ABSTRACT
The role of Slit/Robo signaling has extended from initial axon repulsion in the developing nervous system to organ morphogenesis, cancer development and angiogenesis. Slit/Robo signaling regulates similar pathways within these processes. Slit/Robo ensures the homeostasis of the dynamic interaction between cell-cell and cell-matrix interactions. The dysregulation of Slit/Robo signaling damages the tissue barrier, resulting in developmental abnormalities or disease. Here, we summarize how Slit/Robo controls kidney morphogenesis and describe the dual roles of Slit/Robo signaling in the regulation of tumorigenesis and angiogenesis.
Introduction
Slit/Robo signaling was first identified as an “axon guidance” cue that plays crucial roles in preventing the commissural axon from recrossing the midline and in regulating sensory axon elongation and branching.Citation1,2 This signaling was later demonstrated to play an important role in the development of multiple tissues, including lung,Citation3,4 kidney,Citation4,5 heartCitation6-8 and mammary gland.Citation9 In addition to kidney agenesis and cardiac defects, Slit3-mutant mice revealed diaphragmatic hernias.Citation10,11 A similar defect of diaphragmatic hernias was also observed in Robo 1/2-mutant mice.Citation12 The authors noted that the primary defect of Slit/Robo signaling is a delayed foregut separation from the dorsal body wall.Citation12 Thus, the Slit/Robo signaling pathway is an important regulator that controls tissue morphogenesis.
Many processes that control embryogenesis are dysregulated during cancer development, such as the epithelial-to-mesenchymal transition (EMT) for embryos causes cancer cells to migrate, and the mesenchymal-to-epithelial transition (MET) for migratory embryonic cells or cancer cells causes the development of organs or tumors when they reach to their destinations.Citation13,14 In addition to normal cellular processes, Slit/Robo signaling has been shown to participate in tumorigenesis, including cell proliferation, cell adhesion, and cell migration.Citation15 However, both oncogenic and tumor suppressor roles of Slit/Robo signaling have been reported in various cancer types. Understanding how Slit/Robo signaling regulates remodeling of tissue barriers during normal morphogenesis would help to understand the roles of this signaling in tumor development and metastasis. This review discusses different consequences of the Slit/Robo signaling with special emphasis on its roles in kidney morphogenesis, cancer cell adhesion and motility, and angiogenesis.
Structure of Slit and Robo proteins
The roundabout gene, robo, was first discovered in Drosophila in a screen for mutants that control axon midline crossing in the central nervous system (CNS).Citation16,17 One Robo ortholog is expressed in C. elegans.Citation18 Three Robos exist in flies, chicken and Xenopus,Citation17,19-23 and 4 are present in zebrafish and mammals.Citation17,24-26 Robos are 1,000–1,600 amino acids long and are highly conserved transmembrane receptors that belong to the IgCAM superfamily. They have neither autocatalytic activity nor enzymatic activity. Robo1–3, but not Robo4, share a high degree of structural and functional similarity. They exhibit a similar ectodomain structure containing 5 immunoglobulin-like (IG) domains followed by 3 fibronectin type 3 (FN3) repeats, except for Robo4, which contains only 2 IG domains and 2 FN3 repeats. The intracellular tail domain of Robos is quite variable, but it contains several conserved motifs for recruiting a variety of adaptor proteins, such as a CC0–3 motif in Robo1–2 molcules, CC0, CC2, and CC3 motifs in Robo3, as well as CC0, and CC2 motifs in Robo4 cytoplasmic tailCitation27,28 ().
Figure 1. The domain structure of Slit and Robo proteins. (A) A schematic diagram of the domain organization of Slit1, Slit2 and Slit3 in humans. The domain structure of Slits includes 4 leucine-rich repeats (D1-D4), followed by 6 epidermal growth factor-like (EGF) domains, a laminin G domain, 3 additional EGF domains and a C-terminal cysteine knot. (B) A schematic diagram of the domain organization of Robo1, Robo2, Robo3 and Robo4 in vertebrates. The ectodomain is conserved among Robo1–Robo3, with 5 immunoglobulin (Ig) and 3 fibronectin type 3 (FNIII) domains. Robo4 contains only 2 Ig domains and 2 FNIII repeats. The intracellular domain of Robos contains several conserved motifs, including CC0-CC3 for Robo1–2, CC0 and CC2–3 for Robo3, and CC0 and CC2 for Robo4.
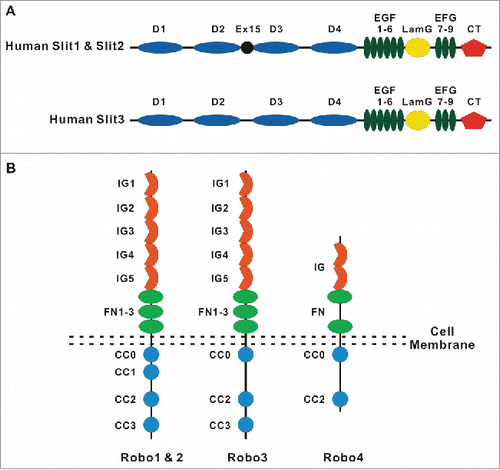
Slit is the ligand of the Robo1-Robo3 receptors in Drosophila, and its homologs have been identified in worms, teleosts and mammals.Citation29-31 While invertebrates express a single Slit protein, vertebrates have 3, Slit1, Slit2 and Slit3.Citation27 Slits are approximately 1,500-amino-acids long. From the N-terminus, the primary structure of mammalian Slits has 4 tandem leucine-rich repeats (LRR, D1-D4), 6 epidermal growth factor (EGF)-like domains, an ALPS (agrin, perlecan, laminin, Slit)/ laminin G-like domain, one (invertebrates) or 3 (vertebrates) EGF-like domains, and a C-terminal cysteine-rich knot (). Genetic and crystallography studies have illustrated that the second LRR (D2) of Slit is required for the interaction with the first Ig domain of Robo1,Citation32 while LLR4 (D4) is required for the dimerization of Slit2.Citation33
In both fly and vertebrate systems, Slits can be cleaved between the 5th and 6th EGF-like domain to form the long membrane-tethered Slit2-N (140 kDa) and diffusible short Slit2-C fragments (55–60 kDa).Citation1,2,34 Recently, the fly homolog of pheromone convertase 2 (PC2), amontillado (Amon), has been shown to cleave Slit.Citation35 Slit2-N, but not Slit2-C, binds to Robo and induces the branching of dorsal root ganglia axons.Citation36 Slit2-N also inhibits the PDGF-induced migration of vascular smooth muscle migration.Citation34,37 Recently, circulating Slit2-C has been shown to have a novel activity: it can activate the thermogenic, PKA-dependent pathway in adipocytes and improve glucose homeostasis.Citation38 In addition to proteolytic cleavage, we have identified alternative splicing variants for Slit2 at exon 15, which is located at the end of LRR2, Slit2-WT (presence of exon 15) and Slit2-ΔE15 (absence of exon 15).Citation39 Slit2-ΔE15 inhibits both the growth and invasion of CL1–5 lung cancer cells, while Slit2-WT possesses only invasion inhibitory activity.Citation39
The slit1, slit2, slit3, robo1 and robo3 genes are frequently downregulated via hypermethylation within the promoter region in cancers, including non-small cell lung, breast, ovarian, glioma, hepatocellular, and colorectal cancers and leukemias, suggesting that Slit/Robo signaling functions as a tumor suppressor.Citation15 However, some reports have revealed the upregulation of Slits in ductal carcinoma, prostate cancer, and lobular breast cancer, suggesting that Slit/Robo signaling may be oncogenic in certain cellular contexts.Citation15 Moreover, miR-218–1 and miR-218–2 are intronic microRNAs that are located in intron 15 of Slit2 and Slit3, respectively. It is expected that the expression of miR-218–1 and miR218–2 is correlated with their host genes, Slit2 and Slit3, respectively. Both miRNAs target and inhibit the expression of Robo1 and Robo2.Citation40,41 Thus, the expression of Robo1 and Robo2 may be negatively correlated with the expression level of Slit2 and Slit3, which adds another layer of complexity to the function of Slit/Robo signaling in cancer. Furthermore, a recent study has shown that there is one enhancer (Slit2-hs1) located 52 kb upstream of miR218 and 2 enhancers located 76 kb (Slit3-hs2) and 130 kb (Slit3-hs1) upstream of miR218–2. Intriguingly, the Slit2-hs1 enhancer is located in intron 5 and exon 6 of Slit2 gene which enhances miR218–1 expression but not that of the host Slit2 gene.Citation42 How an enhancer upregulates the intronic microRNA but not the host gene has not been elucidated. Nonetheless, this study suggested that the relationship between the expression of Slit2 and Robo1/Robo2 may not necessarily be correlated.
