ABSTRACT
Airway and intestinal epithelial permeability barriers are crucial in epithelial homeostasis. High mobility group box 1 (HMGB1), increased by various stimuli, is involved in the induction of airway inflammation, as well as the pathogenesis of inflammatory bowel disease. HMGB1 enhances epithelial hyperpermeability. Two-and-a-half dimensional (2.5D) culture assays are experimentally convenient and induce cells to form a more physiological tissue architecture than 2D culture assays for molecular transfer mechanism analysis. In 2.5D culture, treatment with HMGB1 induced permeability of FITC-dextran into the lumen formed by human lung, nasal and intestinal epithelial cells. The tricellular tight junction molecule angulin-1/LSR is responsible for the epithelial permeability barrier at tricellular contacts and contributes to various human airway and intestinal inflammatory diseases. In this review, we indicate the mechanisms including angulin-1/LSR and multiple signaling in dysfunction of the epithelial permeability barrier induced by HMGB1 in 2.5D culture of human airway and intestinal epithelial cells.
KEYWORDS:
- 2.5d matrigel cultures
- epithelial permeability barriers
- hmgb1
- proinflammatory cytokines
- tight junctions
- claudins
- angulin-1/LSR
- tricellulin
- TGF-β
- EW-7197
- PYK2
- PF431396
- tnfα-antibody
- p63
- normal human pulmonary epithelial cells
- normal human nasal epithelial cells
- human intestinal epithelial cell line CACO-2
- cell metabolism
Introduction
The airway and gut epithelia interface with the environment, where they act as a physiological barrier critical for protecting the body from environmental allergens, pathogenic microbes and viruses, toxins, volatile organic compounds and other pollutants.1–Citation3 The epithelial permeability barriers are crucial in epithelial homeostasis. High mobility group box 1 (HMGB1), increased by various stimuli, is involved in the induction of airway inflammation and inflammatory bowel disease. Two-and-a-half dimensional (2.5D) culture assays are convenient and induce cells to form a more physiological tissue architecture than 2D culture assays for molecular transfer mechanism analysis (). In 2.5D culture, treatment with HMGB1 induced permeability of FITC-dextran into the lumen formed by human lung, nasal and intestinal epithelial cells (, ). The tricellular tight junction molecule angulin-1/LSR is responsible for the epithelial permeability barrier at tricellular contacts and contributes to various human airway and intestinal inflammatory diseases as well as other tight junction molecules (, ). In this review, we demonstrated the mechanisms including angulin-1/LSR, multiple signaling and cellular metabolism in dysfunction of the epithelial permeability barrier induced by HMGB1 in 2.5D culture of human airway and intestinal epithelial cells (primary cultures of human lung epithelial (HLE) cells and human nasal epithelial (HNE) cells and human colorectal adenocarcinoma cell line Caco-2).
Figure 1. Schematic representation of (a) methods and (b) localization of tight junction proteins in 2D and 2.5D cultures. (c) Schematic representation and images in localization of OCLN and angulin-1/LSR in 2.5D culture of Caco-2.
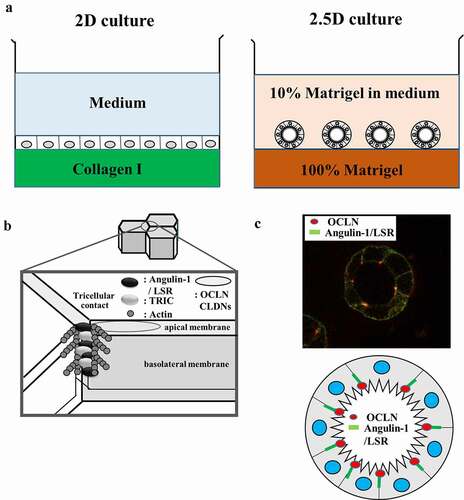
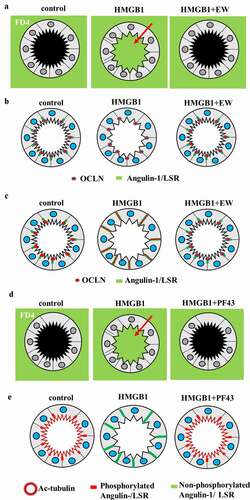
Figure 2. (a) Schematic representation of FD4 in 2.5D culture of HLE cells and HNE cells pretreated with EW-7197 (EW) before treatment with HMGB1. (b) Schematic representation of OCLN and LSR in 2.5D culture of HLE cells and HNE cells pretreated with EW before treatment with HMGB1. (c) Schematic representation of OCLN and angulin-1/LSR in 2.5D culture of Caco-2 cells pretreated with EW before treatment with HMGB1. (d) Schematic representation of FD4 in 2.5D culture of Caco-2 cells pretreated with PF431396 (PF43) before treatment with HMGB1. (e) Schematic representation of phosphorylated and non-phosphorylated angulin-1/LSR and Ac-tubulin in 2.5D culture of Caco-2 cells pretreated with PF43 before treatment with HMGB1.
Tight junctions
The tight junction (TJ) is an epithelial cell–cell junction that regulates the flow of solutes through paracellular pathways and maintains cell polarity.Citation4 TJs are involved in various signal transduction pathways that regulate epithelial cell proliferation, gene expression, differentiation and morphogenesis.Citation5,Citation6 TJ proteins are closely involved in cancer, innate immunity and infectious diseases.Citation7 They are composed of claudins (CLDNs), occludins (OCLN), JAMs and scaffold proteins such as ZO.Citation8,Citation9 CLDNs are major components of TJs in epithelial and endothelial cells.Citation10,Citation11 CLDN-2 is expressed in the tight junctions of leaky epithelia, where it forms cation-selective and water permeable paracellular channels.Citation12 Its expression is modulated by a variety of conditions.Citation13
Angulin-1/lipolysis-stimulated lipoprotein receptor (LSR) and tricellulin (TRIC) seal the extracellular spaces where three epithelial cells come into contact and have various functions.Citation14 The family of angulins consists of angulin-1/LSR, angulin-2, (immunoglobulin-like domain-containing receptor 1 [ILDR1]) and angulin-3 (ILDR2).Citation15 LSR recruits TRIC to tTJs, and both proteins are required for the full barrier function of epithelial cellular sheets.Citation15 Downregulation of LSR influences not only the barrier function but also various kinds of signaling.Citation16–19 The c-Jun N-terminal kinase (JNK) pathway is involved in the regulation of tricellular tight junctions, including TRIC expression and the barrier function.Citation16,Citation20 Protein tyrosine kinase 2β (Pyk2) phosphorylates angulin-1/LSR and enhances the localization of angulin-1/LSR and TRIC at tTJs.Citation21,Citation22 PF431396 (PF43), which is an inhibitor of Pyk2, suppresses the recruitment of LSR and TRIC from tTJs to bTJs and cytoplasmic regions.Citation21
Tight junctions in lung
The airways are broadly classified into conducting and respiratory regions. The conducting airways consist of the trachea, bronchi, and bronchioles, while respiratory regions consist of respiratory bronchioles and alveoli. The conducting airways are lined by a continuous layer of bronchiolar epithelial cells, which act as a first barrier for inhaled materials and play an important role in protection from external pathogens such as bacteria, viruses, chemical substances and allergic components.Citation23,Citation24 On the other hand, alveolar epithelial cells, which are vital for gas exchanges, form a barrier for an air-liquid interface.Citation25 To maintain the air-liquid interface and control gas exchanges, alveolar epithelial cells provide a proper permeability barrier.Citation26 The main characteristic of epithelial tissues is that epithelial cells are arranged in monolayers or stratified layers and tightly adhere to each other. This sheet-like structure provides a barrier function that separates the apical and basolateral compartments of various tissues, and maintains homeostasis.
