?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lutein is a kind of natural carotenoids possessing many pharmacological effects. The application of lutein was limited mainly due to its low oral bioavailability caused by poor aqueous solubility. Nanocrystal formulation of lutein was developed to improve the oral bioavailability in this study. The nanosuspension was prepared by the anti-solvent precipitation-ultrasonication method and optimized by Box–Behnken design, followed by freeze-drying to obtain lutein nanocrystals. The nanocrystals were characterized on their physical properties, in vitro dissolution and in vivo absorption performance. Lutein nanocrystals showed as tiny spheres with an average particle size of 110.7 nm. The result of diffractograms indicated that the percent crystallinity of lutein was 89.4% in coarse powder and then declined in nanocrystal formulation. The saturated solubility of lutein in water increased from 7.3 μg/ml for coarse powder up to 215.7 μg/ml for lutein nanocrystals. The dissolution rate of lutein nanocrystals was significantly higher than that of coarse powder or the physical mixture. The Cmax and AUC0–24 h of lutein nanocrystals after oral administration in rats was 3.24 and 2.28 times higher than those of lutein suspension, respectively. These results indicated that the nanocrystal formulation could significantly enhance the dissolution and absorption of lutein and might be a promising approach for improving its oral bioavailability.
Introduction
Lutein is one of the carotenoids mainly extracted from the marigold [Citation1]. It is an important macular pigment found in retina and possesses a wide range of pharmacological functions, such as protection and improvement of vision, prevention of age-related macular degeneration and cataracts, prophylaxis of cardiovascular disease [Citation2]. Lutein could not be synthesized in human body and entirely gained from the diet [Citation3]. But its absorption is limited by poor solubility [Citation4] and low stability caused by degradation easily at high temperature and light [Citation5]. Several strategies, including nanoemulsions [Citation6], microcapsules [Citation7] and inclusion complex [Citation4], have been applied to improve solubility and/or stability of lutein. And some of these technologies also showed preferable in vivo performance [Citation8].
Nanocrystals was defined as a sub-micron colloidal dispersion and consisted of stabilizers and drugs [Citation9]. It could improve the solubility of poorly soluble drugs through reducing particle size of drug. Some of the stabilizers presented in the nanocrystal formulation showed a solubilizing effect, which might further enhance the in vivo absorption of drug [Citation10,Citation11]. In addition, nanocrystals could be used as an pharmaceutical intermediates to make full use of the advantages of solubilization [Citation12]. Moreover, nanocrystals with high drug loading and dispersion could be used at a lower dosage for oral administration, which provides a better compliance and lower risk for patients [Citation13]. Therefore, the nanocrystal technology might be applied as a preferable strategy to enhance the solubility and oral bioavailability of lutein.
In the present study, lutein nanosuspension was prepared using anti-solvent precipitation method and optimized by employing Box–Behnken design (BBD) of response surface method, followed by freeze-drying to obtain lutein nanocrystals. The morphology, crystallinity, in vitro dissolution and in vivo pharmacokinetic of drug nanocrystals were then investigated and compared with those of coarse lutein.
Materials and methods
Materials
Lutein (purity ≥80%) was provided by Jingzhu Biological Technology Co., Ltd. (Nanjing, China). Soy phosphatidylcholine (SPC) was purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). Polyvinyl pyrrolidone (PVP) K30 was from Hayashibara Co., Ltd. (Henan, China). Methanol (Chromatographic grade) was supplied by Fisher Co., Ltd. (New Jersey, USA). Tetrahydrofuran (THF) was provided by Damao Pharmaceutical Co., Ltd. (Tianjin, China). Mannitol was from Shanghai Macro Chemical Preparation Materials Technology Co., Ltd. (Shanghai, China). Double-distilled water for all experiments was prepared by a Milli-Q Plus integral water purification system (Millipore, Merck, Germany). All other reagents were of analytical grade.
Methods
Preparation of lutein nanosuspension
According to previous reports [Citation14], lutein nanosuspensions were prepared by an anti-solvent nanoprecipitation method as follows: lutein was completely dissolved in THF to obtain a concentrated drug solution of 50 mg/ml. Aqueous solution was prepared using SPC as stabilizer. Then 1 ml lutein solution was rapidly injected into a certain amount of aqueous solution under intense sonication by a JY99-IIDN ultrasonic processor (Scientz Biotechnology Co., Ltd. Ningbo, China) at 800 W in an ice-water bath. Finally, PVP K30 was added into the obtained nanosuspension at a level of 0.5% (w/v) for inhibition of particle aggregation and/or sedimentation.