The roles of adhesion molecules and the actin cytoskeleton in the regulation of tissue barriers
In multicellular organisms, formation of tissue barriers is critical for the establishment of distinct body compartments with unique cellular architecture and chemical composition. The formation of functional barriers requires the assembly of different types of adhesive contacts between neighboring cells (intercellular junctions), as well as contacts between the cells and the extracellular matrix (ECM). The most important types of intercellular junctions are tight junctions (TJs) and adherens junctions (AJs). TJs are located at the most apical part of the lateral plasma membrane, and they regulate paracellular permeability and the maintenance of the apico-basal cell polarity in epithelial and endothelial layersCitation43 (). AJs mediate initial cell-cell attachments via trans-interaction of different cell adhesion molecules (CAMs), and cadherins. Furthermore, cadherins are retained at the plasma membrane and are attached to the cortical actin cytoskeleton by forming a complex with β-catenin, p120-catenin and α-catenin.Citation44-46 AJs are thought to be required for TJ assembly. During the embryonic development and wound healing, epithelial junctions undergo a robust remodeling to enable cell movement. Even in adult tissue, TJs and AJs are constantly remodeled without compromising barrier function.Citation47 The deregulation of the expression of cadherins (e.g., downregulation of E-cadherin) and the switching of the expression among family members (e.g., switching from E-cadherin to N-cadherin) could significantly contribute to tumor progression and metastasis.Citation48-50
Figure 2. Molecular organization of intercellular junctions and cell-matrix adhesions. The Figure shows key adhesion structures formed by epithelial and endothelial cells. These structures include 2 junctional complexes, tight junctions and adherens junctions and as well as focal adhesions that mediate cell interactions with ECM. Tight junctions, adherens junctions and focal adhesions are linked to a prominent cortical actomyosin cytoskeleton that regulates the organization and dynamics of intercellular junctions and ECM adhesions.
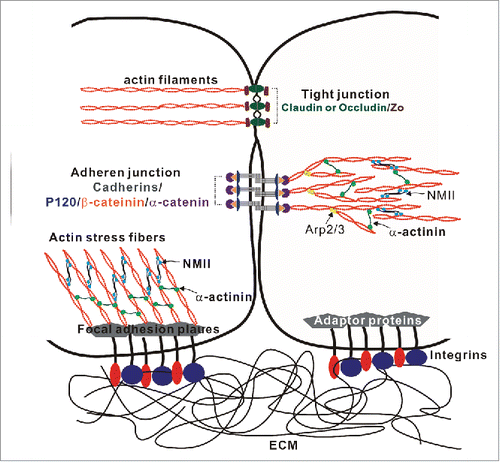
The extracellular matrix provides mechanical support and anchorage for cells. Cells interact with ECM components via various adhesion molecules, including integrins, to regulate cell adhesion, migration, differentiation, tissue organization, polarity, and tumorigenesis. In mammals, 18 α-subunits and 8 β-subunits have been identified to form 24 distinct heterodimers that bind to extracellular components such as fibronectin, collagen, and laminin.Citation51,52 Integrins link the ECM and cytoskeleton. The activation of integrins by binding to matrix ligands induces conformational changes in the integrins that recruit talin, paxillin, integrin-linked kinase (ILK), vinculin, α-actinin, the focal adhesion kinase (FAK), and approximately 30 other adaptor proteins to form “focal adhesion plaque” that is connected to actin stress fibersCitation53 ().
Cells are constantly facing dynamic mechanical stress and strain while maintaining the homeostasis of functional barriers in living organs. These dynamic forces are sensed and transduced to neighboring cells by intercellular junctions and ECM adhesions, which are coupled to the actomyosin cytoskeleton.Citation54,55 Therefore, signaling events that affects actomyosin structure associated with intercellular junctions and ECM adhesions have significant impact on the integrity and function of tissue barriers. RhoA, Rac1 and Cdc42, which belong to a family of Rho small GTPases, are key regulators of cellular adhesions by controlling actin polymerization and myosin II activity.Citation56 These Rho GTPases have distinct effects on epithelial and endothelial barriers. Their activities are tightly regulated in terms of space and time. Active Rac1 promotes actin polymerization and controls lamellipodia and membrane ruffles. Cdc42 enhances actin polymerization and induces filopodia formation, while RhoA establishes contractile actomyosin by inducing actin polymerization via formins and activating myosin II via Rho-associated coiled-coil kinase (ROCK).Citation57,58 The initial cell-cell contact is mediated by lamellipodia with the Rac1-induced branched actin network via Arp2/3, and subsequently, the formation of filopodia by Cdc42 promote actin bundles and connection by adhesion molecules. Both Rac1 and Cdc42 are involved in junction maturation, while RhoA is required for maintaining stable junctions through the formation of contractile actomyosin.Citation59,60
The activation of Slit/Robo signaling recruits Slit-Robo GTPase-activating protein 1 (srGAP1) or Vilse/Cross GAP to the intracellular domain of the Robo receptor and inactivates Rho GTPases (Cdc42, RhoA or Rac1), thus inhibiting actin polymerization and stress fiber formation.Citation61 This review focuses on how Slit/Robo signaling affects tissue barriers via cell-cell and cell-matrix interactions, particularly in kidney morphogenesis, angiogenesis and tumorigenesis.
Roles of the Slit/Robo signaling in kidney development
Early kidney development requires the outgrowth and invasion of the ureteric bud from the nephric duct into the metanephric mesenchyme. The invading ureteric bud tip induces the polarization of metanephric mesenchyme cells to undergo the MET to generate the epithelial cells of the nephron.Citation62 It has been shown that Slit1–3 and Robo1–2 are expressed in discrete patterns in the developing murine metanephros.Citation4 A lack of Slit2 or Robo2 causes the supernumerary buds to form ectopic ureteric buds and inappropriate connections to the nephric ductCitation63 (). In humans, a de novo translocation that disrupts Robo2 and 2 independent Robo2 intracellular missense variants in 2 unrelated families were found to be associated with urinary tract anomalies and a risk of vesicoureteral reflux.Citation5 In addition, mutations in the Slit/Robo effector srGAP1 were found in patients with congenital anomalies of the kidney and urinary tract, further augmenting the importance of Slit/Robo signaling in kidney development.Citation64 How Slit2/Robo2 signaling functions to avoid supernumerary buds is not yet clear. However, it has been hypothesized that Slit2/Robo2 signaling may avoid the interaction of the Wolffian duct and nephrogenic cord and restrict ureteric bud induction, suggesting that the inappropriate association of 2 cell populations induces cell proliferation for ectopic UB induction ().Citation65
Figure 3. Roles of the Robo-Slit signaling and the development and functions of the glomerular filtration barrier. (A) Downregulation of Robo2 permits the interactions between the Wolffian duct (WD) and the nephrogenic cord (NC), resulting in the increased cell proliferation and formation of the ectopic ureteric bud (UB). This figure is adopted from CitationRef. 65. (B) Podocytes cover the outer surface of glomerular capillaries to form the glomerular filtration barrier that contains 3 structural elements: (1) a layer of fenestrated endothelial cells; (2) the glomerular basement membrane (GBM); (3) specialized epithelial cells with extended foot processes covering the outer face of the GBM. (C) The interplay between the Robo-Slit signaling and integrins mediates the establishing of podocyte-GBM adhesions. The laminin-binding integrins α3/β1 are linked to actin-based stress fibers through adaptor proteins. RhoA induces stress fiber formation through Rho-associated protein kinase (ROCK)-dependent phosphorylation of non-muscle myosin IIA (NMIIA). Slit2/Robo2 signaling inhibits NMIIA activity by sequestering myosin II regulatory light chain (MRLC) through srGAP1 (GAP) to the intracellular domain of Robo2. The “slit diaphragm” is the junction between adjacent podocyte foot processes. Nephrin is the major adhesive molecule within slit diaphragm that triggers actin polymerization through adaptor protein Nck. Slit2/Robo2 signaling inhibits nephrin-induced actin polymerization by forming the Robo2/Nck/nephrin complex.