In the normal lung, there are various reports about expression of CLDNs in bronchiolar epithelial cells and alveolar epithelial cells. In human bronchiolar epithelium, expression of CLDN-1, −2, −3, −4, −5, −7 and −8 is reported,Citation27–31 whereas 14 CLDNs are expressed in alveolar epithelium and CLDN-3, −4 and −18 are the major components of type II pneumocytes.Citation30,Citation32,Citation33 Angulin-1/LSR, expressed at tTJ of most epithelial tissues,Citation18,Citation19,Citation34 is closely associated with lung epithelial permeability barriers.Citation35,Citation36
Asthma
Asthma is characterized by chronic airway inflammation induced by allergens, chemical mediators and other external pathogens, and the airway epithelial barrier plays an important role in protection against these factors.Citation24,Citation37 This airway epithelial barrier is attenuated by changing expression of CLDNs in asthma. Some reports have discussed the relationship of the lung-specific TJ protein CLDN-18.1 and airway epithelial barrier dysfunction in asthma.Citation37–39 Interleukin-13 (IL-13), which is a cytokine secreted by T helper type 2 (Th2) cells, is highly expressed in Th2 cells in asthma, and decreases trans-epithelial electronical resistance (TEER) and increases the permeability of FITC-conjugated dextran by decreasing expression of CLDN-18.1 in 16HBE cells.Citation38,Citation39 Another report showed that overexpression of orosomucoid-like protein isoform 3 (ORMDL3), which is a gene closely associated with childhood onset asthma, decreased expression of CLDN-18.1 in human bronchial epithelial 16HBE cells.Citation40 As for the other CLDNs, high-mobility group box 1 protein (HMGB1), a non-histone chromatin-associated protein involved in pathological process in asthma, decreases TEER and increases permeability to 4 kDa FITC-dextran by decreasing expression of CLDN-141. Transforming growth factor-β (TGF-β), which contributes to epithelial permeability,Citation41,Citation42 is a major mediator of airway tissue remodeling during atopic asthma, and it increases epithelial permeability via redistribution of CLDN-3 from TJ into cell nuclei.Citation25 Furthermore, house dust mite allergen Der p1, an important factor in asthma, increases expression of CLDN-4 and decreases TEER in normal human bronchial epithelial cells.Citation43 In addition, exposure to diesel exhaust particles, a major component of airborne particulate matter (PM), decreases expression of the tTJ protein tricellulin, decreases TEER and increases permeability to 4 kDa FITC-dextran in 16HBE cells.Citation44
Chronic obstructive pulmonary disease (COPD)
Chronic obstructive pulmonary disease (COPD), which is one of the most common chronic respiratory diseases, causes slow progressive and irreversible alveolar emphysema and airway inflammation. The main risk factor for COPD is cigarette smoking, including indirect and passive exposures, and some cases are caused by inhalation of toxic gases and particulates due to air pollution and occupational diseases. The epithelial barrier disruption induced by cigarette smoke is associated with not only the pathogenesis of COPD, but also viral and bacterial infections in COPD.Citation45 In COPD, some reports have revealed the relationship between cigarette smoke exposure and disruption of the epithelial barrier inducing by changing expressions of TJ proteins. The cigarette smoke exposure induces expression of CLDN-3 as an early inflammatory reaction.Citation46
Idiopathic pulmonary fibrosis (IPF)
Idiopathic pulmonary fibrosis (IPF) is one of the idiopathic interstitial pneumonias, a pulmonary disease in which severe fibrosis of the lungs is the main pathology, resulting in restrictive ventilatory impairment.Citation43 The mechanisms leading to IPF are unclear, but one of the causes of pathogenesis in IPF is considered to be epithelial injury, and disruption of epithelial barrier is important in the pathogenesis of IPF. Recent reports have focused on TGF-β and HMGB1 as factors in the pathogenesis of IPF. TGF-β is highly expressed in fibrotic lungs,Citation47 and HMGB1 levels in sputum and serum are higher in patients with asthma, COPD and IPF than in the healthy lung.Citation48 As we mentioned in the previous section, both TGF-β and HMGB1 contribute to epithelial permeability.Citation41,Citation42,Citation49 In addition, TGF-β induces differentiation of alveolar epithelial cells into fibroblasts via epithelial-to-mesenchymal transition (EMT)Citation50 and this process also plays a crucial role in the progression of IPF.Citation51 HMGB1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway.Citation49 Furthermore, expression of HMGB1 is increased by TGF-β and knockdown of HMGB1 reverses EMT induced by TGF-β in human alveolar epithelial cell line A549 and normal human bronchial epithelial cell line BEAS-2B.Citation52 Importantly, these two factors may affect the epithelial barrier via TJ proteins in IPF.
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS)
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are acute and severe respiratory failures secondary to various diseases. Injury of the alveolar epithelium in ALI/ARDS increases epithelial permeability and leads to pulmonary edema.Citation53,Citation54 Therefore, the disruption of the TJ complex in alveolar epithelial cells can be one of the major causes. Most of the investigations about the relationship between changing TJ molecules and epithelial hyperpermeability in ALI/ARDS have focused on the expression of CLDN-4 and CLDN-18.1, which are highly expressed in alveolar epithelial cells. CLDN-4 is a sealing type of CLDN that plays an important role in the alveolar epithelial barrier.Citation32,Citation55 Studies have revealed that an increase of CLDN-4 expression decreases paracellular permeability,Citation27 whereas knockout of CLDN-4 increases solute permeability of the alveolar epithelium and, as a result, increases susceptibility to lung injury.Citation56 In an experiment using a ventilator-induced lung injury model in mice, expression of CLDN-4 was increased after 3 h of moderate or high tidal ventilation, indicating that expression of CLDN-4 is increased in the early stage of ALI.Citation57 The increase of CLDN-4 expression in ARDS may be a compensatory response of the alveolar epithelium and alteration of CLDN-4 expression can be a potential target for therapy. On the other hand, CLDN-18.1 is a lung-specific TJ molecule that is highly expressed in normal alveolar epithelium and the downregulation of its expression impairs epithelial barrier function.Citation58 Some studies have indicated that expression of CLDN-18.1 is decreased in ALI/ARDS.Citation42,Citation59,Citation60 Knockout of CLDN-18.1 expression increases solute permeability and alveolar fluid clearance, and furthermore, expression of CLDN-3 and CLDN-4 is remarkably increased.Citation61 CLDN-18.1 may be associated with permeability and alveolar fluid homeostasis, either directly or by altering expression of other CLDNs.
