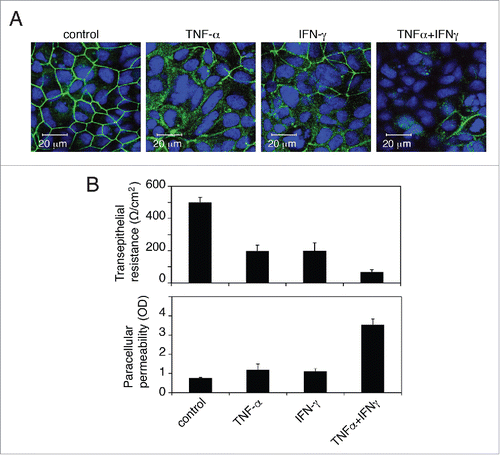
To determine the role of TNF-α and IFN-γ in the disruption of oral epithelial tight junctions, we established monostratified, polarized buccal epithelial cells. Primary buccal epithelial keratinocytes were isolated from buccal biopsies of HIV-negative donors. The purity of the epithelial cells was determined by detection of pankeratin using a cocktail of anti-keratin antibodies containing Ab-1 and Ab-2 (Thermo Fisher Scientific), and only those epithelial cell populations that were 100% positive for keratin were used. Polarized cells were established in 0.45-µm Transwell 2-chamber filter inserts. The polarity of the cells was verified by immunodetection of the tight junction protein occludin and measurement of transepithelial resistance (TER) and paracellular permeability, as described in our previous work.Citation3 Polarized cells were treated with recombinant TNF-α and IFN-γ, independently or in combination, for 48 h. Analysis of the integrity of tight junctions of untreated control cells revealed ring-shaped membrane localization of occludin (), development of a TER of 450 Ω/cmCitation2 (, upper panel) and lack of paracellular leakage (, lower panel). In contrast, in TNF-α- and IFN-γ-treated cells, occludin staining in approximately 40 to 50% of the cells became diffuse cytoplasmic (). These cells also lost their TER by approximately 60% and allowed paracellular leakage of immunoglobulin G (IgG) from the upper chamber of Transwell inserts into the lower chamber (). Furthermore, cells treated with a combination of TNF-α and IFN-γ had a substantial loss of occludin expression, reduction of TER and increase of paracellular leakage (≈ 80 to 90%) compared to untreated control cells. It has been shown that treatment of intestinal epithelial cells with a combination of TNF-α and IFN-γ also synergistically reduces epithelial permeability.Citation135 Together, the data from oral epithelial tissues and polarized cells indicate that HIV-associated upregulation of TNF-α and IFN-γ by infiltrating immune cells may play a critical role in the disruption of oral epithelial tight junctions. The combination of TNF-α and IFN-γ synergistically induced the disruption of tight junctions in oral epithelial cells as they did in intestinal epithelial cells.
Figure 4. Proinflammatory cytokines TNF-α and IFN-γ disrupt tight junctions of oral epithelial cells. (A) Polarized oral epithelial cells were treated with recombinant TNF-α and IFN-γ at 10 ng/ml each, independently and in combination, for 48 h. Cells were immunostained for the tight junction protein occludin (green). Nuclei are stained in blue. Cells were analyzed by confocal microscopy. (B) (Upper panel) Transepithelial resistance was measured with an epithelial Millicell-ERS voltohmmeter. (Lower panel) Paracellular permeability was evaluated by adding horseradish peroxidase-conjugated goat anti-donkey IgG to the upper compartments of Transwell inserts and, after 1 h, photometrically assaying horseradish peroxidase in the medium from the lower compartment using o-phenylenediamine dihydrochloride as the substrate. The values are expressed as optical density (OD, 450 nm). Detection of IgG in the lower chamber indicated leakage of IgG from the upper chamber via disrupted tight junctions. Error bars show the mean ± SE taken from a total of 3 samples.
HIV-associated disruption of mucosal junctions facilitates the penetration and spread of HPV and HSV
HIV/AIDS disease promotes the initiation and progression of multiple bacterial, viral and fungal infections. Multiple oncogenic HPVs, including HPV16 and HVP18, have been identified as the etiological agents of most oral and genital mucosal epithelial cancer. Although HPV vaccination is effective in preventing HPV infection, its value is limited in HIV-positive individuals who have already had multiple exposures to HPV. The incidence of HPV-associated oropharyngeal cancer is increased in HIV-infected individuals, who have about a sixfold greater risk for oropharyngeal and tonsillar cancers than do HIV-uninfected individuals.Citation136 In addition to oral cancer, the incidence of HPV-associated anal and cervical cancer is 80 and 22 times higher, respectively, in HIV-infected individuals than in HIV-uninfected individuals.Citation137
Development of HPV-associated neoplasia is initiated upon HPV entry into basal and parabasal cells of the epithelium. Animal model work suggests that HPV penetration through multiple layers of the stratified squamous epithelium requires a mechanical breach or tear.Citation138 Analysis of oral and anal mucosal epithelia from HIV-infected individuals showed that approximately 60% of tissues have disrupted epithelial junctions; i.e., the epithelium lost its barrier integrity.Citation26 HIV-1-infected CD4+ T lymphocytes, LC and macrophages expressing viral gp120 and tat proteins were detected within the disrupted oral and anal mucosal epithelia. Treatment of polarized oral and anal epithelial cells and tissue explants isolated from HIV-negative individuals in vitro with recombinant HIV-1 tat and/or gp120 proteins caused the disruption of epithelial junctions. Adding HPV-16 pseudovirions (PsV) to disrupted tissues from their mucosal surface led to paracellular penetration of PsV into the epitheliumCitation26 (). HPV-16 PsV entry was observed in the basal/parabasal cells, in which the HPV life cycle is initiated. These findings indicate that HIV-associated disruption of mucosal epithelial junctions facilitates paracellular penetration by oncogenic HPV viruses through strata granulosum and spinosum layers and infection of basal/parabasal cells. This may lead to the subsequent development of HPV-associated neoplasia and may explain, at least in part, the mechanism of increased HPV infection in HIV-infected individuals; i.e., HIV-induced disruption of epithelial junctions may play a critical role in the development of HPV-associated cancer.