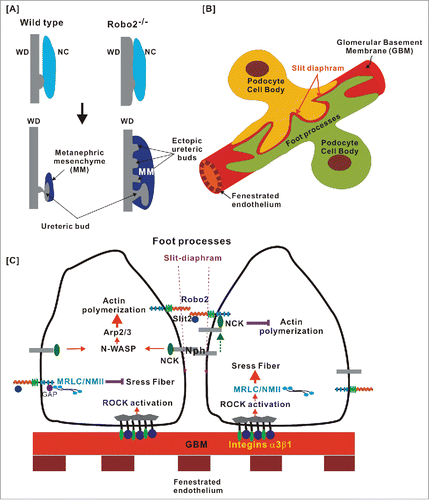
Slit2-Robo2 signaling not only plays an important role in suppressing ectopic ureteric bud but also functions in podocyte structures, which covers the outer surface of glomerular capillaries. The glomerular filtration barrier is a very complex system that has 3 structures: (1) a spherical bundle of capillaries that are lined by fenestrated endothelial cells and their associated glycocalyx; (2) the glomerular basement membrane (GBM), which provides a scaffold structure for endothelial cells and podocytes on either side; and (3) specialized epithelial cells, podocytes, that cover the outer surface of the GBMCitation66 (). The complex cellular architecture of the glomerulus warrants the existence of podocytes, which provide a special tissue barrier that is a major component of the glomerular filter. Podocytes extend major processes from the cell body to cover the outer face of the GBM that are further extended to form branches, also known as foot processes. Foot processes of neighboring podocytes interdigitate regularly to form a network of zipper-like structures with a narrow slit space (slit-diaphragm). The diameter of the slits is 25–30 nm, enabling them to exclude proteins larger than serum albumin.Citation67-69 The cell body and the primary process of podocytes contain microtubules and intermediate filaments, while the foot processes use an actin-based contractile apparatusCitation70,71 ( and ).
The mutation of the genes encoding actin-associated proteins induces foot process effacement and results in proteinuria, suggesting that the formation and maintenance of the structure of complex foot processes require the dynamic organization of the actin cytoskeleton.Citation72 Nephrin (Nph), a key slit-diaphragm protein, belongs to the Ig superfamily and functions as a signaling adhesion molecule to connect foot processes between neighboring podocytes ().Citation73 The knockout of nephrin causes a thicker GBM, broader foot processes, and a loss of the slit diaphragm.Citation74,75 It has been found that phosphorylated nephrin induces actin polymerization via binding to the adaptor protein Nck, which, in turn, activates the down-stream effector proteins N-WASP and Arp2/3 for actin branching polymerization.Citation71,76-78 In addition to their role in kidney formation, Slit2/Robo2 signaling has been shown to be involved in the foot process formation of podocytes. Upon binding with Slit2, Robo2 activation inhibits nephrin-induced actin polymerization by forming the Robo2/Nck/nephrin complexCitation79 (). The loss of Robo2 in podocytes generated short, broader, irregular and disorganized foot processes and elevated the albumin level in tissue-specific knockout mice.Citation79 Intriguingly, the authors also found that the loss of Robo2 alleviated abnormal foot process architecture in nephrin-knockout mice. Although the precise mechanism involved in the Robo2-mediated inhibition of nephrin-induced actin polymerization remains unclear, Slit2/Robo2 signaling clearly modulates foot process plasticity in podocytes to maintain the functional glomerular filtration barrier.
In addition to the integrity of cell-cell adhesion between podocyte foot processes, the cell-matrix adhesion between podocytes and ECM is also important for the proper functioning of the glomerulus. Integrins are primary receptors for the ECM, and they play a pivotal role in the interaction between cells and the extracellular matrix. Podocytes express a high level of integrin α3/β1, which binds to laminin-α5β2γ1 (laminin-521) in the GBM and forms the focal adhesion complex that links the actin cytoskeleton through multiple linker proteins, including talin, vinculin, and α-actinin.Citation80 These focal complex molecules sense forces from either the ECM or actin-myosin II-based contractile actomyosin stress fibers and form a stable focal adhesive complex.Citation81,82 Non-muscle myosin II (NMII) molecules contain 2 heavy chains, 2 regulatory light chains (MRLCs) and 2 essential light chains (MELCs). Three isoforms of heavy chain NMII, NMIIA, NMIIB and NMIIC, are present in mammals. NMII interacts with actin filaments and mediates filament movement and bundling thereby regulating fundamental cell processes such as cell division, cell adhesion and migration.Citation83 In podocytes, NMIIA-based actin stress fibers are critical for the maintenance of podocyte structure, and the deletion or dysfunction of the NMIIA gene results in susceptibility to kidney disease.Citation84,85
The Rho family of GTPases regulates the actin cytoskeleton. They also play an important role in regulating the downstream responses of Slit/Robo signaling. RhoA induces actin polymerization and stress fiber formation via the Rho-associated protein kinase (ROCK)-dependent phosphorylation of myosin II.Citation83 On the other hand, Slit2/Robo2 signaling inhibits NMIIA activity, which, in turn, decreases focal adhesion formation and attachment. Robo2 forms a complex with myosin II regulatory light chain (MRLC), a subunit of NMIIA, through srGAP1 in the presence of Slit2 (). The conditional knockout of NMIIA in podocytes decreases the podocyte number; however, the loss of both NMIIA and ROBO2 partially rescues the podocyte-loss phenotype.Citation86
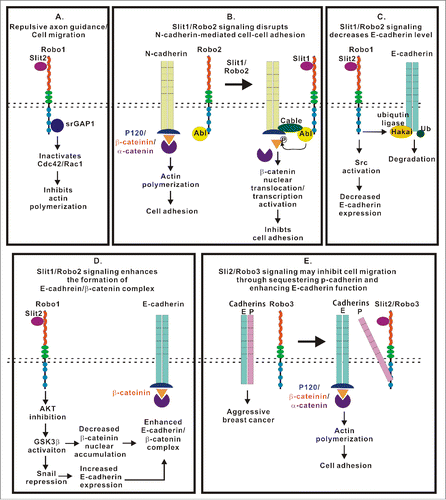
Figure 4. Multiple roles of the Slit/Robo signaling in the regulation of intercellular junctions, ECM adhesion and cell motility. This figure summarizes current knowledge regarding signaling mechanisms initiated by Slit/Robo complexes and regulating integrity and remodeling of intercellular junctions and ECM adhesions. (A) Slit/Robo signaling inhibits actin polymerization through srGAP1 to inactivate Rho GTPases. (B) Slit1/Robo2 signaling inhibits N-cadherin-mediated cell adhesion by forming Robo2/N-cadherin complex through the Abl kinase and its adaptor proteins. The Abl kinase subsequently phosphorylates β-catenin resulting in its dissociation from the cadherin complex and nuclear translocation. (C) Slit1/Robo2 signaling induces E-cadherin degradation by recruiting the ubiquitin ligase Hakai to E-cadherin. (D) Slit1/Robo2 signaling enhances the formation of the E-cadherin/β-catenin complex through AKT/GSK3β/Snail pathway. (E) Slit2/Robo3 signaling promotes the interaction between Robo3 and P-cadherin, which, in turn, enhances the formation of E-cadherin homodimer and inhibits cell migration.
The adhesion molecule involved in the cell-cell connection between the slit diaphragm of neighboring podocytes is nephrin, not cadherin. Similar to cadherin, the activation of nephrin upon cell-cell interactions also transduces the signal to actin/myosin stress fibers via a variety of adaptor proteins.Citation71 In addition to the regulation of actin dynamics by the nephrin-mediated cell-cell interaction, the interaction of foot process and GBM also regulates actin cytoskeleton remodeling via integrin. Furthermore, intercellular junctions crosstalk with ECM adhesions via the actin cytoskeleton, allowing cells to actively respond to changes in the environment.Citation87 For example, abnormal organization of actomyosin complexes associated with either intercellular junctions or ECM adhesions could contributes to the abnormal podocyte morphology. Slit2/Robo2 signaling may regulate the assembly of podocyte junctions and ECM adhesions by balancing actin polymerization and actomyosin contractility at the perijunctional actin filaments and basal stress fibers. Therefore, Slit2/Robo2 signaling has a crucial role in the formation of the glomerulus filtration barrier, including podocyte attachment to the GMB and the structural integrity of foot processes.