Tight junctions in nasal epithelium
In the human nasal epithelium, OCLN, JAM-A, ZO-1, ZO-2, CLDN-1, −4, −7, −8, −12, −13, −14 at bicellular TJs (bTj) and TRIC and angulin-1/LSR at tricellular TJs (tTJ) are detected on the surfaces of cells.Citation1Citation2,Citation6 Nasal epithelial barrier dysfunction contributes to various nasal diseases, including allergies.Citation62–66
Tight junctions in intestine
TJs play a major role in maintaining the integrity and impermeability of the intestinal barrier, which is required for the maintenance of mucosal homeostasis. In the human small intestine, CLDN-1, −2, −3, −4, −5, −7, −8, −12 and −15 are expressed.Citation67 In addition, CLDN-2 is expressed along the crypt-villus axis, whereas in the fetal colon the expression is limited to the crypt base and in the adult colon tissue it is even absent.Citation68 Tricellular junctions regulate intestinal stem cell behavior to maintain homeostasis.Citation69
Inflammatory bowel disease (IBD)
TJ molecules play important roles in the intestinal epithelial barrier of inflammatory bowel disease (IBD).Citation70 In addition, an abundance of CLDN-2 has been widely observed in ulcerative colitis (UC) and Crohn’s disease (CD).Citation71,Citation72 TNFα and IL-13 have been linked to the observed CLDN-2 increase.Citation71,Citation72 TNFα also causes dislocalization of CLDN-5 and CLDN-8 from the TJ to sub-TJ membrane components and into endosomes.Citation72 CLDN-4 and −7 are downregulated in UC.Citation7 In CD, CLDN-5 and −8 are downregulated.Citation71 In IBD, recent studies have indicated that zinc is important for the maintenance of the mucosal barrier.Citation73 In UC, downregulation of TRIC and CLDN-4, and upregulation of CLDN-2 are observed.Citation74,Citation75 The downregulation of TRIC in active UC is recovered in remission from UC, while angulin-1/LSR expression is in remission UC at the same level as in the control.Citation76 In CD, TRIC expression level is unchanged, but its localization shifts from crypts to surface epithelium.Citation76
HMGB1
High mobility group box 1 (HMGB1), a chromatin-associated protein, is one of the damage-associated molecular patterns (DAMPs) and is also a proinflammatory mediator that belongs to the alarmin family.Citation77 HMGB1 is abundantly and widely expressed in a variety of cell nuclei and plays a role in gene transcription in various human diseases, including autoimmune diseases, inflammatory diseases and cancer.Citation8 It promotes the induction of inflammatory cytokines in the pathogenesis of various inflammatory diseases.Citation78
HMGB1 is involved in the induction of airway inflammation and injury in patients with allergy, asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF) and respiratory virus infections.Citation79–81 The HMGB1 levels in sputum and serum are higher in patients with asthma, COPD, and IPF than in healthy subjects.Citation48,Citation79 It impairs airway epithelial barrier function through activation of the receptor for advanced glycation end-products (RAGE)/ERK pathway.Citation49
HMGB1 is also involved with allergic rhinitis (AR), chronic rhinosinusitis (CRS) and eosinophilic chronic rhinosinusitis (ECRS).Citation82–84 It induces hyperpermeability of human nasal epithelial cells by downregulation of ZO-1, OCLN and CLDN-1 via hypoxia.Citation85
HMGB1 protein is involved in the development of IBD.Citation86,Citation87 In a UC mouse model prepared with dextran sulfate sodium (DSS), colonic expression of HMGB1 and its receptor, RAGE, was significantly higher than in control mice.Citation88 Coincubation of the cytokines TNFα, IFNγ and IL-1β with human colon cancer Caco-2 cells and rat intestinal epithelial cells (IECs) revealed that supernatant concentrations of HMGB1 in both cell cultures were elevated.Citation89 Incubation of Caco-2 enterocytic monolayer cells with HMGB1 or B-box increases NO expression and hyperpermeability of Caco-2 cells.Citation90
TGF-β and EW-7197
TGF-β1 induces epithelial permeability in respiratory epithelial cells.Citation41 HMGB1 expression is increased by TGF-β1 and knockdown of HMGB1 reverses TGF-β1-induced epithelial–mesenchymal transition (EMT) in human alveolar epithelial cell line A549 and normal human bronchial epithelial cell line BEAS-2B.Citation91 The disruption of the epithelial barrier induced by HMGB1 and inflammatory cytokines contributes to TGF-β/EGF signaling in Caco-2 cells.Citation92 EW-7197 is a TGF-β type I receptor kinase inhibitor with potential anti-inflammatory and antifibrotic properties.Citation93 EW-7197 prevents ulcerative colitis-associated fibrosis and inflammationCitation94 and prevents changes in the distribution of angulin-1/LSR and the epithelial barrier function by TGF-β in a pancreatic cancer cell line.Citation19
p63
Transcription factor p63, which is a member of the p53 family, plays a crucial role in the proliferation and differentiation of various epithelial basal cells.Citation95 It regulates the epithelial barrier via various signaling pathways in the upper airway epithelium.Citation96,Citation97 p63 is upregulated in the epithelium of CRS and nasal polyps and contributes to the formation and maintenance of differentiated pseudostratified bronchial epithelium and epithelial remodeling.Citation98 In bronchioles of IPF lungs, the numbers of p63-positive cells are increased.Citation99 Some p63-positive basal cells undergo EMT, and knockdown of p63 prevents the phenotypic switch in bronchial epithelial cells.Citation100
Analysis of epithelial permeability barriers in 2.5 D culture of human airway and intestinal epithelial cells
The addition of basement membrane proteins such as Matrigel to the medium in 2D cultures is sufficient to induce tissue-specific differentiation of diverse epithelial cells, whereas epithelial tissues often lose their differentiated state and migrate individually when cultured in a stromal matrix such as collagen I (). Two-and-a-half dimensional (2.5D) culture in which the cells are plated with additional 10% Matrigel in the medium on top of 100% Matrigel, induces cells to form a more physiological tissue architecture than 2D assays and the cells remain accessible for molecular analysis (, ). In our system, 35-mm culture glass-coated dishes were coated with 100% Matrigel at 4 C° and incubated at 37 C° for 30 min. The cells (5 × 104) were plated in the medium with 10% Matrigel and cultured for 4 days. The epithelial permeability barrier and the factors controlling it can be assessed in vitro by measurement of transepithelial electrical resistance (TEER) and fluorescein isothiocyanate (FITC) permeability assay. The permeability of fluorescein isothiocyanate (FITC)-dextran (FD4, MW 4.0 kDa) from the outside into the spheroid lumen was examined by using 2.5D Matrigel culture of the cells on 35-mm glass-coated dishes (, ). In our culture system, the cells were incubated in the medium with 1% FD4 at 37 C° for 2 h. Ten spheroids of all experiments were photographed and measured using a confocal laser scanning microscope with imaging software.
Dysfunction of epithelial permeability barriers induced by HMGB1 in 2.5D culture of human lung epithelial (HLE) cells
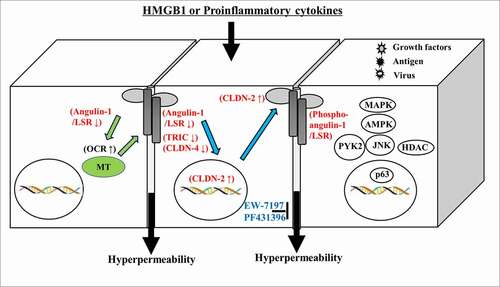
In 2.5D culture of HLE cells, multi-row columnar epithelial cells were observed to have bronchial-like morphology. In 2.5D culture of HLE cells, treatment with HMGB1 induced permeability of FITC-dextran (FD4) into the lumen, whereas pretreatment with EW-7197 prevented this hyperpermeability (). In treatment with HMGB1, LSR was decreased at the membranes, while OCLN was detected at the luminal surface (). Treatment with HMGB1 decreased expression of angulin-1/LSR, TRIC and CLDN-1, −4, −7 and increased that of CLDN-2 (). Pretreatment with EW-7197 prevented the changes of all tight junction molecules induced by HMGB1 (). In 2.5D culture, treatment with TGF-β1 induced permeability of FD-4 and HDAC inhibitors TSA and Quisinostat prevented the hyperpermeability induced by TGF-β136.
In 2.5D culture, knockdown of transcription factor p63 prevents the hyperpermeability induced by HMGB1 as well as pretreatment with EW-719735. In 2D culture of HLE cells with HMGB1, knockdown of p63 increases the levels of angulin-1/LSR and CLDN-4, while pretreatment with EW-7197 enhances the increase of CLDN-4 induced by knockdown of p6335. Immunohistochemical analysis of IPF, CLDN-2, HMGB1 and p63 has revealed that their levels are higher in regenerative epithelium of the terminal bronchial region than in normal epithelium.Citation35 HMGB1 induces epithelial permeability of HLE cells via tight junctions and p63/TGF-β and HDAC signaling. Thus, EW-7197 and HDAC inhibitors may have potential for use in therapy for IPF.