Figure 5. HIV/AIDS-associated disruption of mucosal epithelium facilitates HPV and HSV infection. (A) In HIV-positive individuals, HIV-infected CD4+ T lymphocytes, LC/DC and macrophages infiltrate the mucosal epithelium, releasing virions and viral proteins gp120 and tat. Interaction of virions and viral proteins with one or more HIV coreceptors (CCR5, CXCR4, GalCer, HSPG), TLR-2/4, integrins and/or mannose receptor of epithelial cells induces the activation of MAPK, PI3K, TLR and NF-κB signalings, which leads to the expression of proinflammatory cytokines (TNF-α, IFN-γ, IL-1, IL-1β, IL-2, IL-6, IL-8, and IL-13 ), MMPs (MMP-2 and -9) and caspase (caspase-3 and -6). This mediates the disruption of epithelial tight and adherens junctions by downregulating their expression, aberrant internalization and/or degradation. HIV-associated disruption of tight junctions of oral and genital epithelium facilitates paracellular penetration by HPV toward basal epithelial cells, where HPV initiates lytic infection and the neoplastic process. (B) HIV-associated immune dysfunction leads to the reactivation of HSV-1 from sensory neurons. Reactivated virus infects epithelial cells through the interaction of viral envelope glycoprotein D with its receptor nectin-1. Nectin-1 is sequestered within the intact adherens junctions region of lateral membranes of epithelial cells. HIV-induced disruption of adherens junctions liberates nectin-1 from its sequestered areas, binding HSV glycoprotein D and thereby promoting HSV infection and cell-to-cell spread from neurons to epithelial cells and within the oral epithelium. This leads to the rapid progression of HSV-mediated mucosal lesions and ulcers. GR, granulosum; SP, spinosum; BL, basal.
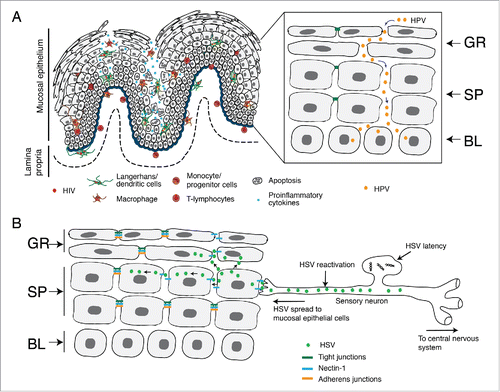
HSV-1 is a common viral pathogen that causes multiple oral and anogenital disorders, such as ulcers and necrotic lesions. Although the increased risk of HSV infection and reactivation may be mediated in part by HIV-induced immune dysfunction, prolonged interaction of the HIV proteins tat and gp120 and cell-free HIV virions with oral epithelial cells induces the disruption of tight and adherens junctions and thereby facilitates the rapid spread of HSV within the epithelium.Citation24 This is due to the liberation of HSV receptor nectin-1 that is hidden within the epithelial junctions. Nectin-1, an adhesion protein that binds to HSV glycoprotein D, facilitates the entry of virions into epithelial cells and cell-to-cell spread of progeny virions.Citation139 Nectin-1 is sequestered in the intercellular junctions, limiting the access of HSV.Citation140 HIV-associated disruption of adherens junctions exposes sequestered nectin-1 to HSV envelope glycoprotein D.Citation24 Exposure of nectin-1 facilitates binding of HSV-1 glycoprotein D, which substantially increases HSV-1 infection in epithelial cells with disrupted junctions over that of cells with intact junctions. HIV-induced liberation of nectin-1 substantially promotes cell-to-cell spread of HSV to uninfected oral epithelial cells.Citation24 In most individuals with normal immune surveillance, HSV is latent in sensory neurons;Citation141 however, HIV/AIDS disease may induce reactivation of HSV in neurons.Citation142 HIV-mediated release of nectin-1 from disrupted adherens junctions may also increase the spread of HSV-1 from neurons to epithelial cells. Thus, HIV-associated disruption of oral epithelial junctions potentiates HSV-1 infection and its paracellular and cell-to-cell spread within the oral mucosal epithelium (). This could be one of the possible mechanisms of the rapid development of HSV-associated oral lesions in HIV-infected individuals.
Assembled intercellular tight junctions may hide receptors for several viruses. The tight junction proteins occludin and claudins 1, 6 and 9 serve as receptors for hepatitis C virus (HCV).Citation143,144 Coxsackievirus and adenovirus receptor (CAR) is also associated with tight junctions.Citation145 In addition, entry of coxsackievirus also requires occludin.Citation146 Reoviruses use JAM-1,Citation147 and JAM-1, occludin and ZO-1 are important for entry of rotaviruses.Citation148 All these receptors are sequestered in intercellular junctions, and disruption of junctions exposes them to the appropriate viruses, leading to their rapid entry and spread. It is possible that HIV-associated disruption of epithelial junctions may occur in multiple organs that have epithelial tissues, including lung, kidney and liver, and this may liberate receptors for multiple pathogenic human viruses. For example, accumulating evidence indicates that HIV and HCV coininfection is becoming more common in HIV-infected individuals with the development of mutual pathogenesis for both infections.Citation149 HIV-induced liberation of occludin and claudins may facilitate HCV infection and spread, promoting the progression of liver disease, including HCV-associated cirrhosis and neoplasia.