Formulation optimization of lutein nanosuspension
Both particle size and polydispersity index (PDI) were key variables for assessing drug nanocrystals. Small particle size and good homogeneity may enhance the oral absorption of lutein. To obtain a lutein suspension with nanoscale particle size and narrow size distribution, a three-factor (including ratio of two phases, ultrasonication time and the concentration of SPC), three-level BBD was employed to optimize the formulation. The design and analysis of experiment were performed by Design-Expert software (Version 8.0.6.1, Stat-Ease Inc., Minneapolis, MN). The variables and their levels were listed in .
Table 1. Factors and responses in the Box–Behnken Design.
Preparation of lutein nanocrystals
Based on the optimized formulation, the freshly prepared lutein nanosuspensions were mixed with mannitol (5%, w/v), then pre-frozen for 24 h at –80 °C before freeze-dried for 48 h in a FD-1 C freeze-drier (Beijing, China). The obtained powders were further characterized.
Characterization of lutein nanocrystals
Particle size and size distribution analysis
Lutein nanocrystals were re-dispersed in water and evaluated on their mean particle size using a NICOMPTM 380 particle analyzer (PSS Nicomp, Santa Barbara, CA, USA) at a detection angle of 90°. The size distribution of nanocrystals was expressed by their PDI values.
Morphology observation
Lutein nanocrystals were observed on their appearance before and after freeze-drying, respectively. The sample was dropped on the copper grid, dried and recorded by an H-7650 transmission electron microscope (TEM, Hitachi, Japan). The dry powder of lutein nanocrystals was secured onto a stub, coated with platinum and then analysed under an S-3400 scanning electron microscope (SEM, Intec International Co., Ltd. Hitachi, Japan).
Differential scanning calorimetry (DSC)
The thermograms of raw lutein, excipients (mixture of SPC, mannitol and PVP K30), their physical mixture and lutein nanocrystals were recorded on a SETSYS-1750 CS Evolution thermogravimetry analyzer (SERARAM, France). The accurately weighed samples were tipped into an aluminium pan and analysed at 10 °C/min from 25 to 300 °C under nitrogen atmosphere. The heating curves were recorded using an empty pan as the reference.
X-ray powder diffraction
The X-ray powder diffractogram (XRPD) was performed by a D/MARX2200/PC instrument (Rigaku Co., Tokyo, Japan). The diffractograms of different samples were recorded at 40 mA and 40 kV with a scanning rate of 8°/min over a 2θ range of 3–60°.
Determination of saturation solubility
The solubility of lutein, physical mixture and lutein nanocrystals in different media (0.1 N HCl, water or pH 6.8 PBS) was analysed according to a reported method [Citation15]. Briefly, an excess quantity of sample powder was added into 0.5 ml aqueous medium, and then shaken for 72 h at 100 rpm and 37 °C in the THZ-100B shaking-air bath (Yiheng Scientific Instruments Co., Ltd, Shanghai, China). The supernatant was obtained after centrifugation at 12 000 rpm for 8 min and filtered through 0.22 μm syringe filter. Finally, the content of lutein in samples was detected by a L-2000 liquid chromatography system (Hitachi, Japan) equipped with an ODS Hypersil C18 column (250 mm ×4.6 mm ×3 μm, Thermo, Bellefonte, PA, USA). The mixture of methanol and water (97:3, v/v) was applied as the mobile phase and maintained at a flow rate of 0.7 ml/min. The detection was performed at 445 nm using an UV-Vis detector (Model L7420, Hitachi, Japan).
Dissolution test
The dissolution profiles of raw lutein, physical mixture and lutein nanocrystals were tested by a paddle apparatus (RCZ-8 M, TiandaTianfa Technology Co., Ltd, Tianjin, China). The medium was stirred at 100 rpm and the temperature was set at 37 ± 0.5 °C. The accurately weighed powders (containing 8 mg lutein) were added into 900 ml phosphate buffer solution with 0.5% Tween 20 (PBST, pH 6.8). Aliquots of 5 ml were withdrawn at 2, 4, 6, 8, 10, 15, 30, 45, 60, 90 and 120 min and replaced with an equal volume of fresh medium. The samples were filtered through a 0.22 μm syringe filter and quantified by the above-mentioned High Performance Liquid Chromatography (HPLC) method. The experiments were performed in triplicate and the average ± S.D. was calculated.