Dysfunction of epithelial permeability barriers induced by HMGB1 in 2.5D culture of human nasal epithelial (HNE) cells
2.5D culture of HNE cells was formed by simple columnar epithelial cells. In 2.5D culture, treatment with HMGB1 induced the permeability of FD4 into the lumen and pretreatment with EW-7197 prevented the hyperpermeability of FD4 into the lumen induced by HMGB1 (). Citation101 Treatment with EW-7197 prevented the downregulation of angulin-1/LSR, TRIC, CLDN-4 and p63 induced by treatment with HMGB1104. HMGB1 induces epithelial permeability of HNE cells via tight junctions and p63/TGF-β signaling.Citation101 EW-7197 may have potential for use in therapy for AR, CRS and ECRS, to which HMGB1 is closely related.
Dysfunction of epithelial permeability barriers induced by HMGB1 in 2.5D culture of human intestinal epithelial cells Caco-2
2.5D culture of Caco-2 cells was formed by simple columnar epithelial cells. In the spheroid cells, OCLN and LSR were strongly expressed at the luminal surface and LSR was also expressed throughout the basolateral membranes (). In 2.5D culture, EW-7197 prevented hyperpermeability of FD4 induced by HMGB1 (). In treatment with HMGB1, the cells expressed OCLN and LSR throughout the basolateral membranes from the luminal surfaces (). Pretreatment with EW-7197 prevented the changes in expression of TJs caused by HMGB195. Transmission electron microscopic (TEM) analysis revealed that “kissing points”, indistinguishable distances between two adjacent cell membranes, were loosened by treatment with HMGB1 in spheroid cells, and this change was prevented by treatment with EW-719795. Treatment with HMGB1 decreased the expression of TRIC and CLDN-1, and pretreatment with EW-7197 prevented the change in expression induced by HMGB195.
HMGB1 affected cilia formation in 2.5D culture. Treatment with HMGB1 decreased Ac-tubulin-positive cilia at the luminal surfaces (). TEM analysis showed that the number of cilia was decreased by treatment with HMGB195. Treatment with EW-7197 prevented these changes caused by HMGB195. The disruption of the epithelial barrier including cilia formation induced by HMGB1 contributed to TGF-β signaling in Caco-2 cells.
To investigate the roles of Pyk2 phosphorylated angulin-1/LSR and TRIC in the intestinal epithelial barrier, 2D and 2.5D cultures of Caco-2 cells were treated with the Pyk2 inhibitor PF43 with or without HMGB1. Treatment with PF43 increased expression of angulin-1/LSR, phosphorylated AMPK and phosphorylated MAPK and decreased that of phosphorylated JNK, with upregulation of the epithelial barrier.Citation22 Treatment with PF43 prevented the downregulation of the epithelial barrier by HMGB in 2D culture.Citation22 Treatment with PF43 prevented the epithelial hyperpermeability and the decrease of cilia induced by HMGB in 2.5D culture (). Treatment with HMGB1 inhibited phosphorylation of the serine of angulin-1/LSR (). Treatment with PF-43 induced phosphorylation of the serine of angulin-1/LSR ().
HMGB1 induces epithelial permeability of Caco-2 cells via tight junctions and TGF-β and Pyk2 signaling. The immunohistochemical results for IBD showed that in the ductal structural area of regenerative colonic epithelium, the expression of HMGB1 was higher than that of the normal region and angulin-1/LSR was not detected in regenerative colonic epithelium.Citation92 EW-7197 and PF43 may have potential for use in therapy for IBD.
Effects of HMGB1 on epithelial permeability barriers via cellular metabolism
In bronchial epithelial Calu-3 cells, knockdown of angulin-1/LSR upregulates the expression of the tight junction molecule claudin-2, AMPK activity, and the cellular metabolism indicated as mitochondrial respiration, and downregulates the epithelial barrier.Citation102 To examine how HMGB1 contributes to the cellular metabolism indicated as mitochondrial respiration, bronchial epithelial Calu-3 cells and intestinal epithelial Caco-2 cells were pretreated with EW-7197 before treatment with HMGB1.95,Citation102 In Calu-3 cells, treatment with HMGB1 increased baseline OCR, maximal OCR, proton leak and ATP production, and decreased spare respiratory capacity with downregulation of the epithelial barrier (Supplemental ). EW7197 prevented the changes induced by HMGB1 (Supplemental ). In Caco-2 cells, treatment with HMGB1 increases baseline OCR, proton leaks and ATP production with downregulation of the epithelial barrier.Citation92 EW-7197 prevents the changes caused by treatment with HMGB195. In Caco-2 cells, PF-43 increased baseline OCR and ATP production with upregulation of the epithelial barrier.Citation92 HMGB1 in part induces the dysfunction of the epithelial permeability barriers of airway and intestinal epithelial cells via cellular metabolism.
Conclusions
In conclusion, our findings indicate dysfunction of the epithelial permeability barriers induced by HMGB1 via expression of phosphorylated angulin-1/LSR, TRIC and claudins dependent on various signaling pathways (MAPK, AMPK, JNK, Pyk2, HDAC, p63/TGF-β) in 2.5D culture of human airway and intestinal epithelial cells (). Furthermore, cell metabolism induced by HMGB1 may also contribute to dysfunction of the epithelial permeability barriers (). TGF-β type I receptor kinase inhibitor EW7197 and Pyk2 inhibitor PF431396 have potential for use in therapy for inflammatory diseases such as IPF, nasal allergies and IBD via not only epithelial barrier function but also cellular metabolism.Citation103–105 The 2.5D culture is similar to human organoids in vivo and the changes induced by various stimuli are sensitive compared to those of 2D culture. Thus, 2.5D culture may be a useful in vitro model for studying the mechanisms of human diseases.
Future considerations
In patients with severe case of COVID-19, serum HMGB is elevated and exogenous HMGB1 induces the expression of SARS-CoV-2 entry receptor ACE2 in alveolar epithelial cells in an AGER-dependent manner.Citation106 An increased concentration of HMGB1 in the serum may contribute to several infectious and inflammatory diseases of the airway and intestine.Citation107 In HNECs treated with HMGB1, upregulation of the COVID-19-related genes cathepsin L (CTSL) and furin is observed104. Cysteine protease CTSL expression is upregulated during chronic inflammation and is involved in processing the COVID-19 spike protein.Citation108Citation109 The protease furin is also involved in mediating SARS-CoV-2 entry.109 It is possible that HMGB1 induced by COVID-19 infection may increase in HNECs and disrupt the epithelial permeability barriers. Thus, HMGB1 in the airway and intestine may be a therapeutic target in the severe inflammation of COVID-19.
Ethics statement
The protocol for human study was reviewed and approved by the ethics committee of the Sapporo Medical University School of Medicine. Written informed consent was obtained from each patient who participated in the investigation. All experiments were carried out in accordance with the approved guidelines and the Declaration of Helsinki.
Supplemental Material
Download TIFF Image (1.5 MB)Disclosure Statement
The authors declare no competing financial interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009 Nov 9;9(11):1–15. PMID: 19855405. doi:https://doi.org/10.1038/nri2653.
- Kojima T, Go M, Takano K, Kurose M, Ohkuni T, Koizumi J, Kamekura R, Ogasawara N, Masaki T, Fuchimoto J, et al. Regulation of tight junctions in upper airway epithelium. Biomed Res Int. 2013;2013:947072. Epub 2012 Dec 29. PMID: 23509817; PMCID: PMC3591135. doi:https://doi.org/10.1155/2013/947072.
- Vllasaliu D, Fowler R, Garnett M, Eaton M, Stolnik S. Barrier characteristics of epithelial cultures modelling the airway and intestinal mucosa: a comparison. Biochem Biophys Res Commun. 2011 Dec 2;415(4):579–585. Epub 2011 Nov 2. PMID: 22079636. doi:https://doi.org/10.1016/j.bbrc.2011.10.108.