Conclusions and future perspectives
Healthy oral, intestinal and genital mucosal epithelia with tight and adherens junctions have highly efficient biological barrier functions that prevent paracellular penetration by viral pathogens, including HIV. Upon initial interaction of HIV with oral, intestinal and genital mucosal surfaces, viral envelope protein gp120 interacts with one or more viral coreceptors (GalCer, CCR5, CXCR4, HSPG) as well as integrins, TLR2/4 and mannose receptors on the mucosal surface. This interaction activates multiple signaling pathways, including MAPK, PI3K, NF-κB and/or apoptosis. These events lead to the induction of expression of proinflammatory cytokines, which disrupt epithelial junctions by reducing the expression of tight junction proteins ZO-1, occludin, and claudins and/or their aberrant internalization. Apoptosis-associated activation of MMPs and caspases may cause degradation of junctional proteins. Disruption of tight junctions opens the intercellular space between epithelial cells, allowing paracellular penetration by HIV and other pathogens.
During systemic HIV/AIDS disease, the infiltration of HIV-infected CD4+ T lymphocytes, LC/DC and macrophages into the oral, intestinal and genital mucosal environment causes the secretion of HIV virions and viral proteins gp120 and tat, which interact with epithelial cells through HIV coreceptors and integrins. This may activate MAPK, PI3K, inflammatory and/or apoptotic signalings, leading to disruption of tight and adherens junctions. Disruption of the barrier integrity of mucosal epithelium facilitates bacterial translocation and penetration of their product via the paracellular gate, further activating HIV-associated inflammation and disease progression. HIV-associated disruption of oral and genital epitheliia facilitates penetration by HPV into basal and parabasal layers of epithelial cells, which initiates lytic HPV infection, increasing the risk of oral and genital cancer. HIV-associated disruption of tight and adherens junctions of mucosal epithelium may liberate the hidden HSV-1 and -2 receptor nectin-1 in the assembled junctions. This may promote the rapid dissemination of HSV-1 and -2 in the oral and genital epithelia, causing ulcers and necrotic lesions. HIV-induced disruption of epithelial junctions may release sequestered receptors for other viruses, including HCV, reoviris, rotavirus, coxsackievirus and adenovirus, promoting their infection and spread.
HIV-associated disruption of epithelial junctions is a complex process involving the activation of multiple signalings. Therefore, more molecular studies are necessary to identify the target molecules for efficient therapeutic and prophylactic approaches to repair and to reassemble the disrupted junctions and prevent their further disruption. The generation of efficient drugs against HIV-activated MAPK, PI3K, NF-kB, apoptosis and inflammatory responses may protect mucosal epithelia from disruption and could substantially reduce the frequency of new HIV, HPV and HSV infection, as well as infection by other viral, bacterial, and other pathogens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank Drs. Deborah Greenspan and Piri Veluppillai for providing biopsy samples, Dr. Joel Palefsky for discussion, and Rossana Herrera for excellent technical assistance.
Funding
This project was supported by the NIH/NIDCR R01DE023315 grant.
References
- Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 2007; 81, 395-405; PMID:17050597; http://dx.doi.org/10.1128/JVI.01303-06
- Howell AL, Asin SN, Yeaman GR, Wira CR HIV-1 infection of the female reproductive tract. Current HIV/AIDS reports 2005; 2, 35-8; PMID:16091247; http://dx.doi.org/10.1007/s11904-996-0007-0
- Tugizov, SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 2011; 409:211-22; PMID:21056450; http://dx.doi.org/10.1016/j.virol.2010.10.004
- Tugizov, SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol 2012; 86:2556-70; PMID:22205732; http://dx.doi.org/10.1128/JVI.06578-11
- Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 1999; 117:359-67; PMID:10419917; http://dx.doi.org/10.1053/gast.1999.0029900359
- Liu X, Zha J, Chen H, Nishitani J, Camargo P, Cole SW, Zack JA. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. J Virol 2003; 77:3470-6; PMID:12610122; http://dx.doi.org/10.1128/JVI.77.6.3470-3476.2003
- Herrera R, Morris M, Rosbe K, Feng Z, Weinberg A, Tugizov S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2015; 487:172-87 ; PMID:26539799; http://dx.doi.org/10.1016/j.virol.2015.09.025
- Zheng J, Xie Y, Campbell R, Song J, Wang RQ, Chiu R, Berenson J, Razi M, Massachi S, Yang OO, et al. gp120-independent HIV infection of cells derived from the female reproductive tract, brain, and colon. J Acquir Immune Defic Syndr 2006; 43:127-36; PMID:16951651; http://dx.doi.org/10.1097/01.qai.0000228149.17669.08
- Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol 1997; 71:3498-506; PMID:9094621
- Micsenyi AM, Zony C, Alvarez RA, Durham ND, Chen BK, Klotman ME. Postintegration HIV-1 infection of cervical epithelial cells mediates contact-dependent productive infection of T cells. J Infect Dis 2013; 208:1756-67; PMID:23908485; http://dx.doi.org/10.1093/infdis/jit362