Pharmacokinetic study in rats
A total of 12 male Sprague-Dawley rats (7–9 weeks, 200 ± 20 g) were provided by the Laboratory Animal Center of Ningxia Medical University (Yinchuan, China). All procedures were approved by the Animal Research Ethics Committee, General Hospital of Ningxia Medical University. The rats were maintained in a specific pathogen-free environment at 23 ± 2 °C with free access to water and kept fasting for 12 h before experiments.
The rats were randomly divided into two groups and orally administrated with the suspension of raw lutein (the control group) or lutein nanocrystals (the test group) at a dose of 10 mg/kg body weight, respectively. Blood samples of 0.5 ml were obtained from orbital veins and transferred into heparinized tubes at 5, 15, 30 and 45 min, and 1, 3, 5, 8, 12 and 24 h after administration. The samples were centrifuged at 3000 rpm for 10 min and 200 μl supernatant was mixed with 400 μl dichloromethane. The mixture was centrifuged at 12 000 rpm for 10 min and the organic phase was collected and volatilized under nitrogen gas flow. The residue was completely re-dissolved by 100 μl absolute ethanol. The supernatant was collected by centrifugation at 12 000 rpm for 10 min and then directly measured by a HPLC method. The mobile phase was composed of acetonitrile, methanol and dichloromethane at a volume ratio of 60:20:20 and at a flow rate of 0.7 ml/min. The other chromatographic conditions were the same as mentioned above.
The pharmacokinetic parameters were evaluated using non-compartmental model with a DAS 3.0 software package (Bojia Corp., Shanghai, China). The results were expressed as the average and standard deviation. Student’s t-test and one-way analysis of variance (ANOVA) were used to analyse the data using SPSS software (version 17.0, Chicago, USA). p < .05 was considered as statistically significant.
Results and discussion
Analysis of response surfaces
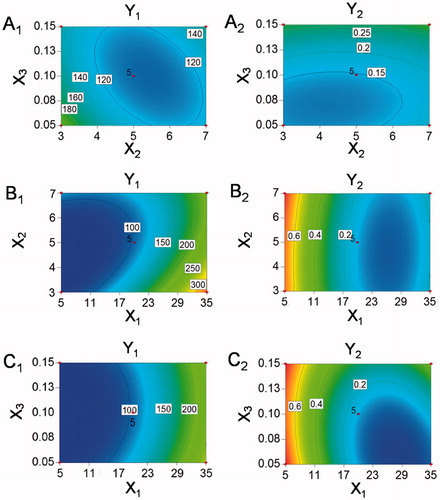
The design matrix and response values were listed in . The particle size (Y1) of nanosuspensions was ranged from 68.2 to 358.3 nm, and PDI (Y2) was from 0.011 to 0.801.
Table 2. Arrangement and results of Box–Behnken design.
All data obtained from the responses of 17 runs were simultaneously fitted to linear, two-factor (2 F) and quadratic model using Design Expert software. The results were depicted in for each response, the best fitted model for particle size and PDI were quadratic models with their coefficient of multiple determinations (r2) valued 0.9462 and 0.9337, respectively. Therefore, the quadratic equations were used to analyse and predict the best formulation.
Table 3. Results of model summary statistics analysis for responses.
The polynomial equations of quadratic model for particle size and PDI were showed in EquationEquations (1)(1)
(1) and Equation(2)
(2)
(2) , the p values of .0012 and .0023 implied the significance of the models (). Therefore, comprehensive analyses of two responses were carried out by the quadratic model.
(1)
(1)
(2)
(2)
Table 4. The quantitative factor effects and associated p values for the responses.
The contour plots were used to analyse the effects of two independent variables on response values. As shown in ), the particle sizes and PDI values of nanosuspensions became smaller as the ultrasonication time was closer to 5 min. The reason might lay in the fact that the degree of particle breakage increased with the extension of ultrasonication time. But the re-aggregation of particles was caused by the heat generated from longer ultrasonication time. The particle size of obtained nanosuspensions decreased with the increase of SPC concentration, but the PDI value was opposite ()), suggesting the particles of nanocrystals showed an inhomogeneous distribution when excess stabilizer was added. The particle sizes increased and PDI values decreased as the ratio of aqueous phase ascending ()), which might be due to the aggregation and precipitation of particles caused by poor dispersion of nanocrystals in a small proportion of aqueous phase.