The role of Slit/Robo signaling in tumor metastasis: affecting cell adhesion by regulating cadherin functions
Slit/Robo signaling plays a major role in repulsive axon guidance by increasing the interaction between Robo1 and srGAP1, which subsequently inactivates Cdc42 and reduces actin polymerization.Citation88 Slit2 inhibits vascular smooth muscle cell migration by inactivating another small GTPase, Rac1.Citation34 The primary function of these pathways is in cell motility. The loss of this pathway has been observed in a variety of cancers. Slit2 has been shown to inhibit glioma cell migration by reducing Cdc42 activity through the Robo1 receptor.Citation89 The deregulation of the activities of Slit2-mediated srGAP1 and Cdc42 is associated with poor prognoses in esophageal cancer, breast cancer, and colorectal cancer.Citation90-92 Thus, Slit/Robo signaling controls cell migration by regulating small GTPase-mediated actin polymerization ().
In addition to increased actin polymerization, the disruption of intercellular junctions could accelerate cell migration. The Slit/Robo signaling pathway has been shown to affect AJ integrity by targeting different cadherins (). For example, Slit1/Robo2 signaling affects the aggregation of placode-derived cranial sensory neurons via the post-translational downregulation of the N-cadherin level.Citation93 Upon binding to Slit, Robo forms a multimeric complex with Abl kinase, and adaptor protein, cable, N-cadherin and β-catenin. Abl kinase subsequently phosphorylates β-catenin, which results in the nuclear translocation of β-catenin. Nuclear β-catenin binds to Tcf/Lef for transcriptional activation.Citation94,95 Thus, Slit/Robo signaling reduces cell adhesion by decreasing the affinity of β-catenin to N-cadherin, which inhibits N-cadherin-mediated cell adhesion ().
The EMT is a process that allows epithelial cell migration during embryogenesis. The re-activation of this process is a key event that leads to cell-cell dissociation and promotes the motility of tumor cells. This process is characterized by the reduced expression of E-cadherin.Citation96 E-cadherin is a cell-cell adhesion molecule. The intracellular domain of E-cadherin interacts with underlying actin filaments by forming a complex with cytoplasmic scaffolds β-catenin, p120 and α-catenin.Citation97,98 Slit2/Robo1 signaling has been shown to trigger both upregulation and downregulation of E-cadherin expression in different types of cancers ( and ). Decreased Slit2 and Robo1 expression have been observed in various cancers.Citation15 The loss of Slit2/Robo1 signaling in breast cancer and lung cancer increases the level of nuclear β-catenin and decreases the level of E-cadherin, thus causing AJ disassembly and enhancing cancer cell migration.Citation99,100 On the other hand, the upregulation of Slit2 and Robo1 has also been reported in colorectal cancer.Citation101,102 Recently, Slit2/Robo1 signaling was shown to induce E-cadherin degradation via the recruitment of the ubiquitin ligase Hakai to E-cadherin in colorectal cancerCitation103 (). Thus, the role of Slit2/Robo1 signaling in cell adhesion may be dependent on the cellular context of the cancer.
P-cadherin is another classical cadherin playing essential role in the maintenance of epithelial barriers. However, P-cadherin is a double-edged sword in that it may function as both a tumor suppressor and a tumor-promoting molecule. P-cadherin is downregulated in lung cancer, melanoma, oral cancer, and liver cancer, and P-cadherin possesses a tumor suppressor function similar to E-cadherin. On the other hand, P-cadherin is upregulated in breast, gastric, endometrial, ovarian, prostate, skin, pancreatic, and colon cancers.Citation104,105 The role of P-cadherin in tumor progression depends on the molecular context, including, in part, the expression level of E-cadherin.Citation106 It has been hypothesized that P-cadherin shares similar signaling pathways with E-cadherin. The ectopic expression of either cadherin has a similar phenotype of cell invasion for cadherin-free cells.Citation107 However, in the presence of E-cadherin, the expression of P-cadherin increases the aggressiveness of breast cancer cells, presumably by disrupting the E-cadherin/catenin complex.Citation106 Interestingly, it has been shown that Slit2 enhances the interaction between Robo3 and P-cadherin, thus inhibiting cell migration.Citation108 It is possible that the binding of Robo3 to P-cadherin alleviates the P-cadherin-induced disruption of the E-cadherin/catenin complex and maintains E-cadherin function ().
Actin remodeling not only regulates cell motility but also affects cell-cell interactions. Slit/Robo signaling regulates cancer cell motility by modulating actin remodeling and the activity of various cadherins. It is possible that mechanisms of Slit/Robo signaling identified in cancers are also present in normal tissues to regulate cell-cell adhesion. However, 3 members of the Slit family and 4 members of the Robo family are present in mammals. Thus, it is conceivable that the mechanisms of Slit/Robo signaling in the cell-cell interaction would be much more complicated in normal tissues than in cancers.
The role of Slit/Robo signaling in angiogenesis
Angiogenesis is the growth of blood vessels from existing vasculature. It occurs under both physiologic (like in the uterus and ovaries)Citation109 and pathological (such as in diabetic retinopathy and cancer) conditions. Angiogenesis is tightly regulated by both activators (e.g., growth factors, cytokines, matrix metalloproteinases, tumor necrosis factor, and angiopoietins) and inhibitors (e.g., angiostatin, interferon, endostatin, interleukins, and inhibitors of matrix metalloproteinases).Citation110 The deregulation of angiogenesis is a hallmark of cancer.Citation111 Without angiogenesis, tumor volume is limited to 1–2 mm3.Citation112,113 Interestingly, axon guidance signals with attractive and repulsive properties are involved in angiogenesis, such as Slits/Robos, netrins/UNC5, semaphorins/plexin, neuropilin, and ephrin/Eph.Citation114,115
The role of Slit/Robo signaling in angiogenesis is context-dependent, as it plays both pro- and anti-angiogenic roles (). Slit2 and Slit3 are expressed by endothelial cells, smooth muscle cells and brain vascular pericytes in blood vessels.Citation34,116,117 Robo4 is highly expressed by all endothelial cells, while the expression of Robo1 is weaker in endothelial cells.Citation15,118 The expression of Robo1 is inhibited by miR-218, which is located at intron 15 of the Slit2 and Slit3 genes. Thus, the expression of Robo1 may negatively correlate with Slit2/Slit3 expression in endothelial cells.Citation40,41 There are many contradictory reports regarding the role of Slits, Robo1 and Robo4 in endothelial migration. Slit2 has been shown to promote angiogenesis via the Robo1 receptorCitation102,119,120 or the Robo1/Robo4 heterodimer ( and ).Citation121 In contrast, Slit2 functions as an inhibitor in endothelial cell migration through Robo4 ().Citation28,122,123 Interestingly, a recent study revealed that Slit2 promotes HUVEC migration through Robo1 but only when Robo4 is knocked down. Thus, the authors hypothesized that the effect of Slit2 on HUVEC migration is dependent on the Robo1/Robo4 ratio ().Citation124 The nature of the interaction between Slit2 and Robo4 is under debate. Although the interaction between Slit2 and Robo4 has been shown in co-immunoprecipitation experiments,Citation28,116 no direct interaction has been observed using a Biacore assay.Citation125 In addition, Robo4 lacks the Ig domain that is present in the other Robo receptors interacting with Slit2.Citation32 Therefore, it is possible that a co-receptor, such as Robo1, syndecans, or heparin sulfate, is required for the Slit2/Robo4 interaction.Citation126,127 It is possible that Slit2/Robo1 signaling promotes endothelial migration; however, the interaction between Robo1/Robo4 inhibits cell migration. In addition to Slit2, Slit3 has been shown to induce endothelial cell migration through Robo4, which activates Rac1 and RhoA (). Slit3 promotes angiogenesis in vitro and ex vivo, and the knockout of Slit3 disrupts angiogenesis during embryogenesis.Citation116
Figure 5. Complex and context dependent functions of the Slit/Robo signaling in angiogenesis. (A) Slit2/Robo1 signaling promotes angiogenesis by activating Robo1-EGFR2-ERK1/2 signaling or by yet to be determined intracellular events. (B) Slit2/Robo4 signaling blocks VEGF-induced angiogenesis by inhibiting the Ras/Raf/Mek/Erk pathway. (C) Slit2 binds to the Robo1/Robo4 heterodimer to promote HUVEC motility by recruiting WASP and Arp2/3 for actin polymerization. (D) Assembly of the Robo1/Robo4 heterodimer reduces Slit2/Robo1 signaling-mediated pro-angiogenesis activity. (E) Slit3/Robo4 signaling activates Rho GTPases and promotes HUVEC motility and angiogenesis. (F) Slit2/Robo4 signaling activates GIT/GAP and inhibits VEGF-induced permeability of blood vessels and angiogenesis by inactivating Arf6 GTPase. (G) Slit2N/Robo4 signaling inhibits endothelial permeability caused by cytokine production in sepsis and influenza by increasing VE-cadherin recruitment to the plasma membrane. (H) The Slit2N/Robo4 signaling attenuates increased permeability of lung lymphatic vessels caused by HIV-1-derived gp120 glycoprotein. (I) Robo4 stabilizes blood vessel through Slit2-independent pathway. Intercellular interaction of Robo4 and UNC5 inhibits VEGF-induced vessel permeability.