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009 Aug;1(2):a002584. doi:https://doi.org/10.1101/cshperspect.a002584. PMID: 20066090; PMCID: PMC2742087.
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005 Oct;17(5):453–458. PMID: 16098725. doi:https://doi.org/10.1016/j.ceb.2005.08.003.
- Takano K, Kojima T, Sawada N, Himi T. Role of tight junctions in signal transduction: an update. EXCLI J. 2014 Oct;13(13):1145–1162. PMID: 26417329; PMCID: PMC4464418.
- Sawada N. Tight junction-related human diseases. Pathol Int. 2013 Jan;63(1):1–12. Epub 2013 Jan 7. PMID: 23356220; PMCID: PMC7168075. doi:https://doi.org/10.1111/pin.12021.
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994 Dec;127(6):1617–1626. PMID: 7798316; PMCID: PMC2120300. doi:https://doi.org/10.1083/jcb.127.6.1617.
- Heinemann U, Schuetz A. Structural features of tight-junction proteins. Int J Mol Sci. 2019 Nov 29;20(23):6020. PMID: 31795346; PMCID: PMC6928914. doi:https://doi.org/10.3390/ijms20236020.
- Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci. 2000;915(1):129–135. doi:https://doi.org/10.1111/j.1749-6632.2000.tb05235.x. PMID: 11193568
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008 Mar;1778(3):631–645. Epub 2007 Oct 25. PMID: 18036336. doi:https://doi.org/10.1016/j.bbamem.2007.10.018.
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002 Dec 15;115(24):4969–4976. PMID: 12432083. doi:https://doi.org/10.1242/jcs.00165.
- Venugopal S, Anwer S, Szászi K. Claudin-2: roles beyond permeability functions. Int J Mol Sci. 2019 Nov 12;20(22):5655. PMID: 31726679; PMCID: PMC6888627. doi:https://doi.org/10.3390/ijms20225655.
- Furuse M, Oda Y, Higashi T, Iwamoto N, Masuda S. Lipolysis-stimulated lipoprotein receptor: a novel membrane protein of tricellular tight junctions. Ann N Y Acad Sci. 2012 Jun;1257(1):54–58. PMID: 22671589. doi:https://doi.org/10.1111/j.1749-6632.2012.06486.x.
- Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013 Feb 15;126(\(Pt4)):966–977. Epub 2012 Dec 13. Erratum in: J Cell Sci. 2013 Aug 15;126\(Pt16): 3797.PMID: 23239027. doi:https://doi.org/10.1242/jcs.116442.
- Konno T, Kohno T, Kikuchi S, Shimada H, Satohisa S, Saito T, Kondoh M, Kojima T. Epithelial barrier dysfunction and cell migration induction via JNK/cofilin/actin by angubindin-1. Tissue Barriers. 2020;8(1):1695475. doi:https://doi.org/10.1080/21688370.2019.1695475. Epub 2019 Nov 29. PMID: 31782346; PMCID: PMC7063864
- Shimada H, Abe S, Kohno T, Satohisa S, Konno T, Takahashi S, Hatakeyama T, Arimoto C, Kakuki T, Kaneko Y, et al. Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci Rep. 2017 Jan;10(7):37049. PMID: 28071680; PMCID: PMC5223122. doi:https://doi.org/10.1038/srep37049.
- Kohno T, Konno T, Kojima T. Role of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor (LSR) in cancer cells. Int J Mol Sci. 2019 Jul 20;20(14):3555. PMID: 31330820; PMCID: PMC6679224. doi:https://doi.org/10.3390/ijms20143555.
- Kyuno T, Kyuno D, Kohno T, Konno T, Kikuchi S, Arimoto C, Yamaguchi H, Imamura M, Kimura Y, Kondoh M, et al. Tricellular tight junction protein LSR/angulin-1 contributes to the epithelial barrier and malignancy in human pancreatic cancer cell line. Histochem Cell Biol. 2020 Jan;153(1):5–16. Epub 2019 Oct 24. PMID: 31650247. doi:https://doi.org/10.1007/s00418-019-01821-4.
- Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010 Nov;225(3):720–733. doi:https://doi.org/10.1002/jcp.22273.PMID:20533305.
- Nakatsu D, Kano F, Shinozaki-Narikawa N, Murata M. Pyk2-dependent phosphorylation of LSR enhances localization of LSR and tricellulin at tricellular tight junctions. PLoS One. 2019 Oct 1;14(10):e0223300. PMID: 31574128; PMCID: PMC6773211. doi:https://doi.org/10.1371/journal.pone.0223300.
- Konno T, Kohno T, Miyakawa M, Tanaka H, Kojima T. Pyk2 inhibitor prevents epithelial hyperpermeability induced by HMGB1 and inflammatory cytokines in Caco-2 cells. Tissue Barriers. 2021 Apr 3;9(2):1890526. Epub 2021 Mar 4. PMID: 33660567; PMCID: PMC8078543. doi:https://doi.org/10.1080/21688370.2021.1890526.
- Inoue H, Akimoto K, Homma T, Tanaka A, Sagara H. Airway epithelial dysfunction in asthma: relevant to epidermal growth factor receptors and airway epithelial cells. J Clin Med. 2020 Nov 18;9(11):3698. PMID: 33217964; PMCID: PMC7698733. doi:https://doi.org/10.3390/jcm9113698.
- Ganesan S, At C, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013 Oct 1;1(4):e24997. Epub 2013 May 30. PMID: 24665407; PMCID: PMC3783221. doi:https://doi.org/10.4161/tisb.24997.
- Schilpp C, Lochbaum R, Braubach P, Jonigk D, Frick M, Dietl P, Wittekindt OH. Wittekindt OH. TGF-β1 increases permeability of ciliated airway epithelia via redistribution of claudin 3 from tight junction into cell nuclei. Pflugers Arch. 2021 Feb 2;473(2):287–311. Epub 2021 Jan 2. PMID: 33386991; PMCID: PMC7835204. doi:https://doi.org/10.1007/s00424-020-02501-2.
- Schlingmann B, Molina SA, Claudins: KM. Gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015 Jun;42:47–57 . Epub 2015 May 4. PMID: 25951797; PMCID: PMC4562902. doi:https://doi.org/10.1016/j.semcdb.2015.04.009.
- Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017 Jan;469(1):135–147. Epub 2016 Dec 5. PMID: 27921210; PMCID: PMC5203840. doi:https://doi.org/10.1007/s00424-016-1917-3.
- Cb C, Tm G, Rc B, JL C, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003 Nov;285(5):L1166–78. Epub 2003 Aug 8. PMID: 12909588. doi:https://doi.org/10.1152/ajplung.00182.2003.
- Kaarteenaho-Wiik R, Soini Y. Claudin-1, −2, −3, −4, −5, and −7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009 Mar;57(3):187–195. Epub 2008 Oct 27. PMID: 18955738; PMCID: PMC2664931. doi:https://doi.org/10.1369/jhc.2008.951566.
- Kaarteenaho R, Merikallio H, Lehtonen S, Harju T, Soini Y. Divergent expression of claudin −1, −3, −4, −5 and −7 in developing human lung. Respir Res. 2010 May 17;11(1):59. PMID: 20478039; PMCID: PMC2886022. doi:https://doi.org/10.1186/1465-9921-11-59.
- Kielgast F, Schmidt H, Braubach P, Winkelmann VE, Thompson KE, Frick M, Dietl P, Wittekindt OH. Glucocorticoids regulate tight junction permeability of lung epithelia by modulating Claudin 8. Am J Respir Cell Mol Biol. 2016 May;54(5):707–717. PMID: 26473470. doi:https://doi.org/10.1165/rcmb.2015-0071OC.
- Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol. 2013;75(1):551–567. doi:https://doi.org/10.1146/annurev-physiol-030212-183809. Epub 2012 Oct 15. PMID: 23072447
- Overgaard CE, Mitchell LA, Koval M. Roles for claudins in alveolar epithelial barrier function. Ann N Y Acad Sci. 2012 Jun;1257(1):167–174. PMID: 22671603; PMCID: PMC3375852. doi:https://doi.org/10.1111/j.1749-6632.2012.06545.x.
- Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014 May 1;2(3):e28960. PMID: 25097825; PMCID: PMC4117683. doi:https://doi.org/10.4161/tisb.28960.
- Kodera Y, Kohno T, Konno T, Arai W, Tsujiwaki M, Shindo Y, Chiba H, Miyakawa M, Tanaka H, Sakuma Y, et al. HMGB1 enhances epithelial permeability via p63/TGF-β signaling in lung and terminal bronchial epithelial cells. Tissue Barriers. 2020 Oct 1;8(4):1805997. Epub 2020 Aug 28. PMID: 32857676; PMCID: PMC7714505. doi:https://doi.org/10.1080/21688370.2020.1805997.
- Shindo Y, Arai W, Konno T, Kohno T, Kodera Y, Chiba H, Miyajima M, Sakuma Y, Watanabe A, Kojima T. Effects of histone deacetylase inhibitors Tricostatin A and Quisinostat on tight junction proteins of human lung adenocarcinoma A549 cells and normal lung epithelial cells. Histochem Cell Biol. 2021 May 11;155(6):637–653. PMID: 33974136. doi:https://doi.org/10.1007/s00418-021-01966-1.
- Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int. 2018 Jan;67(1):12–17. Epub 2017 Sep 21. PMID: 28941636. doi:https://doi.org/10.1016/j.alit.2017.08.011.
- Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, Kanegai C, Fahy JV, Frank JA. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017 Jan;139(1):72–81.e1. Epub 2016 Apr 20. PMID: 27215490; PMCID: PMC5073041. doi:https://doi.org/10.1016/j.jaci.2016.02.035.
- Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, Georas SN. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013 Apr 1;1(2):e24333. PMID: 24665390; PMCID: PMC3875607. doi:https://doi.org/10.4161/tisb.24333.
- Yang R, Tan M, Xu J, Zhao X. Investigating the regulatory role of ORMDL3 in airway barrier dysfunction using in vivo and in vitro models. Int J Mol Med. 2019 Aug;44(2):535–548. Epub 2019 Jun 6. PMID: 31173170; PMCID: PMC6605285. doi:https://doi.org/10.3892/ijmm.2019.4233.
- Togami K, Yamaguchi K, Chono S, Tada H. Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β1 in A549, NCI-H441, and Calu-3 cells: development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J Pharmacol Toxicol Methods. 2017 Jul;86:19–27 . Epub 2017 Mar 1. PMID: 28259823. doi:https://doi.org/10.1016/j.vascn.2017.02.023.
- Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012 Jan 15;302(2):L193–205. Epub 2011 Oct 14. PMID: 22003091. doi:https://doi.org/10.1152/ajplung.00349.2010.
- Majewski S, Piotrowski WJ. Air pollution – an overlooked risk factor for idiopathic pulmonary fibrosis. J Clin Med. 2020 Dec 28;10(1):77. PMID: 33379260; PMCID: PMC7794751. doi:https://doi.org/10.3390/jcm10010077.
- Smyth T, Veazey J, Eliseeva S, Chalupa D, Elder A, Georas SN. Diesel exhaust particle exposure reduces expression of the epithelial tight junction protein tricellulin. Part Fibre Toxicol. 2020 Oct 15;17(1):52. PMID: 33059747; PMCID: PMC7560077. doi:https://doi.org/10.1186/s12989-020-00383-x.
- Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018 Feb;58(2):157–169. PMID: 28933915. doi:https://doi.org/10.1165/rcmb.2017-0200TR.
- Cuzić S, Bosnar M, Kramarić MD, Ferencić Z, Marković D, Glojnarić I, Eraković Haber V. Claudin-3 and Clara cell 10 kDa protein as early signals of cigarette smoke-induced epithelial injury along alveolar ducts. Toxicol Pathol. 2012 Dec;40(8):1169–1187. Epub 2012 Jun 1. PMID: 22659244. doi:https://doi.org/10.1177/0192623312448937.
- Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1778–1783. PMID: 10677534; PMCID: PMC26512. doi:https://doi.org/10.1073/pnas.97.4.1778.
- Yamaguchi K, Iwamoto H, Sakamoto S, Horimasu Y, Masuda T, Miyamoto S, Nakashima T, Ohshimo S, Fujitaka K, Hamada H, et al. Serum high-mobility group box 1 is associated with the onset and severity of acute exacerbation of idiopathic pulmonary fibrosis. Respirology. 2020 Mar 3;25(3):275–280. Epub 2019 Jul 3. PMID: 31270920. doi:https://doi.org/10.1111/resp.13634.
- Huang W, Zhao H, Dong H, Wu Y, Yao L, Zou F, Cai S. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int J Mol Med. 2016 May;37(5):1189–1198. Epub 2016 Mar 24. PMID: 27035254; PMCID: PMC4829140. doi:https://doi.org/10.3892/ijmm.2016.2537.
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L525–34. Epub 2007 Jul 13. PMID: 17631612. doi:https://doi.org/10.1152/ajplung.00163.2007.
- Li LC, Li DL, Xu L, Mo XT, Cui WH, Zhao P, Zhou WC, Gao J, Li J. High-mobility group box 1 mediates epithelial-to-mesenchymal transition in pulmonary fibrosis involving transforming growth factor-β1/smad2/3 signaling. J Pharmacol Exp Ther. 2015 Sep;354(3):302–309. Epub 2015 Jun 30. PMID: 26126535. doi:https://doi.org/10.1124/jpet.114.222372.
- Gui Y, Sun J, You W, Wei Y, Tian H, Jiang S. Glycyrrhizin suppresses epithelial-mesenchymal transition by inhibiting high-mobility group box1 via the TGF-β1/Smad2/3 pathway in lung epithelial cells. PeerJ. 2020 Feb 3;8:e8514. doi:https://doi.org/10.7717/peerj.8514. PMID: 32117622; PMCID: PMC7003690.
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001 May;163(6):1376–1383. PMID: 11371404. doi:https://doi.org/10.1164/ajrccm.163.6.2004035.
- Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2018 Jan 6;2:32. doi:https://doi.org/10.21037/atm.2017.12.18. PMID: 29430449; PMCID: PMC5799138.
- Jin W, Rong L, Liu Y, Song Y, Li Y, Pan J. Increased claudin-3, −4 and −18 levels in bronchoalveolar lavage fluid reflect severity of acute lung injury. Respirology. 2013 May;18(4):643–651. PMID: 23253121. doi:https://doi.org/10.1111/resp.12034.
- Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, Kim KJ, Crandall ED, Borok Z. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol. 2014 Oct 1;307(7):L524–36. Epub 2014 Aug 8. PMID: 25106430; PMCID: PMC4187039. doi:https://doi.org/10.1152/ajplung.00077.2014.
- Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009 Aug;297(2):L219–27. Epub 2009 May 15. PMID: 19447895; PMCID: PMC2742793. doi:https://doi.org/10.1152/ajplung.00043.2009.
- Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, Kanegai C, Fahy JV, Frank JA. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017 Jan;139(1):72–81.e1. Epub 2016 Apr 20.PMID: 27215490. doi:https://doi.org/10.1016/j.jaci.2016.02.035.
- Cohen TS, Gray Lawrence G, Margulies SS. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS One. 2010 Jun 25;5(6):e11322. PMID: 20593014; PMCID: PMC2892473. doi:https://doi.org/10.1371/journal.pone.0011322.