Formulation optimization of lutein nanosuspension
According to the results of BBD experiments, the optimized formulation was described as follows: ratio of organic phase and aqueous phase was 1:20.49, ultrasonication time was 5.35 min and the concentration of SPC in aqueous solution was 0.08% (w/v). The optimal predictive values were particle size of 106.9 nm and PDI of 0.12. The actual values for optimal formulation were particle size of 110.7 ± 11.2 nm and PDI of 0.141 ± 0.054, which was consistent with the predicted values and favourable for enhancing saturated solubility and dissolution rate of poorly soluble drugs [Citation16].
Characterization of lutein nanocrystals
Particle size and size distribution analysis
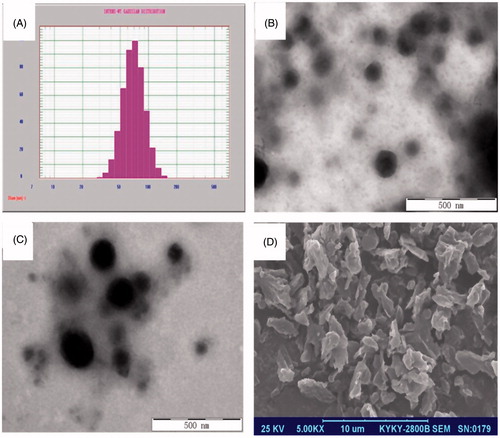
As shown in , the particle size of lutein nanosuspension was 110.7 nm and PDI was 0.141. The reconstituted size of dried nanocrystals (159.5 nm, PDI = 0.079) was slightly larger than that of freshly prepared nanosuspension, indicating certain particle aggregation occurred during the freeze-drying process.
Morphology observation
The dried lutein nanocrystals appeared as an orange powder. As shown in , many irregular particles with their length around 3 μm were observed under SEM. A uniform orange suspension was obtained after re-dispersed in aqueous medium. The nanosuspensions showed as spherical particles under TEM with their diameters ranging from 50 to 200 nm ()). But their size was larger for the reconstituted crystals, which was consistent with the results of particle size analysis.
Differential scanning calorimetry (DSC)
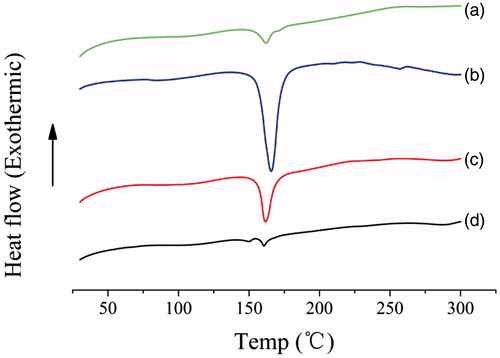
DSC patterns of lutein, excipients, the physical mixture and nanocrystals were shown in . The thermogram of raw lutein powder showed a characteristic peak at 162 °C corresponding to drug melting endotherm. The excipients had one sharp endothermic peak at 168 °C predominantly corresponding to the melting of mannitol [Citation17]. One sharp peak at 165 °C was observed in the physical mixture of drug and excipients, which was possibly due to the overlap of melting endotherms of lutein and mannitol. And this peak still existed but with significantly lower intensity in the drug nanocrystal sample, indicating much loss of its crystallinity [Citation18].
X-ray powder diffraction
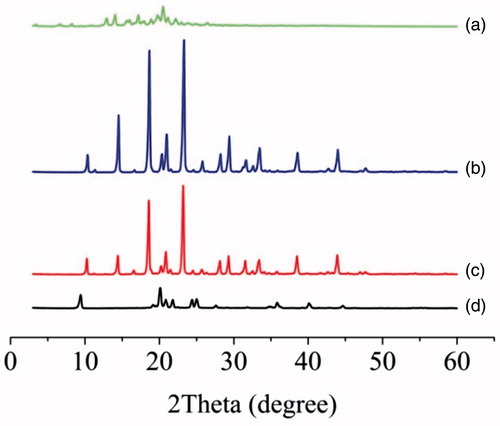
XRPD was performed to determine the physical state of lutein in different samples. As illustrated in , the excipients showed distinct peaks mainly corresponding to mannitol, and these peaks were retained in the physical mixture. The unprocessed lutein powder showed a crystalline compound with intense peaks at 2θ range of 12–25° (12.96°, 14.06°, 15.62°, 17.16°, 18.00°, 19.72°, 20.46°, 21.18°, 22.98°, 23.94° and 24.92°). These peaks of lutein were indiscernible in the diffractogram of physical mixture, which might be assigned to the low content of drug (4.29%). Surprisingly, most characteristic peaks of lutein and mannitol were lost in drug nanocrystals, indicating some interaction between drug and excipients during the drying process [Citation19]. But the exact reason for the change was remained for further study.