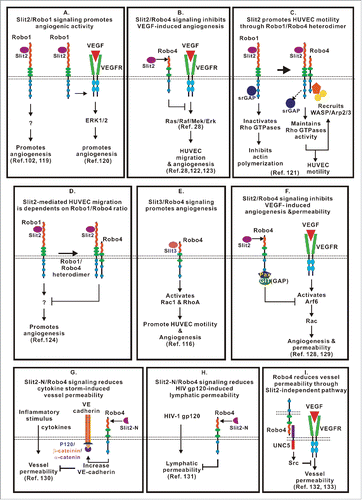
In addition to regulating endothelial cell migration and angiogenesis, Slit2/Robo4 signaling has been shown to inhibit VEGF-induced endothelial permeability and promotes vascular stability by recruiting GTPase-activating proteins (GAPs) to inhibit the activities of the small GTPase Arf6 and Rac ().Citation128,129 Robo4 activated by Slit2-N stabilizes the endothelial barrier by increasing localization of VE-cadherin at the plasma membrane, reduces vascular permeability and increases the survival of animals during a cytokine storm induced by sepsis and influenza ().Citation130 Similarly, the Slit2/Robo4 pathway may also play a role in stabilizing the lymphatic endothelium. Treatment with the HIV-1 envelop glycoprotein gp120 (HIV-1 gp120) induced the permeability of lung lymphatic endothelial cells (L-LECs), which correlated with reduced expression of Slit2. Slit2-N has the ability to protect L-LECs from HIV-1 gp120-induced hyperpermeability ().Citation131 Thus, Slit2/Robo4 signaling is important for maintaining vascular and lymphatic barrier function. A recent study revealed that Robo4 stabilizes vessel integrity via the Slit2-independent pathway (). It has been shown that Robo4 binds UNC5B and activates UNC5B signaling, which, in turn, inhibits VEGF/VEGFR signaling, thus stabilizing blood vessels and inhibiting angiogenesis.Citation132 Further study demonstrated that the cytoplasmic domain of Robo4 is not required for activating UNC5B activation.Citation133 These studies suggest that Robo4 maintains the integrity of the blood vessel barrier through either the Slit2-dependent or Slit2-independent pathways.
Interestingly, Slits expressed by different cell sources may have different functions. For example, in breast cancer, Slit2/Slit3 expressed by mural cells encircling blood vessels in the stroma, but not those expressed by the epithelium, inhibit VEGF-induced angiogenesis via Robo4 but not Robo1.Citation123 The integrity of the newly synthesized blood vessels around the tumor is structurally abnormal and highly permeable. The conditioned medium (CM) of lung cancer cells induces HUVEC permeability, poor-quality tube formation and thin blood vessel formation in a chorioallantoic membrane (CAM) assay.Citation134 Interestingly, we observed that Slit2 splice variants at exon 15, Slit2 WT and Slit2-ΔE15, exhibit differential functions in vessel quality. Both Slit2-WT and Slit2-ΔE15 effectively suppress CM-induced HUVEC permeability via Robo4. However, only the CM containing Slit2-ΔE15, not Slit2-WT, restores the quality of tube formation in HUVECs and the size of the vessels in the CAM. Neither Robo1 nor Robo4 is required for Slit2-ΔE15-enhanced tube quality.Citation134 Thus, the complicated or contradictory effects of Slits on endothelial migration and angiogenesis may greatly depend on the sources and isoforms of Slit2 and the interaction between Slits with different Robo receptors and various downstream effector proteins.
Conclusion
Slit/Robo signaling was initially discovered as an axon guidance cue. However, it is also involved in tissue morphogenesis, tissue plasticity, cancer development and angiogenesis. There are similarities between early embryo development and tumorigenesis, such as the EMT and MET. These events alter the original tissue barrier by changing cell-cell interactions, the cell-matrix interaction and cytoskeletal organization, which are regulated by Slit/Robo signaling. Slit/Robo signaling plays an important role in inhibiting supernumerary buds and is also essential in maintaining the podocyte structure for a normal glomerulus filtration barrier. The role of Slit/Robo in kidney development and function may involve the homeostasis of actin polymerization. Slit/Robo signaling is also required for heart tube lumen formation in Drosophila by preventing E-cadherin-mediated cell adhesion.Citation135 Since one of the major functions of Slit/Robo signaling is the regulation of the actin cytoskeleton and actin cytoskeleton dynamics are crucial for cell-cell adhesion and cell-matrix adhesion, the Slit/Robo signaling pathway likely plays a role in the regulation of tissue barriers in other tissues.
Although most studies have noted that Slit2/Robo4 signaling stabilizes vessel stability, the overexpression of Slit2 in transgenic mice enhances vessel formation, increases permeability in the brain, and induces Alzheimer disease-like changes.Citation136,137 These conflicting phenomena suggested that tight regulation of Slit/Robo signaling is important in the maintenance of stable vessel barriers. Similar circumstances have been observed for podocyte structures. The activation of nephrin adhesion molecules induces actin polymerization, but Slit2/Robo2 signaling counteracts actin polymerization. Nephrin-knockout mice revealed abnormal foot processes for podocytes; however, further loss of Robo2 expression recovered the foot process architecture.Citation79 In addition, the activation of NMIIA enhances stress fiber formation, while Slit2/Robo2 inhibits this process. Similarly, NMIIA knockout decreased the podocyte number, while the double knockout of NMIIA and Robo2 rescued the podocyte number.Citation86 Thus, the Slit/Robo signaling pathway functions to balance actin polymerization and stress fiber formation to maintain stable and functional tissue barriers.
Slit/Robo signaling participates in many processes in tumorigenesis, such as invasion, metastasis, proliferation, apoptosis and angiogenesis. Thus, this signaling pathway is an attractive target for the development of therapeutic agents against cancer at different levels. However, there are 3 Slits and 4 Robos in vertebrates, and they are important for physiologic and pathological processes. Thus, it is important to elucidate the conditions in which Slits can interact with different combination of Robo receptors. The downstream signaling pathways that involve monomers, homodimers or heterodimers of Robo receptors are currently unknown. Recently, other Slit receptors, such as dystroglycan,Citation138 Plexin A1,Citation139 and Dscam1 (Down syndrome cell adhesion molecule 1), have been discovered.Citation140 These newly identified receptors may engage in crosstalk with Robo receptors. Thoroughly understanding all these questions are important for the development of therapeutic strategies that treat cancer but do not affect the homeostasis of normal tissues.
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed.
Funding
This work was supported by Chung Shan Hospital Institutional Grant CSH-2013-C-030 (Taiwan, R.O.C.) to Ming-Fang Wu and a Ministry of Science and Technology grant (MOST 105–2320-B-040–031; Taiwan, R.O.C.) to Jinghua Tsai Chang.