- Liu R, Huang L, Li J, Zhou X, Zhang H, Zhang T, Lei Y, Wang K, Xie N, Zheng Y, et al. HIV Infection in gastric epithelial cells. J Infect Dis 2013; 208:1221-30; PMID:23852124; http://dx.doi.org/10.1093/infdis/jit314
- Vacharaksa A, Asrani AC, Gebhard KH, Fasching CE, Giacaman RA, Janoff EN, Ross KF, Herzberg MC. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology 2008; 5:66; PMID:18637194; http://dx.doi.org/10.1186/1742-4690-5-66
- Dezzutti CS, Guenthner PC, Cummins JE Jr, Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis 2001; 183:1204-13; PMID:11262202; http://dx.doi.org/10.1086/319676
- Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog 2013; 9:e1003776; PMID:24278022; http://dx.doi.org/10.1371/journal.ppat.1003776
- Bomsel M Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 1997; 3:42-7; PMID:8986739; http://dx.doi.org/10.1038/nm0197-42
- Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med 2002; 8:150-6; PMID:11821899; http://dx.doi.org/10.1038/nm0202-150
- Kohli A, Islam A, Moyes DL, Murciano C, Shen C, Challacombe SJ, Naglik JR. Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. PloS One 2014; 9:e98077; PMID:24857971; http://dx.doi.org/10.1371/journal.pone.0098077
- Kinlock BL, Wang Y, Turner TM, Wang C, Liu B Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PloS One 2014; 9:e96760; PMID:24830293; http://dx.doi.org/10.1371/journal.pone.0096760
- Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852; PMID:20386714; http://dx.doi.org/10.1371/journal.ppat.1000852
- Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol 2013; 87:11388-400; PMID: 23966398; http://dx.doi.org/10.1128/JVI.01377-13
- Dinh, MH, Anderson MR, McRaven MD, Cianci GC, McCoombe SG, Kelley ZL, Gioia CJ, Fought AJ, Rademaker AW, Veazey RS, et al. Visualization of HIV-1 interactions with penile and foreskin epithelia: clues for female-to-male HIV transmission. PLoS Pathog 2015; 11:e1004729; PMID:25748093; http://dx.doi.org/10.1371/journal.ppat.1004729
- Maher D, Wu X, Schacker T, Larson M, Southern P A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. J Infect Dis 2004; 190:1989-97; PMID:15529264; http://dx.doi.org/10.1086/425423
- Izquierdo-Useros N, Lorizate M, McLaren PJ, Telenti A, Kräusslich HG, Martinez-Picado J. HIV-1 capture and transmission by dendritic cells: the role of viral glycolipids and the cellular receptor Siglec-1. PLoS Pathog 2014; 10:e1004146; PMID:25033082; http://dx.doi.org/10.1371/journal.ppat.1004146
- Sufiawati I, Tugizov SM. HIV-Associated Disruption of Tight and Adherens Junctions of Oral Epithelial Cells Facilitates HSV-1 Infection and Spread. PloS One 2014; 9:e88803; PMID:24586397; http://dx.doi.org/10.1371/journal.pone.0088803
- Ferreira VH, Nazli A, Dizzell SE, Mueller K, Kaushic C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PloS One 2015; 10:e0124903; PMID:25856395; http://dx.doi.org/10.1371/journal.pone.0124903
- Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, Pilcher CD, Shiboski CH, Jay N, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013; 446:378-88; PMID: 24074602; http://dx.doi.org/10.1016/j.virol.2013.08.018
- Schluter H, Wepf R, Moll I, Franke WW Sealing the live part of the skin: the integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur J Cell Biol 2004; 83:655-65; PMID:15679110; http://dx.doi.org/10.1078/0171-9335-00434
- Langbein L, Pape UF, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW. Tight junction-related structures in the absence of a lumen: occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol 2003; 82:385-400; PMID:14533737; http://dx.doi.org/10.1078/0171-9335-00330
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 2002; 81:419-35; PMID:12234014; http://dx.doi.org/10.1078/0171-9335-00270
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 2002; 81:253-63; PMID:12067061; http://dx.doi.org/10.1078/0171-9335-00244
- Takano, K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, Sawada N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem 2005; 53:611-9; PMID: 15872054; http://dx.doi.org/10.1369/jhc.4A6539.2005
- Blaskewicz CD, Pudney J, Anderson DJ Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod 2011; 85:97-104; PMID:21471299; http://dx.doi.org/10.1095/biolreprod.110.090423
- Go, M, Kojima T, Takano K, Murata M, Ichimiya S, Tsubota H, Himi T, Sawada N. Expression and function of tight junctions in the crypt epithelium of human palatine tonsils. J Histochem Cytochem 2004; 52:1627-38; PMID:15557217; http://dx.doi.org/10.1369/jhc.4A6339.2004
- Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc 2003; 36:147-56; PMID:14505058; http://dx.doi.org/10.1007/s00795-003-0219-y
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 2007; 127:2525-32; PMID:17934504; http://dx.doi.org/10.1038/sj.jid.5700865
- Naik UP, Eckfeld K Junctional adhesion molecule 1 (JAM-1). J Biol Regul Homeost Agents 2003; 17:341-7; PMID:15065765
- Hartsock A, Nelson WJ Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica et Biophysica Acta 2008; 1778:660-9; PMID:17854762; http://dx.doi.org/10.1016/j.bbamem.2007.07.012
- Rodriguez-Boulan E, Macara IG Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 2014; 15:225-42; PMID:24651541; http://dx.doi.org/10.1038/nrm3775
- UNAIDS. Criminalisation of HIV non-disclosure, exposure and transmission: scientific, medical, legal and human right issues. Genewa, Switzeland (2011).