Determination of saturation solubility
The saturated solubility of lutein samples in different media was listed in . No drug was detected for all samples when 0.1 N HCl was used as the medium, which possibly due to the acidic nature of lutein [Citation14] or stability of lutein in strong acid solvent [Citation20]. The coarse lutein powder was practically insoluble in water or PBS, whereas it became soluble in the physical mixture or nanocrystals. In comparison to the coarse lutein, the solubility of lutein in water was significantly increased by 13.1 folds for the physical mixture and 29.6 folds for the nanocrystals, and that in PBS was increased 14.1 folds for physical mixture and 37.6 folds for nanocrystals, respectively. The increased solubility of lutein could mainly be assigned to the solubilizing effect of SPC in the physical mixtures [Citation21]. And it was further enhanced in the nanocrystals as a result of the dramatic reduction of particle size [Citation22]. The lower solubility in PBS than in water might be due to the acidic nature of lutein itself [Citation14].
Table 5. Saturation solubility of the coarse lutein, physical mixture and nanocrystals in different media (mean ± S.D., n = 3).
Dissolution test
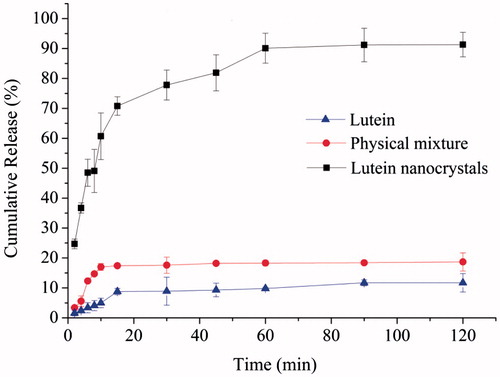
The drug release performances of coarse lutein, physical mixture and nanocrystals were investigated and illustrated in . Compared to the coarse lutein, both physical mixture and drug nanocrystals showed significantly improved dissolution performance (one-way ANOVA, p < .05), and the nanocrystal formulation remained the best. The lutein nanocrystals dissolved 37% within 4 min and 71% within 15 min. However, only 6% of the physical mixture and 3% of coarse lutein dissolved in the first 4 min. And the maximum percentage of dissolved drug for the physical mixture was 18% after 45 min, and that for the coarse lutein was only 10%. These results revealed that the formation of nanocrystal strongly enhanced the dissolution rate and extent of lutein, partly due to the existence of surfactant like SPC in the formulation [Citation21], but mostly relying on the huge reduction of particle size via nanocrystal technology [Citation23,Citation24].
Pharmacokinetic study in rats
To further confirm the advantage of nanocrystal formulation, the in vivo pharmacokinetic performance of lutein nanocrystals was studied in rats and compared to that of the coarse lutein. As shown in , the nanocrystal formulation significantly improved oral absorption of lutein. The results can also be seen in , both formulations reached the maximum plasma drug concentration at around 3 h, but the Cmax and AUC0–24 h value of lutein nanocrystals were 3.24 and 2.28 folds higher than those of the coarse lutein, respectively. These results suggested an improved oral bioavailability of lutein via nanocrystal formulation, which could be mainly attributed to higher solubility and faster dissolution of lutein in nanocrystal form. Besides, the surfactant like SPC may also facilitate the oral absorption of lutein. Similar results were widely observed in previous reports [Citation18,Citation23,Citation25].
Figure 6. Plasma concentration-time curves of the coarse lutein and lutein nanocrystals in SD rats after oral administration (mean ± S.D., n = 6).
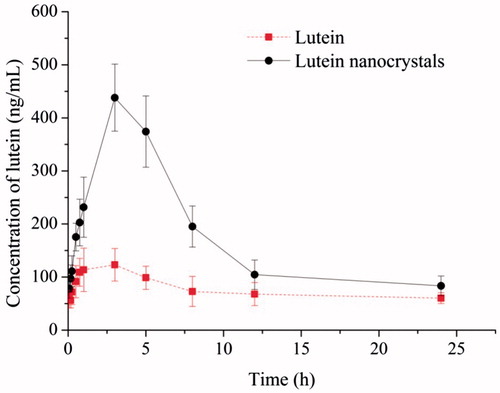
Table 6. Pharmacokinetic parameters following oral administration of the coarse lutein and lutein nanocrystals (mean ± S.D., n = 6).