- Ma X, Yu X, Zhou Q. The IL1β-HER2-CLDN18/CLDN4 axis mediates lung barrier damage in ARDS. Aging (Albany NY). 2020 Feb 15;12(4):3249–3265. Epub 2020 Feb 15. PMID: 32065780; PMCID: PMC7066891. doi:https://doi.org/10.18632/aging.102804.
- Li G, Flodby P, Luo J, Kage H, Sipos A, Gao D, Ji Y, Beard LL, Marconett CN, DeMaio L, et al. Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis. Am J Respir Cell Mol Biol. 2014 Aug;51(2):210–222. PMID: 24588076; PMCID: PMC4148039. doi:https://doi.org/10.1165/rcmb.2013-0353OC.
- Wynne M, Atkinson C, Schlosser RJ, Mulligan JK. Contribution of epithelial cell dysfunction to the pathogenesis of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019 Nov;33(6):782–790. Epub 2019 Aug 5. PMID: 31382760; PMCID: PMC6843741. doi:https://doi.org/10.1177/1945892419868588.
- Jiao J, Wang C, Zhang L. Epithelial physical barrier defects in chronic rhinosinusitis. Expert Rev Clin Immunol. 2019 Jun;15(6):679–688. doi: https://doi.org/10.1080/1744666X.2019.1601556. Epub 2019 Apr 9. PMID: 30925220.
- Siti Sarah CO, Md Shukri N, Mohd Ashari NS, Wong KK. Zonula occludens and nasal epithelial barrier integrity in allergic rhinitis. PeerJ. 2020 Sep 4;8:e9834. doi:https://doi.org/10.7717/peerj.9834. PMID: 32953271; PMCID: PMC7476493.
- Rinaldi AO, Korsfeldt A, Ward S, Burla D, Dreher A, Gautschi M, Stolpe B, Tan G, Bersuch E, Melin D, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy. 2021 Apr 8. Epub ahead of print. PMID: 33830511. doi:https://doi.org/10.1111/all.14842.
- Nur Husna SM, Tan H-T-T, Md Shukri N, Mohd Ashari NS, Wong KK. Nasal epithelial barrier integrity and tight junctions disruption in allergic rhinitis: overview and pathogenic insights. Front. Immunol 2021;12:663626. doi:https://doi.org/10.3389/fimmu.2021.663626.
- Lu Z, Ding L, Lu Q, YH C. Claudins in intestines: distribution and functional significance in health and diseases. Tissue Barriers. 2013;1(3):e24978. doi:https://doi.org/10.4161/tisb.24978.
- Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015 Apr 3;3(1–2):e977176. eCollection 2015.PMID: 25838982. doi:https://doi.org/10.4161/21688370.2014.977176.
- Resnik-Docampo M, Koehler CL, Clark RI, Schinaman JM, Sauer V, Wong DM, Lewis S, D’Alterio C, Walker DW, Jones DL. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat Cell Biol. 2017 Jan;19(1):52–59. doi: https://doi.org/10.1038/ncb3454. Epub 2016 Dec 19. PMID: 27992405; PMCID: PMC6336109. 19
- Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017 Mar 23;4(1):33–46. PMID: 28560287; PMCID: PMC5439240. doi:https://doi.org/10.1016/j.jcmgh.2017.03.007.
- Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007 Jan;56(1):61–72. Epub 2006 Jul 5. PMID: 16822808; PMCID: PMC1856677. doi:https://doi.org/10.1136/gut.2006.094375.
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005 Aug;129(2):550–564. PMID: 16083712. doi:https://doi.org/10.1016/j.gastro.2005.05.002.
- Ohashi W, Fukada T. Contribution of zinc and zinc transporters in the pathogenesis of inflammatory bowel diseases. J Immunol Res. 2019 Mar 10 2019; 8396878. https://doi.org/10.1155/2019/8396878 PMID: 30984791; PMCID: PMC6431494
- Kjærgaard S, MMB D, Chang J, Lb R, Hb R, Hytting-Andreasen R, Sm K, Jd S, Bindslev N, Hansen MB. Altered structural expression and enzymatic activity parameters in quiescent ulcerative colitis: are these potential normalization criteria? Int J Mol Sci. 2020 Mar 10;21(5):1887. PMID: 32164249; PMCID: PMC7084207. doi:https://doi.org/10.3390/ijms21051887.
- Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M, Schulzke JD. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018 Mar;11(2):345–356. Epub 2017 Jun 14. PMID: 28612843; PMCID: PMC5730503. doi:https://doi.org/10.1038/mi.2017.52.
- Hu JE, Weiß F, Bojarski C, Branchi F, Schulzke JD, Fromm M, Krug SM. Expression of tricellular tight junction proteins and the paracellular macromolecule barrier are recovered in remission of ulcerative colitis. BMC Gastroenterol. 2021 Mar 31;21(1):141. PMID: 33789594; PMCID: PMC8010963. doi:https://doi.org/10.1186/s12876-021-01723-7.
- Yang H, Wang H, Chavan SS, Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med. 2015 Oct 27;21(Suppl 3):S6–S12. PMID: 26605648; PMCID: PMC4661054. doi:https://doi.org/10.2119/molmed.2015.00087.
- Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, et al. 3rd, Lotze MT, Tang D. HMGB1 in health and disease. Mol Aspects Med. 2014 Dec;40:1–116 . Epub 2014 Jul 8. PMID: 25010388; PMCID: PMC4254084. doi:https://doi.org/10.1016/j.mam.2014.05.001.
- Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, Zou F. High mobility group protein B1 (HMGB1) in asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17(7–8):807–815. doi:https://doi.org/10.2119/molmed.2010.00173. Epub 2011 Mar 3. PMID: 21380479; PMCID: PMC3146613
- Hosakote YM, Brasier AR, Casola A, Garofalo RP, Kurosky A, Lyles DS. Respiratory syncytial virus infection triggers epithelial HMGB1 release as a damage-associated molecular pattern promoting a monocytic inflammatory response. J Virol. 2016 Oct 14;90(21):9618–9631. PMID: 27535058; PMCID: PMC5068515. doi:https://doi.org/10.1128/JVI.01279-16.
- Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, Nakanishi Y. The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008 Oct;39(4):440–447. Epub 2008 Apr 25. PMID: 18441281. doi:https://doi.org/10.1165/rcmb.2007-0330OC.
- Yuan Y, Liu Q, Zhao J, Tang H, Sun J. SIRT1 attenuates murine allergic rhinitis by downregulated HMGB 1/TLR4 pathway. Scand J Immunol. 2018 Jun;87(6):e12667. PMID: 29701897. doi:https://doi.org/10.1111/sji.12667.
- Bellussi LM, Cocca S, Passali GC, Passali D. HMGB1 in the pathogenesis of nasal inflammatory diseases and its inhibition as new therapeutic approach: a review from the literature. Int Arch Otorhinolaryngol. 2017 Oct 4;21(4):390–398. Epub 2017 Jan 4. PMID: 29018504; PMCID: PMC5629088. doi:https://doi.org/10.1055/s-0036-1597665.
- Choi MR, Xu J, Lee S, Yeon SH, Park SK, Rha KS, Kim YM. Chloroquine treatment suppresses mucosal inflammation in a mouse model of eosinophilic chronic rhinosinusitis. Allergy Asthma Immunol Res. 2020 Nov;12(6):994–1011. PMID: 32935491; PMCID: PMC7492509. doi:https://doi.org/10.4168/aair.2020.12.6.994.
- Zheng J, Wei X, Zhan JB, Jiang HY. [High mobility group box1 contributes to hypoxia-induced barrier dysfunction of nasal epithelial cells]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017Aug5; 3115: 1178–1181 Chinese. doi: https://doi.org/10.13201/j.1001-1781.2017.15.009. PMID: 29798353.