- Page-Shafer K, Sweet S, Kassaye S, Ssali, C. (C2) Saliva, breast milk, and mucosal fluids in HIV transmission. Adv Dent Res 2006; 19:152-7; PMID:16672566; http://dx.doi.org/10.1177/154407370601900127
- UNAIDS. Global report on the global AIDS epidemic 2013. (2013).
- Campo J, Perea MA, del Romero J, Cano J, Hernando V, Bascones A. Oral transmission of HIV, reality or fiction? An update. Oral Dis 2006; 12:219-28; PMID:16700731; http://dx.doi.org/10.1111/j.1601-0825.2005.01187.x
- Baggaley RF, Dimitrov D, Owen BN, Pickles M, Butler AR, Masse B, Boily MC. Heterosexual anal intercourse: a neglected risk factor for HIV? Am J Reprod Immunol 2013; 69(Suppl 1):95-105; PMID:23279040; http://dx.doi.org/10.1111/aji.12064
- Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 2013; 191:4246-58; PMID:24043886; http://dx.doi.org/10.4049/jimmunol.1301482
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 2004; 286:G367-376; PMID:14766535; http://dx.doi.org/10.1152/ajpgi.00173.2003
- Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 2005; 288:G422-430; PMID: 15701621; http://dx.doi.org/10.1152/ajpgi.00412.2004
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 2006; 290:G496-504; PMID:16474009; http://dx.doi.org/10.1152/ajpgi.00318.2005
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003; 171:6164-72; PMID:14634132; http://dx.doi.org/10.4049/jimmunol.171.11.6164
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 2010; 189:111-26; PMID:20351069; http://dx.doi.org/10.1083/jcb.200902153
- Acheampong EA, Parveen Z, Muthoga LW, Wasmuth-Peroud V, Kalayeh M, Bashir A, Diecidue R, Mukhtar M, Pomerantz RJ. Molecular interactions of human immunodeficiency virus type 1 with primary human oral keratinocytes. J Virol 2005; 79:8440-53; PMID:15956588; http://dx.doi.org/10.1128/JVI.79.13.8440-8453.2005
- Gitter AH, Bendfeldt K, Schmitz H, Schulzke JD, Bentzel CJ, Fromm M. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann N Y Acad Sci 2000; 915:193-203; PMID:11193576; http://dx.doi.org/10.1111/j.1749-6632.2000.tb05242.x
- Leone AK, Chun JA, Koehler CL, Caranto J, King JM. Effect of proinflammatory cytokines, tumor necrosis factor-alpha and interferon-gamma on epithelial barrier function and matrix metalloproteinase-9 in Madin Darby canine kidney cells. Cell Physiol Biochem 2007; 19:99-112; PMID:17310104; http://dx.doi.org/10.1159/000099198
- Huet E, Vallée B, Delbé J, Mourah S, Prulière-Escabasse V, Tremouilleres M, Kadomatsu K, Doan S, Baudouin C, Menashi S, et al. EMMPRIN modulates epithelial barrier function through a MMP-mediated occludin cleavage: implications in dry eye disease. Am J Pathol 2011; 179:1278-86; PMID:21777561; http://dx.doi.org/10.1016/j.ajpath.2011.05.036
- Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci 2004; 117:2097-107; PMID:15054114; http://dx.doi.org/10.1242/jcs.01071
- Fanibunda SE, Modi DN, Gokral JS, Bandivdekar AH. HIV gp120 binds to mannose receptor on vaginal epithelial cells and induces production of matrix metalloproteinases. PloS One 2011; 6:e28014; PMID:22132194; http://dx.doi.org/10.1371/journal.pone.0028014
- Al-Sadi R, Guo S, Dokladny K, Smith MA, Ye D, Kaza A, Watterson DM, Ma TY. Mechanism of interleukin-1beta induced-increase in mouse intestinal permeability in vivo. J Interferon Cytokine Res 2012; 32:474-84; PMID: 22817402; http://dx.doi.org/10.1089/jir.2012.0031
- Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, Ma TY. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One 2014; 9:e85345; PMID:24662742; http://dx.doi.org/10.1371/journal.pone.0085345
- Schumann M, Kamel S, Pahlitzsch ML, Lebenheim L, May C, Krauss M, Hummel M, Daum S, Fromm M, Schulzke JD. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci 2012; 1258:43-51; PMID:22731714
- Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, Georas SN. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013; 1:e24333; PMID:24665390; http://dx.doi.org/10.4161/tisb.24333
- Wang Y, Zhang J, Yi XJ, Yu FS. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res 2004; 78:125-36; PMID:14667834; http://dx.doi.org/10.1016/j.exer.2003.09.002
- Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS 1997; 11:1311-8; PMID:9302439; http://dx.doi.org/10.1097/00002030-199711000-00004
- Maresca M, Mahfoud R, Garmy N, Kotler DP, Fantini J, Clayton F. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci 2003; 10:156-66; PMID:12566994; http://dx.doi.org/10.1007/BF02256007
- Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol 2003; 74:676-82; PMID:12960231; http://dx.doi.org/10.1189/jlb.0503206
- Del Corno M, Liu QH, Schols D, de Clercq E, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 2001; 98:2909-16; PMID:11698270; http://dx.doi.org/10.1182/blood.V98.10.2909
- Dayanithi G, Yahi N, Baghdiguian S, Fantini J Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: a putative mechanism for HIV-1 enteropathy. Cell Calcium 1995; 18:9-18; PMID:7585886; http://dx.doi.org/10.1016/0143-4160(95)90041-1