Conclusion
In this study, lutein nanocrystals were successfully prepared by a simple ultrasonication-aided nanoprecipitation method and optimized with BBD. The obtained nanocrystals were characterized in terms of their morphology, particle size and physical state. Lutein in nanocrystal formulation exhibited significantly enhanced aqueous solubility and dissolution rate in vitro. The pharmacokinetic study indicated that the nanocrystal formulation greatly improved drug absorption in rats after oral administration. These findings revealed that drug nanocrystal technology is a promising approach for enhanced oral bioavailability of lutein.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Statement of animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Acknowledgements
This work was financially supported from the National Natural Science Foundation of China [81360644, 81660665].
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Lin JH, Lee DJ, Chang JS. Lutein in specific marigold flowers and microalgae. J Taiwan Inst Chem Eng. 2015;49:90–94.
- Nimalaratne C, Lopes-Lutz D, Schieber A, et al. A fast isocratic liquid chromatographymethod for the quantification of xanthophylls and their stereoisomers. J Separat Sci. 2016;38:4159–4292.
- Yoo J, Baskaran R, Yoo BK. Self-nanoemulsifying drug delivery system of lutein: physicochemical properties and effect on bioavailability of warfarin. Biomol Ther (Seoul). 2013;21:173–179.
- Stancanelli R, Løjkner LD, Larsen KL, et al. Structural and spectroscopic features of lutein/butanoyl-β-cyclodextrin nanoassemblies. J Pharm Biomed Anal. 2012;71:214–218.
- Chena CY, Jesisca, Hsieh CY, et al. Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresource Tech. 2016;200:500–505.
- Li Y, Zheng JK, Xiao H, et al. Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: influence of formulation parameters on polymethoxyflavone crystallization. Food Hydrocolloids. 2012;27:517–528.
- Kuang PQ, Zhang HC, Bajaj PR, et al. Physicochemical properties and storage stability of lutein microcapsules prepared with maltodextrins and sucrose by spray drying. J Food Sci. 2015;80:359–369.
- Murillo AG, Aguilar D, Norris GH, et al. Compared with powdered lutein, a lutein nanoemulsion increases plasma and liver lutein, protects against hepatic steatosis, and affects lipoprotein metabolism in guinea pigs. J Nutrit. 2016;146:1961–1969.
- Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–840.
- Gao L, Liu GY, Ma JL, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30:307–324.
- Zhang H, Meng Y, Wang XQ, et al. Pharmaceutical and pharmacokinetic characteristics of different type of fenofibrate nanocrystals prepared by different bottom-up approaches. Drug Deliv. 2013;21:588–594.
- Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:784–796.
- Wang YC, Zheng Y, Zhang L, et al. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172:1126–1141.
- Lei YY, Kong YD, Sui H, et al. Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv Transl Res. 2016;6:519–525.
- Mitria K, Shegokara R, Gohlac S, et al. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420:141–146.
- Vuppalapati L, Cherukuri S, Neeli V, et al. Application of central composite design in optimization of valsartan nanosuspension to enhance its solubility and stability. CDD. 2016;13:143–157.
- Daraghmeh N, Chowdhry BZ, Leharne SA, et al. Co-processed chitin-mannitol as a new excipient for oro-dispersible tablets. Mar Drugs. 2015;13:1739–1764.
- Shelar DB, Pawar SK, Vavia PR. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation–high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv Transl Res. 2013;3:384–391.
- Waard HD, Hinrichs WLJ, Frijlink HW. A novel bottom-up process to produce drug nanocrystals: controlled crystallization during freeze-drying. J Control Release. 2008;128:179–183.
- Li DJ, Fang GZ, Liu CQ, et al. Stability of lutein and its esters. Chem Indus Forest Products. 2007;27:112–116.
- Rasch MR, Rossinyol E, Hueso JL, et al. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: membrane-loaded and janus vesicles. Nano Lett. 2010;10:3733–3739.
- Bajaj A, Rao MRP, Pardeshi A, et al. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS PharmSciTech. 2012;13:1331–1340.
- Kesisoglou F, Panmai S, Wu Y. Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–644.
- Tu LX, Yi YN, Wu W, et al. Effects of particle size on the pharmacokinetics of puerarin nanocrystals and microcrystals after oral administration to rat. Int J Pharm. 2013;458:135–140.
- Liu GP, Zhanga DR, Jiao Y, et al. In vitro and in vivo evaluation of riccardin D nanosuspensions with different particle size. Colloids Surfaces B: Biointerfaces. 2013;102:620–626.