- Hu Z, Wang X, Gong L, Wu G, Peng X, Tang X. Role of high-mobility group box 1 protein in inflammatory bowel disease. Inflamm Res. 2015 Aug;64(8):557–563. Epub 2015 Jun 16. PMID: 26077468. doi:https://doi.org/10.1007/s00011-015-0841-x.
- Palone F, Vitali R, Cucchiara S, Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A, Stronati L. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014 Aug;20(8):1448–1457. PMID: 24983978. doi:https://doi.org/10.1097/MIB.0000000000000113.
- Yamasaki H, Mitsuyama K, Masuda J, Kuwaki K, Takedatsu H, Sugiyama G, Yamada S, Sata M. Roles of high-mobility group box 1 in murine experimental colitis. Mol Med Rep. 2009 Jan-Feb;2(1):23–27. PMID: 21475785. doi:https://doi.org/10.3892/mmr_00000056.
- Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006 Apr;290(4):C990–9. Epub 2005 Nov 9. PMID: 16282196. doi:https://doi.org/10.1152/ajpcell.00308.2005.
- Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002 Sep;123(3):790–802. PMID: 12198705. doi:https://doi.org/10.1053/gast.2002.35391.
- Gui Y, Sun J, You W, Wei Y, Tian H, Jiang S. Glycyrrhizin suppresses epithelial-mesenchymal transition by inhibiting high-mobility group box1 via the TGF- β 1/Smad2/3 pathway in lung epithelial cells. PeerJ. 2020 Feb 3;8:e8514. doi:https://doi.org/10.7717/peerj.8514. eCollection 2020.PMID: 32117622.
- Miyakawa M, Konno T, Kohno T, Kikuchi S, Tanaka H, Kojima T. Increase in epithelial permeability and cell metabolism by high mobility group box 1, inflammatory cytokines and TPEN in Caco-2 cells as a novel model of inflammatory bowel disease. Int J Mol Sci. 2020 Nov 10;21(22):8434. PMID: 33182652. doi:https://doi.org/10.3390/ijms21228434.
- Park SA, Kim MJ, Park SY, Kim JS, Lee SJ, Woo HA, Kim DK, Nam JS. Sheen YY. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol Life Sci. 2015 May;72(10):2023–2039. Epub 2014 Dec 9. PMID: 25487606. doi:https://doi.org/10.1007/s00018-014-1798-6.
- Binabaj MM, Asgharzadeh F, Avan A, Rahmani F, Soleimani A, Parizadeh MR, Ferns GA, Ryzhikov M, Khazaei M. Hassanian SM. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. J Cell Physiol. 2019 Jul;234(7):11654–11661. Epub 2018 Nov 27. PMID: 30478959. doi:https://doi.org/10.1002/jcp.27823.
- Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, Suzuki Y, Sayan BS, Knight RA, Melino G. p63 is upstream of IKK alpha in epidermal development. J Cell Sci. 2006 Nov 15;119(Pt22):4617–4622. PMID: 17093266. doi:https://doi.org/10.1242/jcs.03265.
- Kaneko Y, Kohno T, Kakuki T, Takano KI, Ogasawara N, Miyata R, Kikuchi S, Konno T, Ohkuni T, Yajima R, et al. The role of transcriptional factor p63 in regulation of epithelial barrier and ciliogenesis of human nasal epithelial cells. Sci Rep. 2017 Sep 7;7(1):10935. PMID: 28883651; PMCID: PMC5589951. doi:https://doi.org/10.1038/s41598-017-11481-w.
- Kaneko Y, Konno T, Kohno T, Kakuki T, Miyata R, Ohkuni T, Kakiuchi A, Yajima R, Ohwada K, Kurose M, et al. Induction of airway progenitor cells via p63 and KLF11 by Rho-kinase inhibitor Y27632 in hTERT-human nasal epithelial cells. Am J Transl Res. 2019 Feb 15;11(2):599–611. PMID: 30899365; PMCID: PMC6413250.
- Huang H, Tan KS, Zhou S, Yuan T, Liu J, Ong HH, Chen Q, Gao J, Xu M, Zhu Z, et al. p63+Krt5+ basal cells are increased in the squamous metaplastic epithelium of patients with radiation-induced chronic Rhinosinusitis. Radiat Oncol. 2020 Sep 25;15(1):222. PMID: 32977822; PMCID: PMC7517817. doi:https://doi.org/10.1186/s13014-020-01656-7.
- Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, Piccoli P, Cangi G, Semenzato G, Doglioni C. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest. 2002 Oct;82(10):1335–1345. PMID: 12379768. doi:https://doi.org/10.1097/01.lab.0000032380.82232.67.
- Jonsdottir HR, Arason AJ, Palsson R, Franzdottir SR, Gudbjartsson T, Isaksson HJ, Gudmundsson G, Gudjonsson T, Magnusson MK. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Lab Invest. 2015 Dec;95(12):1418–1428. Epub 2015 Sep 21. PMID: 26390052. doi:https://doi.org/10.1038/labinvest.2015.114.
- Ohwada K, Konno T, Kohno T, Nakano N, Ohkuni T, Miyata R, Kakuki T, Kondoh M, Takano K, Kojima T. Effects of HMGB1 on tricellular tight junctions via TGF-β signaling in human nasal epithelial cells. Int. J. Mol. Sci. 2021;22(16):8390. doi:https://doi.org/10.3390/ijms22168390.
- Kodera Y, Chiba H, Konno T, Kohno T, Takahashi H, Kojima T. HMGB1-downregulated angulin-1/LSR induces epithelial barrier disruption via claudin-2 and cellular metabolism via AMPK in airway epithelial Calu-3 cells. Biochem Biophys Res Commun. 2020 Jun 25;527(2):553–560. Epub 2020 May 15.PMID: 32423802. doi:https://doi.org/10.1016/j.bbrc.2020.04.113.
- Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008 Dec;23(Suppl 2):S146–50. PMID: 19120888. doi:https://doi.org/10.1111/j.1440-1746.2008.05405.x.
- Hu JE, Bojarski C, Branchi F, Fromm M, Krug SM. Leptin downregulates angulin-1 in active crohn’s disease via STAT3. Int J Mol Sci. 2020 Oct 22;21(21):7824. PMID: 33105684; PMCID: PMC7672602. doi:https://doi.org/10.3390/ijms21217824.
- Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015 May 26;4:101–109. doi:https://doi.org/10.2147/ITT.S58064. PMID: 27471716; PMCID: PMC4918250.
- Chen R, Huang Y, Quan J, Liu J, Wang H, Billiar TR, Lotze MT, Zeh HJ, Kang R, Tang D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020 Dec 7;6(12):e05672. PMID: 33313438; PMCID: PMC7720697. doi:https://doi.org/10.1016/j.heliyon.2020.e05672.
- Wyganowska-Swiatkowska M, Nohawica M, Grocholewicz K, Nowak G. Influence of herbal medicines on HMGB1 release, SARS-CoV-2 viral attachment, acute respiratory failure, and sepsis. A literature review. Int J Mol Sci. 2020 Jun 30;21(13):4639. PMID: 32629817; PMCID: PMC7370028. doi:https://doi.org/10.3390/ijms21134639.
- Gomes CP, Fernandes DE, Casimiro F, da Mata GF, Passos MT, Varela P, Mastroianni-Kirsztajn G, Pesquero JB. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front Cell Infect Microbiol. 2020;10(10):589505. doi:https://doi.org/10.3389/fcimb.2020.589505.
- Kumar A, Prasoon P, Kumari C, Pareek V, Faiq MA, Narayan RK, Kulandhasamy M, Kant K. SARS-CoV-2-specific virulence factors in COVID-19. J. Med. Virol 2021;93(3):1343–1350. doi:https://doi.org/10.1002/jmv.26615.