- Bai L, Zhang Z, Zhang H, Li X, Yu Q, Lin H, Yang W. HIV-1 Tat protein alter the tight junction integrity and function of retinal pigment epithelium: an in vitro study. BMC Infect Dis 2008; 8:77; PMID:18538010; http://dx.doi.org/10.1186/1471-2334-8-77
- Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab 2005; 25:1325-35; PMID:15829913; http://dx.doi.org/10.1038/sj.jcbfm.9600125
- Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci 2008; 28:7788-96; PMID:18667611; http://dx.doi.org/10.1523/JNEUROSCI.0061-08.2008
- Chen Y, Lu Q, Schneeberger EE, Goodenough DA Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 2000; 11:849-62; PMID:10712504; http://dx.doi.org/10.1091/mbc.11.3.849
- Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmári E, Traweger A, Wejksza K, Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol 2005; 25:129-39; PMID:15962510; http://dx.doi.org/10.1007/s10571-004-1378-7
- Wu HL, Gao X, Jiang ZD, Duan ZT, Wang SK, He BS, Zhang ZY, Xie HG. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology 2013; 304:41-8; PMID:23220562; http://dx.doi.org/10.1016/j.tox.2012.11.020
- Al-Sadi R, Guo S, Ye D, Ma TY TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 2013; 183:1871-84; PMID:24121020; http://dx.doi.org/10.1016/j.ajpath.2013.09.001
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol 2008; 14:186-95; PMID:18569453; http://dx.doi.org/10.1080/13550280801993630
- Epple HJ, Allers K, Tröger H, Kühl A, Erben U, Fromm M, Zeitz M, Loddenkemper C, Schulzke JD, Schneider T. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 2010; 139:1289-300; PMID:20600014; http://dx.doi.org/10.1053/j.gastro.2010.06.065
- Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009; 58:220-7; PMID:18936106; http://dx.doi.org/10.1136/gut.2008.150425
- Assimakopoulos SF, Dimitropoulou D, Marangos, M, Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection 2014; 42:951-9; PMID: 25070877; http://dx.doi.org/10.1007/s15010-014-0666-5
- Kapembwa MS, Fleming SC, Orr M, Wells C, Bland M, Back D, Griffin GE. Impaired absorption of zidovudine in patients with AIDS-related small intestinal disease. Aids 1996; 10:1509-14; PMID:8931785; http://dx.doi.org/10.1097/00002030-199611000-00008
- Obinna FC, Cook G, Beale T, Dave S, Cunningham D, Fleming SC, Claydon E, Harris JW, Kapembwa MS. Comparative assessment of small intestinal and colonic permeability in HIV-infected homosexual men. Aids 1995; 9:1009-16; PMID:8527072; http://dx.doi.org/10.1097/00002030-199509000-00005
- Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, Griffin GE. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991; 81:327-34; PMID:1655333; http://dx.doi.org/10.1042/cs0810327
- Stockmann M, Fromm M, Schmitz H, Schmidt W, Riecken EO, Schulzke JD. Duodenal biopsies of HIV-infected patients with diarrhoea exhibit epithelial barrier defects but no active secretion. Aids 1998; 12:43-51; PMID:9456254; http://dx.doi.org/10.1097/00002030-199801000-00006
- Toschi E, Bacigalupo I, Strippoli R, Chiozzini C, Cereseto A, Falchi M, Nappi F, Sgadari C, Barillari G, Mainiero F, et al. HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Mol Biol Cell 2006; 17:1985-94; PMID:16436505; http://dx.doi.org/10.1091/mbc.E05-08-0717
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, Modesti A, Nair BC, Cafaro A, Stürzl M, et al. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 1999; 94:663-72; PMID:10397733
- Watson K, Edwards RJ HIV-1-trans-activating (Tat) protein: both a target and a tool in therapeutic approaches. Biochem Pharmacol 1999; 58:1521-28; PMID:10535742; http://dx.doi.org/10.1016/S0006-2952(99)00209-9
- Urbinati C, Mitola S, Tanghetti E, Kumar C, Waltenberger J, Ribatti D, Presta M, Rusnati M. Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 2005; 25:2315-20; PMID:16166568; http://dx.doi.org/10.1161/01.ATV.0000186182.14908.7b
- Vogel BE, Lee SJ, Hildebrand A, Craig W, Pierschbacher MD, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol 1993; 121:461-8; PMID:7682219; http://dx.doi.org/10.1083/jcb.121.2.461
- Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab 2005; 25:1159-70; PMID:15815581; http://dx.doi.org/10.1038/sj.jcbfm.9600115
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res 2003; 74:255-65; PMID:14515355; http://dx.doi.org/10.1002/jnr.10762
- Ju SM, Song HY, Lee JA, Lee SJ, Choi SY, Park J. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Exp Mol Med 2009; 41:86-93; PMID:19287189; http://dx.doi.org/10.3858/emm.2009.41.2.011
- Ben Haij N, Leghmari K, Planes R, Thieblemont N, Bahraoui E HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology 2013; 10:123; PMID:24165011; http://dx.doi.org/10.1186/1742-4690-10-123
- Ben Haij N, Planès R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-kappaB Pathway. PloS One 2015; 10:e0129425; PMID:26090662; http://dx.doi.org/10.1371/journal.pone.0129425
- Zhong Y, Zhang B, Eum SY, Toborek M HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci 2012; 32:143-50; PMID: 22219277; http://dx.doi.org/10.1523/JNEUROSCI.4266-11.2012