References
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 1999; 96(6):771-84; PMID:10102266; https://doi.org/10.1016/S0092-8674(00)80588-7
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999; 96(6):795-806; PMID:10102268; https://doi.org/10.1016/S0092-8674(00)80590-5
- Greenberg JM, Thompson FY, Brooks SK, Shannon JM, Akeson AL. Slit and robo expression in the developing mouse lung. Dev Dyn 2004; 230(2):350-60; PMID:15162513; https://doi.org/10.1002/dvdy.20045
- Piper M, Georgas K, Yamada T, Little M. Expression of the vertebrate Slit gene family and their putative receptors, the Robo genes, in the developing murine kidney. Mech Dev 2000; 94(1-2):213-7; PMID:10842075; https://doi.org/10.1016/S0925-4773(00)00313-0
- Lu W, van Eerde AM, Fan X,Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet 2007; 80(4):616-32; PMID:17357069; https://doi.org/10.1086/512735
- Medioni C, Bertrand N, Mesbah K, Hudry B, Dupays L, Wolstein O, Washkowitz AJ, Papaioannou VE, Mohun TJ, Harvey RP, et al. Expression of Slit and Robo genes in the developing mouse heart. Dev Dyn 2010; 239(12):3303-11; PMID:20941780; https://doi.org/10.1002/dvdy.22449
- Mommersteeg MT, Andrews WD, Ypsilanti AR, Zelina P, Yeh ML, Norden J, Kispert A, Chédotal A, Christoffels VM, Parnavelas JG. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ Res 2013; 112(3):465-75; PMID:23255421; https://doi.org/10.1161/CIRCRESAHA.112.277426
- Mommersteeg MT, Yeh ML, Parnavelas JG, Andrews WD. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc Res 2015; 106(1):55-66; PMID:25691540; https://doi.org/10.1093/cvr/cvv040
- Macias H, Moran A, Samara Y, Moreno M, Compton JE, Harburg G, Strickland P, Hinck L. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev Cell 2011; 20(6):827-40; PMID:21664580; https://doi.org/10.1016/j.devcel.2011.05.012
- Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev 2003; 120(9):1059-70; PMID:14550534; https://doi.org/10.1016/S0925-4773(03)00161-8
- Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci U S A 2003; 100(9):5217-22; PMID:12702769; https://doi.org/10.1073/pnas.0730709100
- Domyan ET, Branchfield K, Gibson DA, Naiche LA, Lewandoski M, Tessier-Lavigne M, Ma L, Sun X. Roundabout receptors are critical for foregut separation from the body wall. Dev Cell 2013; 24(1):52-63; PMID:23328398; https://doi.org/10.1016/j.devcel.2012.11.018
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139(5):871-90; PMID:19945376; https://doi.org/10.1016/j.cell.2009.11.007
- Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science 2013; 342(6159):1234850; PMID:24202173; https://doi.org/10.1126/science.1234850
- Ballard MS, Hinck L. A roundabout way to cancer. Adv Cancer Res 2012; 114:187-235; PMID:22588058
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: Genes necessary for guidance toward or away from the midline. Neuron 1993; 10(3):409-26; PMID:8461134; https://doi.org/10.1016/0896-6273(93)90330-T
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998; 92(2):205-15; PMID:9458045; https://doi.org/10.1016/S0092-8674(00)80915-0
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 1998; 92(2):217-27; PMID:9458046; https://doi.org/10.1016/S0092-8674(00)80916-2
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell 2000; 103(7):1033-45; PMID:11163180; https://doi.org/10.1016/S0092-8674(00)00207-5
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron 2000; 28(3):753-66; PMID:11163264; https://doi.org/10.1016/S0896-6273(00)00151-3
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 2004; 117(2):157-69; PMID:15084255; https://doi.org/10.1016/S0092-8674(04)00303-4
- Vargesson N, Luria V, Messina I, Erskine L, Laufer E. Expression patterns of Slit and Robo family members during vertebrate limb development. Mech Dev 2001; 106(1-2):175-80; PMID:11472852; https://doi.org/10.1016/S0925-4773(01)00430-0
- Connor RM, Key B. Expression and role of Roundabout-1 in embryonic Xenopus forebrain. Dev Dyn 2002; 225(1):22-34; PMID:12203717; https://doi.org/10.1002/dvdy.10130
- Lee JS, Ray R, Chien CB. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell migration. Dev Dyn 2001; 221(2):216-30; PMID:11376489; https://doi.org/10.1002/dvdy.1136
- Challa AK, Beattie CE, Seeger MA. Identification and characterization of roundabout orthologs in zebrafish. Mech Dev 2001; 101(1-2):249-53; PMID:11231085; https://doi.org/10.1016/S0925-4773(00)00570-0
- Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 2002; 79(4):547-52; PMID:11944987; https://doi.org/10.1006/geno.2002.6745
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 2006; 22:651-75; PMID:17029581
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol 2003; 261(1):251-67; PMID:12941633; https://doi.org/10.1016/S0012-1606(03)00258-6
- Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: An EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell 1988; 55(6):1047-59; PMID:3144436; https://doi.org/10.1016/0092-8674(88)90249-8
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell 1999; 96(6):785-94; PMID:10102267; https://doi.org/10.1016/S0092-8674(00)80589-9
- Chedotal A. Slits and their receptors. Adv Exp Med Biol 2007; 621:65-80; PMID:18269211
- Morlot C, Thielens NM, Ravelli RB, Hemrika W, Romijn RA, Gros P, Cusack S, McCarthy AA. Structural insights into the Slit-Robo complex. Proc Natl Acad Sci U S A 2007; 104(38):14923-8; PMID:17848514; https://doi.org/10.1073/pnas.0705310104
- Seiradake E, von Philipsborn AC, Henry M, Fritz M, Lortat-Jacob H, Jamin M, Hemrika W, Bastmeyer M, Cusack S, McCarthy AA. Structure and functional relevance of the Slit2 homodimerization domain. EMBO Rep 2009; 10(7):736-41; PMID:19498462; https://doi.org/10.1038/embor.2009.95
- Liu D, Hou J, Hu X, Wang X, Xiao Y, Mou Y, De Leon H. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circ Res 2006; 98(4):480-9; PMID:16439689; https://doi.org/10.1161/01.RES.0000205764.85931.4b
- Ordan E, Volk T. Amontillado is required for Drosophila Slit processing and for tendon-mediated muscle patterning. Biol Open 2016; 5(10):1530-4; PMID:27628033; https://doi.org/10.1242/bio.020636
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chédotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci 2001; 21(12):4281-9; PMID:11404413
- Ning Y, Sun Q, Dong Y, Xu W, Zhang W, Huang H, Li Q. Slit2-N inhibits PDGF-induced migration in rat airway smooth muscle cells: WASP and Arp2/3 involved. Toxicology 2011; 283(1):32-40; PMID:21315131; https://doi.org/10.1016/j.tox.2011.01.026
- Svensson KJ, Long JZ, Jedrychowski MP, Cohen P, Lo JC, Serag S, Kir S, Shinoda K, Tartaglia JA, Rao RR, et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab 2016; 23(3):454-66; PMID:26876562; https://doi.org/10.1016/j.cmet.2016.01.008
- Lin YY, Yang CH, Sheu GT, Huang CY, Wu YC, Chuang SM, Fann MJ, Chang H, Lee H, Chang JT. A novel exon 15-deleted, splicing variant of Slit2 shows potential for growth inhibition in addition to invasion inhibition in lung cancer. Cancer 2011; 117(15):3404-15; PMID:21264840; https://doi.org/10.1002/cncr.25890
- Davidson MR, Larsen JE, Yang IA, Hayward NK, Clarke BE, Duhig EE, Passmore LH, Bowman RV, Fong KM. MicroRNA-218 is deleted and downregulated in lung squamous cell carcinoma. PLoS One 2010; 5(9):e12560; PMID:20838434; https://doi.org/10.1371/journal.pone.0012560
- Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res 2010; 107(11):1336-44; PMID:20947829; https://doi.org/10.1161/CIRCRESAHA.110.227926
- Punnamoottil B, Rinkwitz S, Giacomotto J, Svahn AJ, Becker TS. Motor neuron-expressed microRNAs 218 and their enhancers are nested within introns of Slit2/3 genes. Genesis 2015; 53(5):321-8; PMID:25864959; https://doi.org/10.1002/dvg.22852
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol Rev 2004; 84(3):869-901; PMID:15269339; https://doi.org/10.1152/physrev.00035.2003
- Ratheesh A, Yap AS. A bigger picture: Classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol 2012; 13(10):673-9; PMID:22931853; https://doi.org/10.1038/nrm3431
- Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 2013; 25(1):39-46; PMID:23127608; https://doi.org/10.1016/j.ceb.2012.10.010
- Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol 2011; 23(5):515-22; PMID:21807490; https://doi.org/10.1016/j.ceb.2011.07.001