- Rodriguez-Inigo E, Jiménez E, Bartolomé J, Ortiz-Movilla N, Bartolomé Villar B, José Arrieta J, Manzarbeitia F, Carreño V. Detection of human immunodeficiency virus type 1 RNA by in situ hybridization in oral mucosa epithelial cells from anti-HIV-1 positive patients. J Med Virol 2005; 77:17-22; PMID:16032727; http://dx.doi.org/10.1002/jmv.20409
- Chou LL, Epstein J, Cassol SA, West DM, He W, Firth JD. Oral mucosal Langerhans' cells as target, effector and vector in HIV infection. J Oral Pathol Med 2000; 29:394-402; PMID:10972348; http://dx.doi.org/10.1034/j.1600-0714.2000.290805.x
- Kakizawa J, Ushijima H, Oka S, Ikeda Y, Schröder HC, Müller WE. Detection of human immunodeficiency virus-1 DNA, RNA and antibody, and occult blood in inactivated saliva: availability of the filter paper disk method. Acta Paediatr Jpn 1996; 38:218-23; PMID:8741309; http://dx.doi.org/10.1111/j.1442-200X.1996.tb03473.x
- Maticic M, Poljak M, Kramar B, Tomazic J, Vidmar L, Zakotnik B, Skaleric U. Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J Dent Res 2000; 79:1496-501; PMID:11005734; http://dx.doi.org/10.1177/00220345000790071101
- Qureshi MN, Barr CE, Hewlitt I, Boorstein R, Kong F, Bagasra O, Bobroski LE, Joshi B. Detection of HIV in oral mucosal cells. Oral Dis 1997; 3(Suppl 1):S73-7; PMID:9456662; http://dx.doi.org/10.1111/j.1601-0825.1997.tb00380.x
- Nuovo GJ, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol 1993; 143:40-8; PMID:8317555
- Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, Plummer F, Ndinya-Achola J, Roberts PL, Hillier S, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA 1993; 269:2860-4; PMID:8497089; http://dx.doi.org/10.1001/jama.1993.03500220046024
- Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis 2010; 202:1543-52; PMID:20925530; http://dx.doi.org/10.1086/656720
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995; 375:497-500; PMID:7539892; http://dx.doi.org/10.1038/375497a0
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A 2000; 97:11466-71; PMID:11027346; http://dx.doi.org/10.1073/pnas.97.21.11466
- Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, Kornfeld H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr 1992; 5:251-6; PMID:1740750; http://dx.doi.org/10.1097/00126334-199203000-00005
- Rychert J, Strick D, Bazner S, Robinson J, Rosenberg E. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res Hum Retroviruses 2010; 26:1139-45; PMID:20722464; http://dx.doi.org/10.1089/aid.2009.0290
- Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis 2009; 200:1050-3; PMID:19698075; http://dx.doi.org/10.1086/605695
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749-59; PMID:15365096; http://dx.doi.org/10.1084/jem.20040874
- Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol 2008; 1:23-30; PMID:19079157; http://dx.doi.org/10.1038/mi.2007.1
- Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633-45; PMID:24138880; http://dx.doi.org/10.1016/j.immuni.2013.10.001
- Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228-38; PMID:24755434; http://dx.doi.org/10.1093/infdis/jiu238
- Smith AJ, Schacker TW, Reilly CS, Haase, AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. J Acquir Immune Defic Syndr 2010; 55:306-15; PMID:20700059; http://dx.doi.org/10.1097/QAI.0b013e3181ecfeca
- Lackner AA, Mohan M, Veazey RS. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology 2009; 136:1965-78; PMID:19462506; http://dx.doi.org/10.1053/j.gastro.2008.12.071
- Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses 2006; 22:757-62; PMID:16910831; http://dx.doi.org/10.1089/aid.2006.22.757
- Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Front Biosci 2004; 9:1073-81; PMID:14977530; http://dx.doi.org/10.2741/1271
- Kobayashi S, Hamamoto Y, Kobayashi N, Yamamoto N. Serum level of TNF alpha in HIV-infected individuals. AIDS 1990; 4:169-70; PMID:2328100; http://dx.doi.org/10.1097/00002030-199002000-00014
- Matsumoto T, Miike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol 1993; 93:149-51; PMID:8348739; http://dx.doi.org/10.1111/j.1365-2249.1993.tb07957.x
- Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, Kishimoto T, Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol 1990; 144:480-4; PMID:2295799
- Schols D, De Clercq E. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J Virol 1996; 70:4953-60; PMID:8764000
- Cheung R, Ravyn V, Wang L, Ptasznik A, Collman, RG Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol 2008; 180:6675-84; PMID:18453587; http://dx.doi.org/10.4049/jimmunol.180.10.6675
- Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol 2005; 78:1016-23; PMID:16081599; http://dx.doi.org/10.1189/jlb.0105056
- Jiang J, Fu W, Wang X, Lin PH, Yao Q, Chen C. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-alpha-activated porcine and human endothelial cells. Cardiovasc Res 87, 366-374 (2010); PMID:20083573; http://dx.doi.org/10.1093/cvr/cvq013
- Mayne, M, Bratanich AC, Chen P, Rana F, Nath A, Power C. HIV-1 tat molecular diversity and induction of TNF-alpha: implications for HIV-induced neurological disease. Neuroimmunomodulation 5, 184-192 (1998); PMID:9730685; http://dx.doi.org/10.1159/000026336
- Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol 2001; 535:541-52; PMID:11533143; http://dx.doi.org/10.1111/j.1469-7793.2001.00541.x
- Stockmann M, Schmitz H, Fromm M, Schmidt W, Pauli G, Scholz P, Riecken EO, Schulzke JD. Mechanisms of epithelial barrier impairment in HIV infection. Ann N Y Acad Sci 2000; 915:293-03; PMID:11193591; http://dx.doi.org/10.1111/j.1749-6632.2000.tb05257.x
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci 2000; 113(Pt 11):2085-90; PMID:10806119
- Schmitz H, Rokos K, Florian P, Gitter AH, Fromm M, Scholz P, Ullrich R, Zeitz M, Pauli G, Schulzke JD. Supernatants of HIV-infected immune cells affect the barrier function of human HT-29/B6 intestinal epithelial cells. Aids 2002; 16:983-91; PMID:11953464; http://dx.doi.org/10.1097/00002030-200205030-00004
- Cicala C, Arthos J, Rubbert A, Selig S, Wildt K, Cohen OJ, Fauci AS. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc Natl Acad Sci U S A 2000; 97:1178-83; PMID:10655504; http://dx.doi.org/10.1073/pnas.97.3.1178
- Fasano A Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91:151-75; PMID:21248165; http://dx.doi.org/10.1152/physrev.00003.2008
- Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015; 3:e977176; PMID:25838982; http://dx.doi.org/10.4161/21688370.2014.977176
- Gaulke CA, Porter M, Han YH, Sankaran-Walters S, Grishina I, George MD, Dang AT, Ding SW, Jiang G, Korf I, et al. Intestinal epithelial barrier disruption through altered mucosal microRNA expression in human immunodeficiency virus and simian immunodeficiency virus infections. J Virol 2014; 88:6268-80; PMID:24672033; http://dx.doi.org/10.1128/JVI.00097-14
- Vyboh K, Jenabian MA, Mehraj V, Routy JP. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res 2015; 2015:614127; PMID:25759844; http://dx.doi.org/10.1155/2015/614127
- Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859-70; PMID:10068581; http://dx.doi.org/10.1086/314660
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365-71; PMID:17115046; http://dx.doi.org/10.1038/nm1511
- Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra191; PMID:23843452; http://dx.doi.org/10.1126/scitranslmed.3006438
- Capini CJ, Richardson MW, Hendel H, Sverstiuk A, Mirchandani J, Régulier EG, Khalili K, Zagury JF, Rappaport J. Autoantibodies to TNFalpha in HIV-1 infection: prospects for anti-cytokine vaccine therapy. Biomed Pharmacother 2001; 55:23-31; PMID:11237281; http://dx.doi.org/10.1016/S0753-3322(00)00018-4
- Jacobson JM, Greenspan JS, Spritzler J, Ketter N, Fahey JL, Jackson JB, Fox L, Chernoff M, Wu AW, MacPhail LA, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med 1997; 336:1487-93; PMID:9154767; http://dx.doi.org/10.1056/NEJM199705223362103
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 2002; 123:163-72; PMID:12105845; http://dx.doi.org/10.1053/gast.2002.34235
- Beachler DC, Sugar EA, Margolick JB, Weber KM, Strickler HD, Wiley DJ, Cranston RD, Burk RD, Minkoff H, Reddy S, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015; 181:40-53; PMID:25480823; http://dx.doi.org/10.1093/aje/kwu247
- Brickman C, Palefsky JM. Cancer in the HIV-Infected Host: Epidemiology and Pathogenesis in the Antiretroviral Era. Current HIV/AIDS reports (2015); Dec;12(4):388-96; PMID: 26475669; http://dx.doi.org/10.1007/s11904-015-0283-7
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857-61; PMID:17603495; http://dx.doi.org/10.1038/nm1598
- Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol 1998; 72:7064-74; PMID:9696799
- Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol 2002; 76:7203-8; PMID:12072519; http://dx.doi.org/10.1128/JVI.76.14.7203-7208.2002
- Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol 2013; 5:1–9; PMID:24167660; http://dx.doi.org/10.3402/jom.v5i0.22766
- Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, Corey L. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 2004; 190:693-6; PMID:15272395; http://dx.doi.org/10.1086/422755
- Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, Song X, Ding M, Deng H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol 2007; 81:12465-71; PMID:17804490; http://dx.doi.org/10.1128/JVI.01457-07
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 2009; 457:882-6; PMID:19182773; http://dx.doi.org/10.1038/nature07684
- Coyne CB, Voelker T, Pichla SL, Bergelson, JM The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem 2004; 279:48079-84; PMID: 15364909; http://dx.doi.org/10.1074/jbc.M409061200
- Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2007; 2:181-92; PMID:18005733; http://dx.doi.org/10.1016/j.chom.2007.07.003
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell 2001; 104:441-51; PMID:11239401; http://dx.doi.org/10.1016/S0092-8674(01)00231-8
- Torres-Flores JM, Silva-Ayala D, Espinoza MA, Lopez S, Arias CF. The tight junction protein JAM-A functions as coreceptor for rotavirus entry into MA104 cells. Virology 2015; 475:172-8; PMID:25481868; http://dx.doi.org/10.1016/j.virol.2014.11.016
- Liberto MC, Zicca E, Pavia G, Quirino A, Marascio N, Torti C, Focà A. Virological Mechanisms in the Coinfection between HIV and HCV. Mediators Inflamm 2015; 2015:320532; PMID:26494946; http://dx.doi.org/10.1155/2015/320532