- Ebnet K. Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 2008; 130(1):1-20; PMID:18365233; https://doi.org/10.1007/s00418-008-0418-7
- Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: Role and clinical significance in cancer. Cancer Invest 2009; 27(10):1023-37; PMID:19909018; https://doi.org/10.3109/07357900902769749
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res 2010; 8(5):629-42; PMID:20460404; https://doi.org/10.1158/1541-7786.MCR-10-0139
- Paredes J, Figueiredo J, Albergaria A, Oliveira P, Carvalho J, Ribeiro AS, Caldeira J, Costa AM, Simões-Correia J, Oliveira MJ, et al. Epithelial E- and P-cadherins: Role and clinical significance in cancer. Biochim Biophys Acta 2012; 1826(2):297-311; PMID:22613680
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell 2002; 110(6):673-87; PMID:12297042; https://doi.org/10.1016/S0092-8674(02)00971-6
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol 2007; 25:619-47; PMID:17201681
- Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell 2013; 24(5):447-58; PMID:23484852; https://doi.org/10.1016/j.devcel.2013.02.012
- Martin AC. Pulsation and stabilization: Contractile forces that underlie morphogenesis. Dev Biol 2010; 341(1):114-25; PMID:19874815; https://doi.org/10.1016/j.ydbio.2009.10.031
- Hoffman BD, Yap AS. Towards a dynamic understanding of cadherin-based mechanobiology. Trends Cell Biol 2015; 25(12):803-14; PMID:26519989; https://doi.org/10.1016/j.tcb.2015.09.008
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9(9):690-701; PMID:18719708; https://doi.org/10.1038/nrm2476
- Hall A. Rho family GTPases. Biochem Soc Trans 2012; 40(6):1378-82; PMID:23176484; https://doi.org/10.1042/BST20120103
- Quiros M, Nusrat A. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin Cell Dev Biol 2014; 36:194-203; PMID:25223584
- Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell 2012; 23(2):310-23; PMID:22090347; https://doi.org/10.1091/mbc.E11-08-0719
- Arnold TR, Stephenson RE, Miller AL. Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function. Exp Cell Res 2017; PMID:28363828; https://doi.org/10.1016/j.yexcr.2017.03.053
- Ypsilanti AR, Zagar Y, Chedotal A. Moving away from the midline: new developments for Slit and Robo. Development 2010; 137(12):1939-52; PMID:20501589; https://doi.org/10.1242/dev.044511
- Dressler GR. Advances in early kidney specification, development and patterning. Development 2009; 136(23):3863-74; PMID:19906853; https://doi.org/10.1242/dev.034876
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 2004; 6(5):709-17; PMID:15130495; https://doi.org/10.1016/S1534-5807(04)00108-X
- Hwang DY, Kohl S, Fan X, Vivante A, Chan S, Dworschak GC, Schulz J, van Eerde AM, Hilger AC, Gee HY, Pennimpede T, et al. Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum Genet 2015; 134(8):905-16; PMID:26026792; https://doi.org/10.1007/s00439-015-1570-5
- Wainwright EN, Wilhelm D, Combes AN, Little MH, Koopman P. ROBO2 restricts the nephrogenic field and regulates Wolffian duct-nephrogenic cord separation. Dev Biol 2015; 404(2):88-102; PMID:26116176; https://doi.org/10.1016/j.ydbio.2015.05.023
- Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. J Cell Biol 2015; 209(2):199-210; PMID:25918223; https://doi.org/10.1083/jcb.201410017
- Farquhar MG, Wissig SL, Palade GE. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med 1961; 113:47-66; PMID:13698249
- Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol 1974; 63(3):883-903; PMID:4612049; https://doi.org/10.1083/jcb.63.3.883
- Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974; 60(2):423-33; PMID:4204974; https://doi.org/10.1083/jcb.60.2.423
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003; 83(1):253-307; PMID:12506131; https://doi.org/10.1152/physrev.00020.2002
- Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 2007; 17(9):428-37; PMID:17804239; https://doi.org/10.1016/j.tcb.2007.06.006
- Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: New strategies are needed to retard progressive renal function decline. Semin Nephrol 2012; 32(5):407-14; PMID:23062980; https://doi.org/10.1016/j.semnephrol.2012.07.002
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 1999; 96(14):7962-7; PMID:10393930; https://doi.org/10.1073/pnas.96.14.7962
- Done SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K. Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 2008; 73(6):697-704; PMID:18046313; https://doi.org/10.1038/sj.ki.5002707
- Hamano Y, Grunkemeyer JA, Sudhakar A, Zeisberg M, Cosgrove D, Morello R, Lee B, Sugimoto H, Kalluri R. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem 2002; 277(34):31154-62; PMID:12039968; https://doi.org/10.1074/jbc.M204806200
- Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 2006; 440(7085):818-23; PMID:16525419; https://doi.org/10.1038/nature04662
- Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T. Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol Cell Biol 2008; 28(6):2035-46; PMID:18212058; https://doi.org/10.1128/MCB.01770-07
- New LA, Keyvani Chahi A, Jones N. Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 2013; 288(3):1500-10; PMID:23188823; https://doi.org/10.1074/jbc.M112.439463
- Fan X, Li Q, Pisarek-Horowitz A, Rasouly HM, Wang X, Bonegio RG, Wang H, McLaughlin M, Mangos S, Kalluri R, et al. Inhibitory effects of Robo2 on nephrin: A crosstalk between positive and negative signals regulating podocyte structure. Cell Rep 2012; 2(1):52-61; PMID:22840396; https://doi.org/10.1016/j.celrep.2012.06.002
- Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol 2013; 9(4):200-10; PMID:23338211; https://doi.org/10.1038/nrneph.2012.291
- Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol 2009; 19(9):475-86; PMID:19716305; https://doi.org/10.1016/j.tcb.2009.07.001
- Hirata H, Sokabe M, Lim CT. Molecular mechanisms underlying the force-dependent regulation of actin-to-ECM linkage at the focal adhesions. Prog Mol Biol Transl Sci 2014; 126:135-54; PMID:25081617
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10(11):778-90; PMID:19851336; https://doi.org/10.1038/nrm2786
- Johnstone DB, Zhang J, George B, Léon C, Gachet C, Wong H, Parekh R, Holzman LB. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol 2011; 31(10):2162-70; PMID:21402784; https://doi.org/10.1128/MCB.05234-11
- Miura K, Kurihara H, Horita S, Chikamoto H, Hattori M, Harita Y, Tsurumi H, Kajiho Y, Sawada Y, Sasaki S, et al. Podocyte expression of nonmuscle myosin heavy chain-IIA decreases in idiopathic nephrotic syndrome, especially in focal segmental glomerulosclerosis. Nephrol Dial Transplant 2013; 28(12):2993-3003; PMID:24042022; https://doi.org/10.1093/ndt/gft350
- Fan X, Yang H, Kumar S, Tumelty KE, Pisarek-Horowitz A, Rasouly HM, Sharma R, Chan S, Tyminski E, Shamashkin M, et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 2016; 1(19):e86934; PMID:27882344; https://doi.org/10.1172/jci.insight.86934
- Mui KL, Chen CS, Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci 2016; 129(6):1093-100; PMID:26919980; https://doi.org/10.1242/jcs.183699
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, et al. Signal transduction in neuronal migration: Roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 2001; 107(2):209-21; PMID:11672528; https://doi.org/10.1016/S0092-8674(01)00530-X
- Yiin JJ, Hu B, Jarzynka MJ, Feng H, Liu KW, Wu JY, Ma HI, Cheng SY. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol 2009; 11(6):779-89; PMID:20008733; https://doi.org/10.1215/15228517-2009-017
- Tseng RC, Chang JM, Chen JH, Huang WR, Tang YA, Kuo IY, Yan JJ, Lai WW, Wang YC. Deregulation of SLIT2-mediated Cdc42 activity is associated with esophageal cancer metastasis and poor prognosis. J Thorac Oncol 2015; 10(1):189-98; PMID:25490006; https://doi.org/10.1097/JTO.0000000000000369
- Bhattacharya R, Mukherjee N, Dasgupta H, Islam MS, Alam N, Roy A, Das P, Roychoudhury S, Panda CK. Frequent alterations of SLIT2-ROBO1-CDC42 signalling pathway in breast cancer: Clinicopathological correlation. J Genet 2016; 95(3):551-63; PMID:27659325; https://doi.org/10.1007/s12041-016-0678-2
- Feng Y, Feng L, Yu D, Zou J, Huang Z. srGAP1 mediates the migration inhibition effect of Slit2-Robo1 in colorectal cancer. J Exp Clin Cancer Res 2016; 35(1):191; PMID:27923383; https://doi.org/10.1186/s13046-016-0443-7 10.1186/s13046-016-0469-x
- Shiau CE, Bronner-Fraser M. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development 2009; 136(24):4155-64; PMID:19934013; https://doi.org/10.1242/dev.034355
- Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol 2002; 4(10):798-805; PMID:12360290; https://doi.org/10.1038/ncb858
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol 2007; 9(8):883-92; PMID:17618275; https://doi.org/10.1038/ncb1614
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. Embo J 1995; 14(24):6107-15; PMID:8557030
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem 1996; 61(4):514-23; PMID:8806074; https://doi.org/10.1002/(SICI)1097-4644(19960616)61:4%3c514::AID-JCB4%3e3.3.CO;2-D 10.1002/(SICI)1097-4644(19960616)61:4%3c514::AID-JCB4%3e3.0.CO;2-R
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol 2007; 23:237-61; PMID:17539752
- Prasad A, Paruchuri V, Preet A, Latif F, Ganju RK. Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J Biol Chem 2008; 283(39):26624-33; PMID:18611862; https://doi.org/10.1074/jbc.M800679200
- Tseng RC, Lee SH, Hsu HS, Chen BH, Tsai WC, Tzao C, Wang YC. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer Res 2010; 70(2):543-51; PMID:20068157; https://doi.org/10.1158/0008-5472.CAN-09-2084
- Grone J, Doebler O, Loddenkemper C, Hotz B, Buhr HJ, Bhargava S. Robo1/Robo4: Differential expression of angiogenic markers in colorectal cancer. Oncol Rep 2006; 15(6):1437-43; PMID:16685377
- Wang B, Xiao Y, Ding BB, Zhang N, Yuan Xb, Gui L, Qian KX, Duan S, Chen Z, Rao Y, Geng JG. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003; 4(1):19-29; PMID:12892710; https://doi.org/10.1016/S1535-6108(03)00164-8
- Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF, Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res 2011; 21(4):609-26; PMID:21283129; https://doi.org/10.1038/cr.2011.17
- Imai K, Hirata S, Irie A, Senju S, Ikuta Y, Yokomine K, Harao M, Inoue M, Tsunoda T, Nakatsuru S, et al. Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers. Clin Cancer Res 2008; 14(20):6487-95; PMID:18927288; https://doi.org/10.1158/1078-0432.CCR-08-1086
- Vieira AF, Paredes J. P-cadherin and the journey to cancer metastasis. Mol Cancer 2015; 14:178; PMID:26438065
- Ribeiro AS, Sousa B, Carreto L, Mendes N, Nobre AR, Ricardo S, Albergaria A, Cameselle-Teijeiro JF, Gerhard R, Söderberg O, et al. P-cadherin functional role is dependent on E-cadherin cellular context: A proof of concept using the breast cancer model. J Pathol 2013; 229(5):705-18; PMID:23180380; https://doi.org/10.1002/path.4143
- Sarrio D, Palacios J, Hergueta-Redondo M, Gomez-Lopez G, Cano A, Moreno-Bueno G. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 2009; 9:74; PMID:19257890
- Bauer K, Dowejko A, Bosserhoff AK, Reichert TE, Bauer R. Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line. Carcinogenesis 2011; 32(6):935-43; PMID:21459757; https://doi.org/10.1093/carcin/bgr059
- Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. Faseb J 1992; 6(3):886-92; PMID:1371260
- Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J Clin Diagn Res 2015; 9(6):XE01-XE05; PMID:26266204
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144(5):646-74; PMID:21376230; https://doi.org/10.1016/j.cell.2011.02.013
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995; 1(2):149-53; PMID:7585012; https://doi.org/10.1038/nm0295-149
- Parangi S, O'Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, Hanahan D. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A 1996; 93(5):2002-7; PMID:8700875; https://doi.org/10.1073/pnas.93.5.2002
- Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev 2005; 16(4-5):535-48; PMID:15979925; https://doi.org/10.1016/j.cytogfr.2005.05.002
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2010; 2(5):a001875; PMID:20452960; https://doi.org/10.1101/cshperspect.a001875
- Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood 2009; 114(19):4300-9; PMID:19741192; https://doi.org/10.1182/blood-2008-12-193326
- Guijarro-Munoz I, Cuesta AM, Alvarez-Cienfuegos A, Geng JG, Alvarez-Vallina L, Sanz L. The axonal repellent Slit2 inhibits pericyte migration: Potential implications in angiogenesis. Exp Cell Res 2012; 318(4):371-78; PMID:22198087; https://doi.org/10.1016/j.yexcr.2011.12.005
- Zhao H, Anand AR, Ganju RK. Slit2-Robo4 pathway modulates lipopolysaccharide-induced endothelial inflammation and its expression is dysregulated during endotoxemia. J Immunol 2014; 192(1):385-93; PMID:24272999; https://doi.org/10.4049/jimmunol.1302021
- Wang LJ, Zhao Y, Han B, Ma YG, Zhang J, Yang DM, Mao JW, Tang FT, Li WD, Yang Y, Wang R, et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci 2008; 99(3):510-7; PMID:18201275; https://doi.org/10.1111/j.1349-7006.2007.00721.x
- Li S, Huang L, Sun Y, Bai Y, Yang F, Yu W, Li F, Zhang Q, Wang B, Geng JG. Slit2 Promotes Angiogenic Activity Via the Robo1-VEGFR2-ERK1/2 Pathway in Both In Vivo and in vitro Studies. Invest Ophthalmol Vis Sci 2015; 56(9):5210-7; PMID:26244297; https://doi.org/10.1167/iovs-14-16184
- Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL, Bicknell R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. Faseb J 2009; 23(2):513-22; PMID:18948384; https://doi.org/10.1096/fj.07-098269
- Seth P, Lin Y, Hanai J, Shivalingappa V, Duyao MP, Sukhatme VP. Magic roundabout, a tumor endothelial marker: Expression and signaling. Biochem Biophys Res Commun 2005; 332(2):533-41; PMID:15894287; https://doi.org/10.1016/j.bbrc.2005.03.250
- Marlow R, Binnewies M, Sorensen LK, Monica SD, Strickland P, Forsberg EC, Li DY, Hinck L. Vascular Robo4 restricts proangiogenic VEGF signaling in breast. Proc Natl Acad Sci U S A 2010; 107(23):10520-5; PMID:20498081; https://doi.org/10.1073/pnas.1001896107
- Enomoto S, Mitsui K, Kawamura T, Iwanari H, Daigo K, Horiuchi K, Minami T, Kodama T, Hamakubo T. Suppression of Slit2/Robo1 mediated HUVEC migration by Robo4. Biochem Biophys Res Commun 2016; 469(4):797-802; PMID:26713366; https://doi.org/10.1016/j.bbrc.2015.12.075
- Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. Faseb J 2005; 19(1):121-23; PMID:15486058
- Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol 2004; 14(3):225-30; PMID:14761655; https://doi.org/10.1016/j.cub.2004.01.006
- Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci 2001; 4(7):695-701; PMID:11426225
- Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 2008; 14(4):448-53; PMID:18345009
- Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol 2009; 11(11):1325-31; PMID:19855388
- London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2010; 2(23):23ra19; PMID:20375003
- Zhang X, Yu J, Kuzontkoski PM, Zhu W, Li DY, Groopman JE. Slit2/Robo4 signaling modulates HIV-1 gp120-induced lymphatic hyperpermeability. PLoS Pathog 2012; 8(1):e1002461; PMID:22241990
- Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell 2011; 20(1):33-46; PMID:21238923
- Zhang F, Prahst C, Mathivet T, Pibouin-Fragner L, Zhang J, Genet G, Tong R, Dubrac A, Eichmann A. The Robo4 cytoplasmic domain is dispensable for vascular permeability and neovascularization. Nat Commun 2016; 7:13517; PMID:27882935
- Yang YC, Chen PN, Wang SY, Liao CY, Lin YY, Sun SR, Chiu CL, Hsieh YS, Shieh JC, Chang JT. The differential roles of Slit2-exon 15 splicing variants in angiogenesis and HUVEC permeability. Angiogenesis 2015; 18(3):301-12; PMID:26021305
- Santiago-Martinez E, Soplop NH, Patel R, Kramer SG. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J Cell Biol 2008; 182(2):241-8; PMID:18663139
- Han HX, Geng JG. Over-expression of Slit2 induces vessel formation and changes blood vessel permeability in mouse brain. Acta Pharmacol Sin 2011; 32(11):1327-36; PMID:21986575
- Li JC, Han L, Wen YX, Yang YX, Li S, Li XS, Zhao CJ, Wang TY, Chen H, Liu Y, et al. Increased permeability of the blood-brain barrier and Alzheimer's disease-like alterations in slit-2 transgenic mice. J Alzheimers Dis 2015; 43(2):535-48; PMID:25114073
- Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 2012; 76(5):931-44; PMID:23217742
- Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci 2015; 18(1):36-45; PMID:25485759
- Dascenco D, Erfurth ML, Izadifar A, Song M, Sachse S, Bortnick R, Urwyler O, Petrovic M, Ayaz D, He H, et al. Slit and receptor tyrosine phosphatase 69D confer spatial specificity to axon branching via Dscam1. Cell 2015; 162(5):1140-54; PMID:26317474
